Abstract
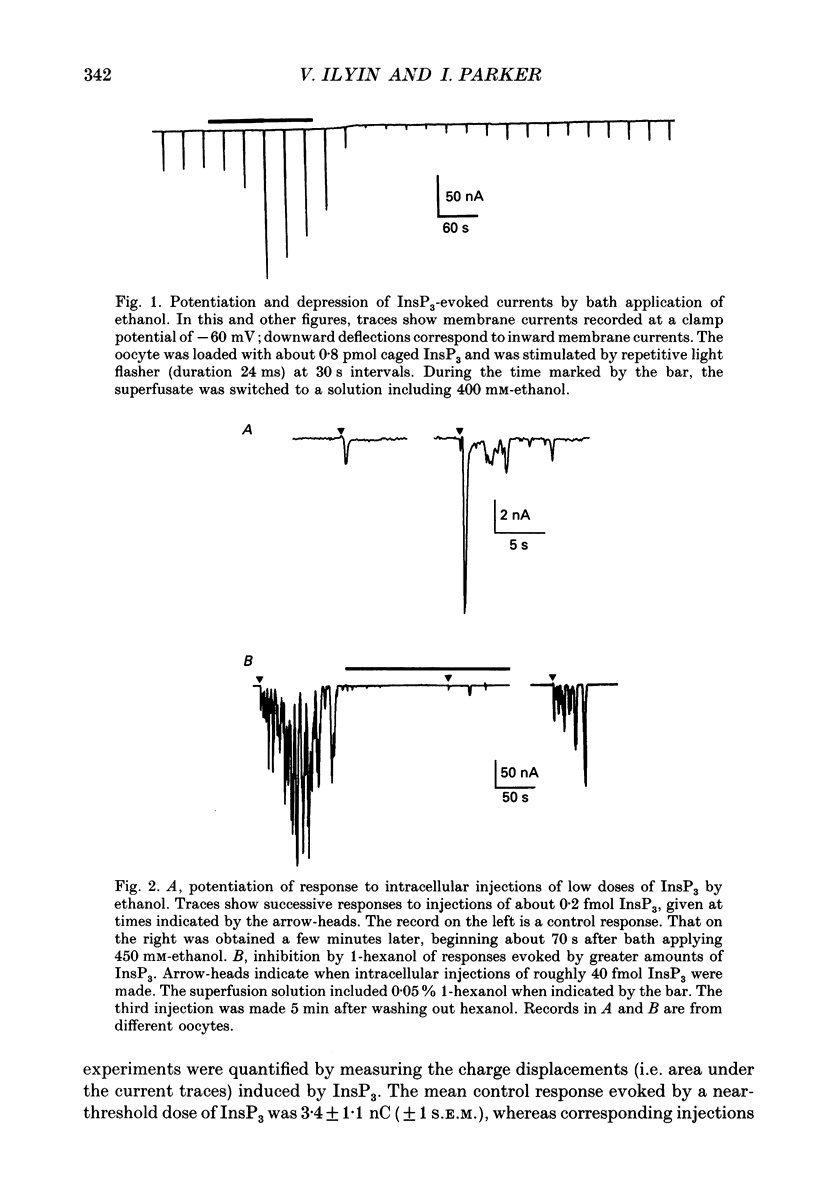
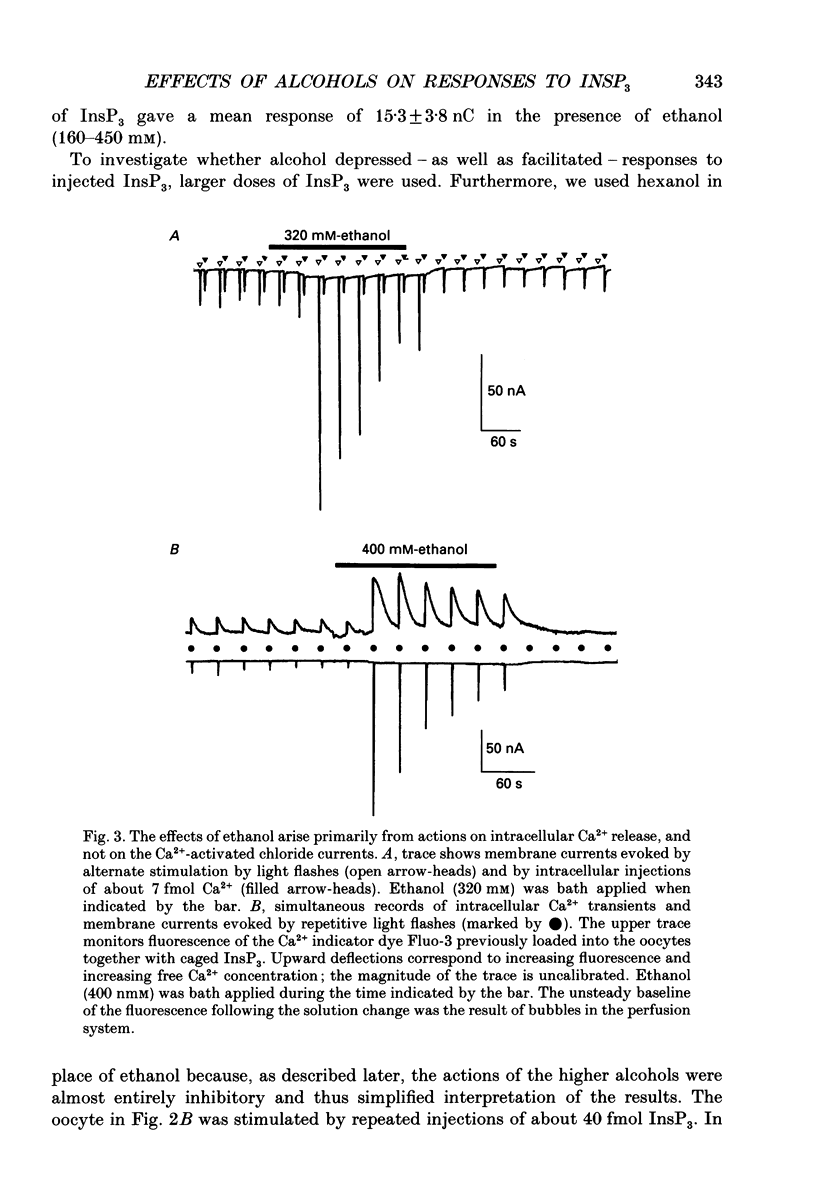
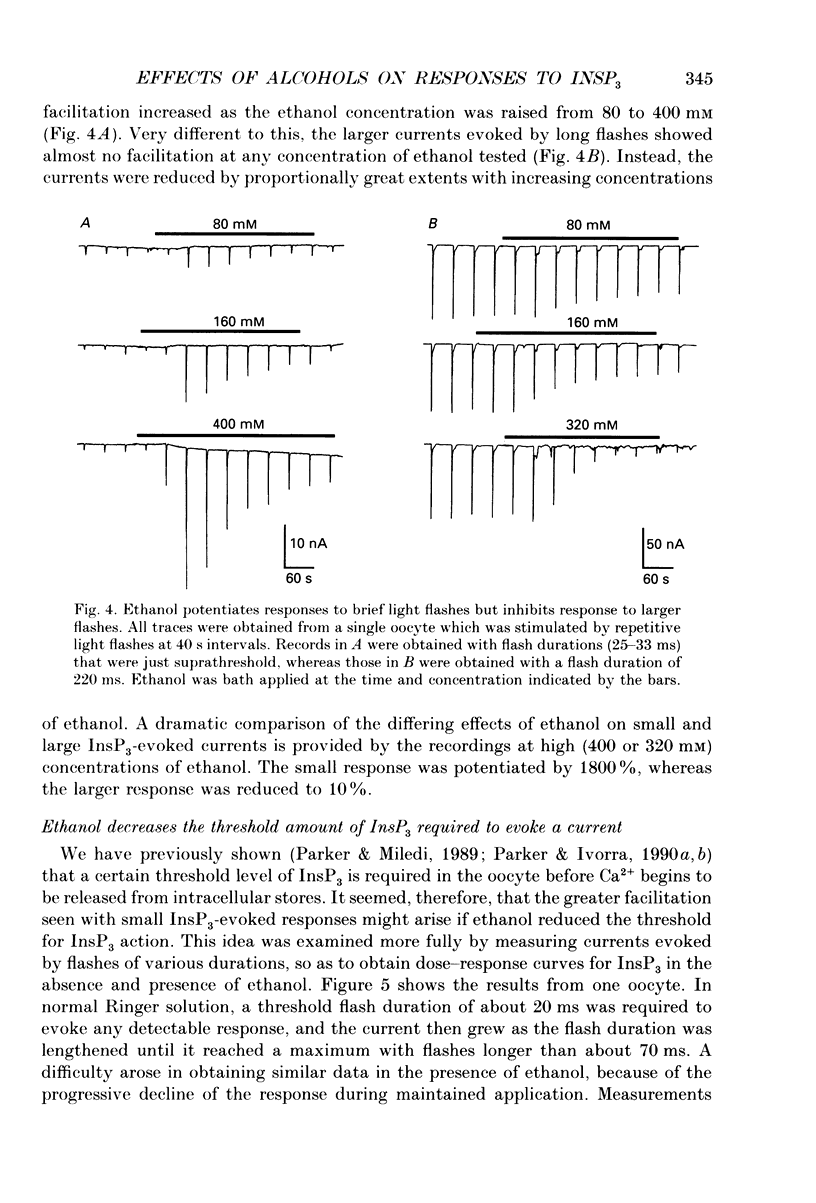
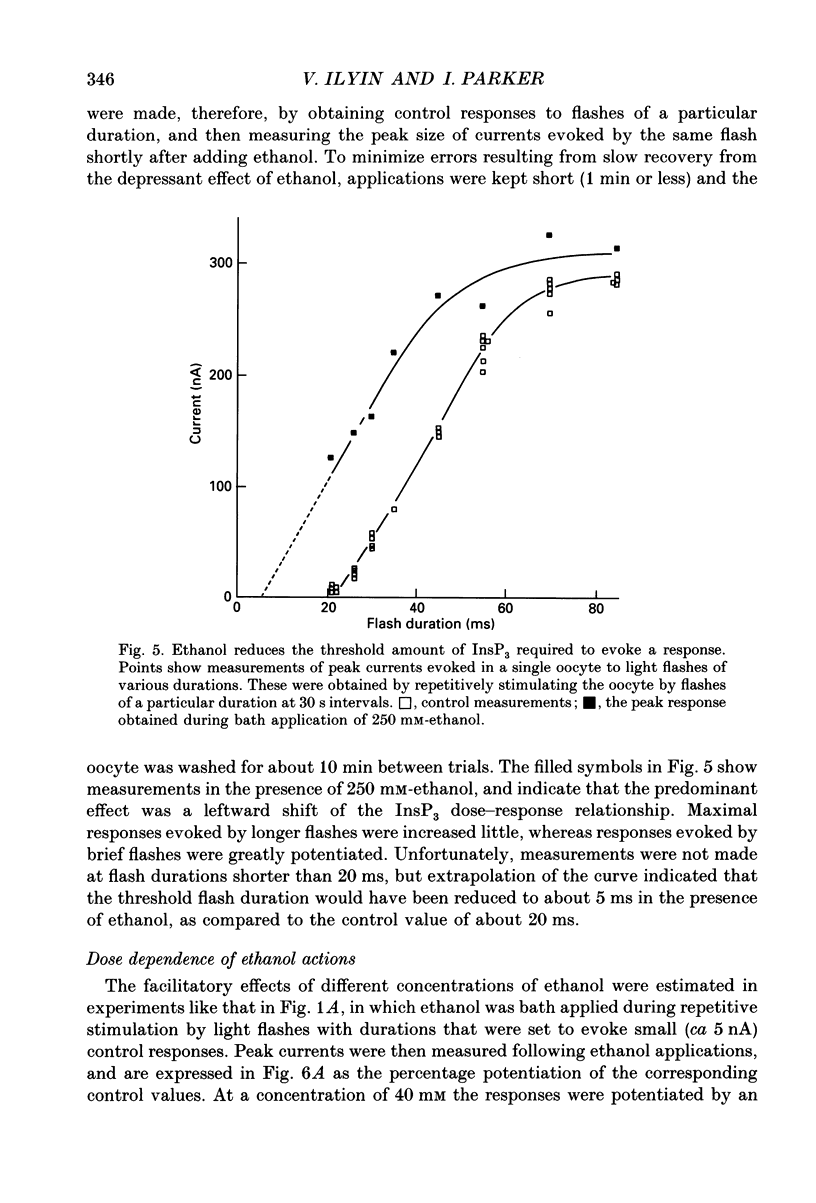
1. The effects of ethanol and other alcohols on inositol 1,4,5-trisphosphate (InsP3) signalling were studied in Xenopus oocytes by the use of flash photolysis of caged InsP3. Calcium liberation induced by InsP3 was monitored by voltage-clamp recording of Ca(2+)-activated membrane currents, and by fluorescence of the Ca2+ indicator Fluo-3. 2. Membrane current and fluorescence Ca2+ signals evoked by light flashes giving small responses were initially potentiated by bath application of ethanol (80-400 mM). However, the responses subsequently declined while ethanol was present and were strongly reduced or suppressed when it was removed. 3. These effects did not arise artifactually from changes in photolysis of caged InsP3, as similar results were seen with responses evoked by intracellular injections of InsP3. Also, the effects on the membrane current did not arise primarily through actions on the Ca(2+)-dependent Cl- channels, since currents evoked by intracellular injections of Ca2+ were little changed by ethanol. 4. Ethanol reduced the threshold level of InsP3 required to cause Ca2+ liberation. Thus, potentiation was most prominent with small responses evoked by brief light flashes, whereas the predominant effect on larger responses was inhibitory. 5. The facilitatory and inhibitory actions of ethanol persisted after removing extracellular Ca2+. 6. Intracellular injections of ethanol produced an initial inhibition of InsP3 responses, followed, in some oocytes, by a potentiation. 7. Methanol had little effect on InsP3 responses, whereas butanol and other long-chain alcohols produced strong inhibition, but little or no potentiation. 8. We conclude that extracellular application of ethanol produces a rapid potentiation of InsP3-mediated Ca2+ liberation, and a more slowly developing inhibition. The potentiation may arise through stimulation of InsP3 formation at the plasma membrane, whereas the inhibition occurs more deeply in the cell. Both actions were evident at relatively low concentrations (a few tens of millimoles per litre), and might thus be important in the behavioural effects of ethanol intoxication.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Whittingham D. G., Cobbold P. H. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature. 1981 Dec 24;294(5843):754–757. doi: 10.1038/294754a0. [DOI] [PubMed] [Google Scholar]
- Daniell L. C., Brass E. P., Harris R. A. Effect of ethanol on intracellular ionized calcium concentrations in synaptosomes and hepatocytes. Mol Pharmacol. 1987 Dec;32(6):831–837. [PubMed] [Google Scholar]
- Davidson M., Wilce P., Shanley B. Ethanol increases synaptosomal free calcium concentration. Neurosci Lett. 1988 Jun 29;89(2):165–169. doi: 10.1016/0304-3940(88)90375-8. [DOI] [PubMed] [Google Scholar]
- Gallaher E. J., Parsons L. M., Goldstein D. B. The rapid onset of tolerance to ataxic effects of ethanol in mice. Psychopharmacology (Berl) 1982;78(1):67–70. doi: 10.1007/BF00470591. [DOI] [PubMed] [Google Scholar]
- Gandhi C. R., Ross D. H. Influence of ethanol on calcium, inositol phospholipids and intracellular signalling mechanisms. Experientia. 1989 May 15;45(5):407–413. doi: 10.1007/BF01952021. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Thomas A. P., Rubin R., Rubin E. Ethanol-induced mobilization of calcium by activation of phosphoinositide-specific phospholipase C in intact hepatocytes. J Biol Chem. 1987 Jan 15;262(2):682–691. [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machu T., Woodward J. J., Leslie S. W. Ethanol and inositol 1,4,5-trisphosphate mobilize calcium from rat brain microsomes. Alcohol. 1989 Nov-Dec;6(6):431–436. doi: 10.1016/0741-8329(89)90047-5. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991 Feb;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Flores T. M., Fein A. Feedback inhibition by calcium limits the release of calcium by inositol trisphosphate in Limulus ventral photoreceptors. Neuron. 1990 Apr;4(4):547–555. doi: 10.1016/0896-6273(90)90112-s. [DOI] [PubMed] [Google Scholar]
- Rabe C. S., Weight F. F. Effects of ethanol on neurotransmitter release and intracellular free calcium in PC12 cells. J Pharmacol Exp Ther. 1988 Feb;244(2):417–422. [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rand M. L., Vickers J. D., Kinlough-Rathbone R. L., Packham M. A., Mustard J. F. Thrombin-induced inositol trisphosphate production by rabbit platelets is inhibited by ethanol. Biochem J. 1988 Apr 1;251(1):279–284. doi: 10.1042/bj2510279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. L. Chronic ethanol consumption reduces [3H]inositol (1,4,5) trisphosphate specific binding in mouse cerebellar membrane fragments. Life Sci. 1987 Dec 28;41(26):2863–2868. doi: 10.1016/0024-3205(87)90433-4. [DOI] [PubMed] [Google Scholar]
- Ticku M. K. Ethanol and the benzodiazepine-GABA receptor-ionophore complex. Experientia. 1989 May 15;45(5):413–418. doi: 10.1007/BF01952022. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Dunwiddie T. V., Harris R. A. Calcium-dependent chloride currents elicited by injection of ethanol into Xenopus oocytes. Brain Res. 1989 Dec 29;505(2):215–219. doi: 10.1016/0006-8993(89)91445-5. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Snyder S. H. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989 Jan;9(1):339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


