Abstract
Introduction
The availability of anti-amyloid therapy for mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s dementia (AD) has underscored the need for realistic estimates of the population with AD/MCI within the healthcare system to assure adequate preparedness. We hypothesize that administrative databases can provide real-world epidemiologic estimates reflecting the population with diagnosed (known) MCI and AD. This study was conducted to estimate diagnostic incidence and prevalence of AD and all-cause MCI among the Medicare fee-for-service (FFS) and Medicare Advantage (MA) beneficiaries in the United States.
Methods
This was a retrospective analysis of Medicare beneficiaries (aged 65 and older) with identified diagnoses of AD/MCI based on ≥ 2 diagnostic codes ≥ 30 days apart. Incidence/prevalence estimates were reported per 10,000 person-years.
Results
In FFS, AD incidence (2008–2018) decreased (138 to 104); MCI incidence increased (8 to 47), but the sum (MCI + AD) was relatively stable (146 to 151). Prevalence (2008–2017) increased for AD (318 to 354), and MCI (13 to 99). In MA (2016) epidemiological estimates were consistent with FFS. In 2017, older age, female sex and the Northeastern region were consistently associated with higher AD/MCI prevalence among FFS beneficiaries.
Conclusion
In FFS, AD/MCI diagnostic prevalence increased over 10 years, especially for MCI; prevalence estimates in MA (2016) were comparable. Diagnostic prevalence in 2016 (FFS + MA) was 3.4% for AD and 0.85% for MCI. Our findings address the reality of Alzheimer’s disease in clinical practice in the United States that is confronted by healthcare professionals, payors, healthcare decision-makers, patients, and caregivers, and may offer a realistic gauge for patient triage for treatment, healthcare resource allocation, and health-systems’ operational prioritization. With the availability of anti-amyloid treatments, we anticipate that the population with diagnosed MCI/AD within the Medicare database may rise over time; therefore, periodic updates of incidence/prevalence estimates may provide support for timely healthcare decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00695-6.
Keywords: Alzheimer’s disease, Incidence, Medicare, Mild cognitive impairment, Prevalence
Key Summary Points
| Why carry out this study? |
| The availability of anti-amyloid treatments (AAT) indicated for initiation in mild cognitive impairment (MCI) due to Alzheimer’s disease (AD) or mild AD dementia has underscored the need to have realistic estimates of the patient population with AD/MCI in the healthcare system to assure adequate preparedness. |
| We hypothesized that administrative databases would provide epidemiologic estimates that reflect the real-world population with diagnosed (known) MCI and AD. |
| What was learned from the study? |
| There were 1,423,401 (3.4%) and 354,751 (0.85%) Medicare beneficiaries with diagnostically coded AD and all-cause MCI, respectively in 2016; estimates of AD/MCI among fee-for-service (FFS) and Medicare Advantage (MA) beneficiaries were comparable. |
| Based on the current study, health care professionals are expected to manage and treat substantially fewer patients with AD/MCI seeking care in the current healthcare system than anticipated based on epidemiologic studies that use active disease detection methodologies. |
| Our findings address the reality of AD in clinical practice that is confronted by healthcare professionals, payors, healthcare decision-makers, patients, and caregivers, and may offer a realistic gauge for patient triage for treatment, healthcare resource allocation, and health-systems’ operational prioritization. |
Introduction
Alzheimer's disease (AD) is a chronic, progressively disabling, neurodegenerative condition that accounts for 60–80% of dementia cases [1]. The Alzheimer’s Association estimates that among people aged 65 years and older in America, approximately 6.9 million and 5–7 million are living with AD and mild cognitive impairment (MCI) due to AD, respectively [1]. Rates of AD rise markedly with age, particularly among people aged 65 years and older [2]. The United States (US) Census projects that 23% of the population will be aged 65 or older by 2060 [3]; likewise, the burden of AD in the US is projected to rise [4].
The availability of anti-amyloid treatments (AAT), which are indicated for initiation in the early stages of AD (MCI due to AD or mild AD dementia), has changed the therapeutic landscape for what has historically been considered a disorder with no disease-modifying therapy options [5–10]. Concerns over the “uncertainty regarding the potential use” of AAT was a contributing factor in the US Centers for Medicare and Medicaid Services (CMS) decision to raise the standard monthly premium for Medicare Part B (medical insurance) enrollees by 14.5% in 2022 [11]. Subsequently it was determined that premiums would be “adjusted downward to account for an overestimate,” based on lower-than-expected Medicare Part B spending on AAT [12]. This episode underscores the potential economic impacts of AAT and the need to have realistic estimates of the patient population with AD/MCI in the healthcare system.
We hypothesize that administrative databases can provide real-world epidemiologic estimates that reflect the population with diagnosed (known) MCI and AD. Recently epidemiologic estimates from the Medicare database have examined rates of dementia, but not AD specifically; additionally, these estimates seldom include MCI [13–17]. We aimed to estimate the incidence and prevalence of clinician-diagnosed AD specifically as well as of all-cause MCI among US Medicare fee-for-service (FFS) plus Medicare Advantage (MA) beneficiaries.
Methods
Study Design and Data Source
This was a retrospective analysis of the CMS Medicare (US government’s health insurance program) administrative database [18], which includes FFS, the traditional Medicare program, plus MA, the capitated program. Collectively, the CMS Medicare (FFS + MA) database includes nationwide healthcare claims for all people qualified for Medicare benefits, with over 85% of beneficiaries aged 65 and older [19]. For FFS beneficiaries, available CMS data allowed estimation of incidence rates over 11 years (2008–2018) and prevalence over 10 years (2008–2017); for MA beneficiaries, epidemiologic estimates could be done for 1 year (2016) based on available MA data from CMS and our case ascertainment method (described below). Of note, MA has experienced rapid growth over recent years [20] and complements FFS; taken together, FFS and MA plans can offer a comprehensive understanding of epidemiology, especially concerning chronic diseases, among the population aged ≥ 65 years in the US. This study does not involve any new research with human participants or animals conducted by the authors.
AD and MCI Case Identification
The study sample was identified using International Classification of Diseases (ICD) 9th or 10th Revision, clinical modification (CM) diagnostic codes. The AD cases were identified using ICD-9-CM 331.0 or ICD-10-CM G30.X, including G30.9, G30.8, and G30.1; the MCI cases were identified using ICD-9-CM 331.83 or ICD-10-CM G31.84 which correspond to MCI of uncertain or unknown etiology (See Table S1 in supplementary material). The case definition was age ≥ 65 years with 2 diagnostic codes (i.e., 2 AD codes or 2 MCI codes) at least 30 days apart within 365 days. Diagnoses codes from all settings were included (e.g., out-patient, in-patient, skilled nursing facilities, etc.). We included beneficiaries who had full yearly membership. We identified individuals across the study period who fulfilled the case definition and flagged them as AD or MCI; these individuals were included in the numerator for incidence and prevalence estimation.
This analysis conformed to CMS privacy requirements; an approval for the overarching database research protocol was obtained through a central institutional review board (IRB). The entire CMS beneficiary population over the selected time period was analyzed to assure diversity, equity, and inclusion. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patient data were de-identified, and due to the study's retrospective design, obtaining formal patient consent was not required.
Statistical Analysis
This was a descriptive analysis. Demographic characteristics (age, sex, and race/ethnicity) and comorbidities [21] were collected and summarized for the beneficiaries who met the case definition for AD or MCI in 2016, as this was the latest year in which data were available to us for both FFS and MA after applying the AD and MCI case definitions. Categorical variables were summarized as numbers and percentages, and continuous variables were summarized using mean, standard deviation (SD), median, and interquartile ratio (IQR).
The epidemiologic estimations of AD and MCI cases per 10,000 person-years were reported. Yearly incidence was calculated as follows: The number of new cases during a year divided by the total population at risk for that year. The “at-risk” population for incidence calculation was defined as the total population minus the already diagnosed population. Yearly prevalence was calculated by dividing the total number of cases in a year by the total at-risk population during that year. The at-risk population for prevalence calculation was defined as the total number of Medicare FFS or MA beneficiaries less those who switched between their Medicare plans or became deceased by that year. Medicare patients may switch between FFS and MA plans due to differences in covered benefits, premiums, and out-of-pocket costs [20]. Medicare beneficiaries with mixed enrollment between FFS and MA in a given year were classified as MA beneficiaries for this study.
The study population was limited to beneficiaries aged ≥ 65 years in each year. Additionally, 95% confidence intervals were calculated using the normal approximation method, but not reported if too narrow.
Incidence and prevalence rates for AD and MCI in 2017 (the latest year with FFS data) were stratified by the following subgroups: age (65–69 years; 70–74 years; 75–79 years; 80–84 years; 85+ years), sex (female; male), race/ethnicity (American Indian; Asian/Pacific Islander; Black; Hispanic; white), and geographic region (each of the 50 United States and the District of Columbia). Analyses of sex and race/ethnicity (Black; Hispanic; white) by age group were also conducted. For the geographic classification, US regions were derived using beneficiary zip codes and categorized by National Center for Chronic Disease Prevention/Health Promotion regions [22].
Subgroups of age ≥ 85 and minority race/ethnicity groups with a small sample size were combined in reporting in conformation to CMS confidentiality requirements. All analyses were conducted using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, North Carolina, USA).
Sensitivity Analyses
We conducted a sensitivity analyses of cumulative prevalence for both AD and MCI in the FFS population over time (2008–2017), based on the following considerations: (1) AD is an absorbing state (i.e., once diagnosed, it will not resolve, because there is no cure); (2) Medicare has a stable plan membership since beneficiaries over 65 years are qualified until death; (3) individuals in the clinical work-up stream may or may not have AD-specific clinical visits each year; and (4) we used a strict definition of AD requiring 2 or more diagnostic codes, 30 days apart, intending to target individuals more likely to fulfil a clinical diagnosis of AD. These factors informed our approach of accumulating prevalent cases (i.e., cumulative prevalence) across the years until the switch to plan type or death. For MCI, we considered that some patients may progress to AD/dementia, Cumulative prevalence was calculated by dividing the total number of prevalence cases identified from prior year(s) plus incidence cases of the current year (year of interest) by the total at-risk population in the year.
Another sensitivity analysis examined use of symptomatic treatments for AD, specifically the acetylcholinesterase inhibitors (AChEIs: donepezil, rivastigmine, galantamine) and memantine among all CMS (FFS + MA) beneficiaries who met the ascertainment criteria for AD and/or MCI in 2016. Additionally, AD and MCI prevalence-related sensitivity analyses were conducted in CMS beneficiaries in 2016 using extended diagnostic coding that may reflect clinical practice where such non-specific codes appear to be used commonly: F01 through F04 were included in addition to the AD diagnostic codes used in the main analysis, and R41.x codes were included in addition to the MCI codes used in the main analysis.
Results
2016 Study Sample: Overall CMS, AD, and MCI
In 2016, there were 41,709,347 Medicare beneficiaries in the CMS database (Table 1), excluding those who had partial membership for the year (10,552,830) and those aged < 65 years (n = 9,310,689). The mean (SD) age of the overall beneficiary population was 75.4 (7.5) years, 56.9% were female, the racial/ethnic distribution was predominantly white (82.1%), followed by Black (8.9%), Asian (2.4%), and Hispanic (2.4%).
Table 1.
Demographics and insurance type of CMS (FFS + MA) beneficiaries aged ≥ 65 years (2016)
| All CMS Beneficiaries Aged ≥ 65 Years | CMS Beneficiaries With AD | CMS Beneficiaries With MCI | |
|---|---|---|---|
| n = 41,709,347 | 1,423,401 | 354,751 | |
| Age, years | |||
| Mean (SD) | 75.4 (7.5) | 82.6 (7.9) | 80.5 (7.8) |
| Median (IQR) | 74 (69, 80) | 83 (77, 89) | 80 (75, 86) |
| Sex, % | |||
| Female | 56.8 | 66.5 | 59.1 |
| Male | 43.2 | 33.5 | 40.9 |
| Race/ethnicity group, % | |||
| White | 82.1 | 82.5 | 85.9 |
| Black | 8.9 | 9.6 | 7.3 |
| Hispanic | 2.4 | 3.7 | 2.8 |
| Asian/Pacific Islander | 2.4 | 1.9 | 1.4 |
| American Indian | 0.4 | 0.2 | 0.2 |
| Other | 2.0 | 1.5 | 1.6 |
| Unknown | 1.6 | 0.5 | 0.8 |
CMS Centers for Medicare and Medicaid Services, FFS Fee-for-service, IQR interquartile range, MA Medicare Advantage, SD standard deviation
A total of 1,423,401 (3.4%) beneficiaries with AD and 354,751 (0.85%) with MCI were identified (Table 1) in 2016, the year where the AD and MCI prevalence could be appropriately estimated with available CMS data for both FFS and MA. The majority were white (AD, 82.5%; MCI, 85.9%), followed by Black (AD, 9.6%; MCI, 7.3%), female (AD, 66.5%; MCI, 59.1%), and enrolled in FFS (AD, 66.5%; MCI, 65.5%). Comorbidities among FFS and MA beneficiaries with AD and MCI are summarized in Table 2. Hypertension was the most common medical comorbidity in beneficiaries with AD and MCI in both the FFS and MA groups (83–84%), followed by hyperlipidemia (69–76%). The most frequent psychiatric comorbidities were depression (39–43%), followed by anxiety (28–31%).
Table 2.
Comorbidities of CMS beneficiaries with AD or MCI (2016)
| FFS | MA | |||
|---|---|---|---|---|
| AD | MCI | AD | MCI | |
| n = 946,746 | n = 232,345 | n = 476,655 | n = 122,406 | |
| Comorbiditya, % | ||||
| Hypertension | 84.3 | 82.9 | 84.4 | 83.6 |
| Hyperlipidemia | 66.8 | 75.7 | 72.1 | 75.6 |
| Depression | 41.4 | 39.3 | 42.5 | 41.6 |
| Diabetes | 34.9 | 34.3 | 38.1 | 37.1 |
| Muscle disorder | 41.1 | 33.8 | 26.0 | 21.8 |
| GERD | 33.1 | 35.2 | 34.5 | 38.4 |
| Hypothyroidism | 32.7 | 32.2 | 32.2 | 29.8 |
| Anxiety | 31.4 | 30.7 | 27.7 | 28.5 |
| Coronary artery disease | 30.3 | 32.6 | 28.7 | 29.2 |
| Cerebrovascular disease | 31.4 | 35.2 | 29.5 | 32.1 |
| Anemia | 32.1 | 28.1 | 27.0 | 26.0 |
| COPD | 27.0 | 26.9 | 26.3 | 26.9 |
| Peripheral vascular disease | 20.0 | 16.1 | 22.3 | 18.1 |
| CHF | 10.2 | 10.2 | 13.1 | 12.5 |
| Parkinson | 7.3 | 8.9 | 6.2 | 7.1 |
| Myocardial infarction | 6.2 | 6.9 | 9.0 | 10.1 |
| Renal disease | 3.5 | 3.1 | 2.7 | 2.5 |
| Mild liver disease | 2.2 | 3.2 | 2.4 | 3.4 |
| Peptic ulcer disease | 2.4 | 2.5 | 2.2 | 2.2 |
| Hyperthyroidism | 1.8 | 1.8 | 1.8 | 1.8 |
| Metastatic carcinoma | 1.4 | 1.9 | 1.3 | 1.6 |
| Rheumatic diseases | 1.0 | 1.5 | 1.0 | 1.3 |
| Moderate or severe liver disease | 0.4 | 0.6 | 0.4 | 0.5 |
| HIV | 0.1 | 0.2 | 0.1 | 0.2 |
CMS Centers for Medicare and Medicaid Services, COPD chronic obstructive pulmonary disease, CHF congestive heart failure, FFS Fee-for-service, GERD gastroesophageal reflux disease, HIV human immunodeficiency virus, MA medicare advantage
aA non-specific dementia diagnostic code was recorded in 85.8% and 84.4% of FFS and MA beneficiaries, respectively, who met the case definition for AD and 43.9% and 41.3% of FFS and MA beneficiaries, respectively who met the case definition for MCI
Annual Trends in Incidence and Prevalence of AD and MCI
Incidence Trends
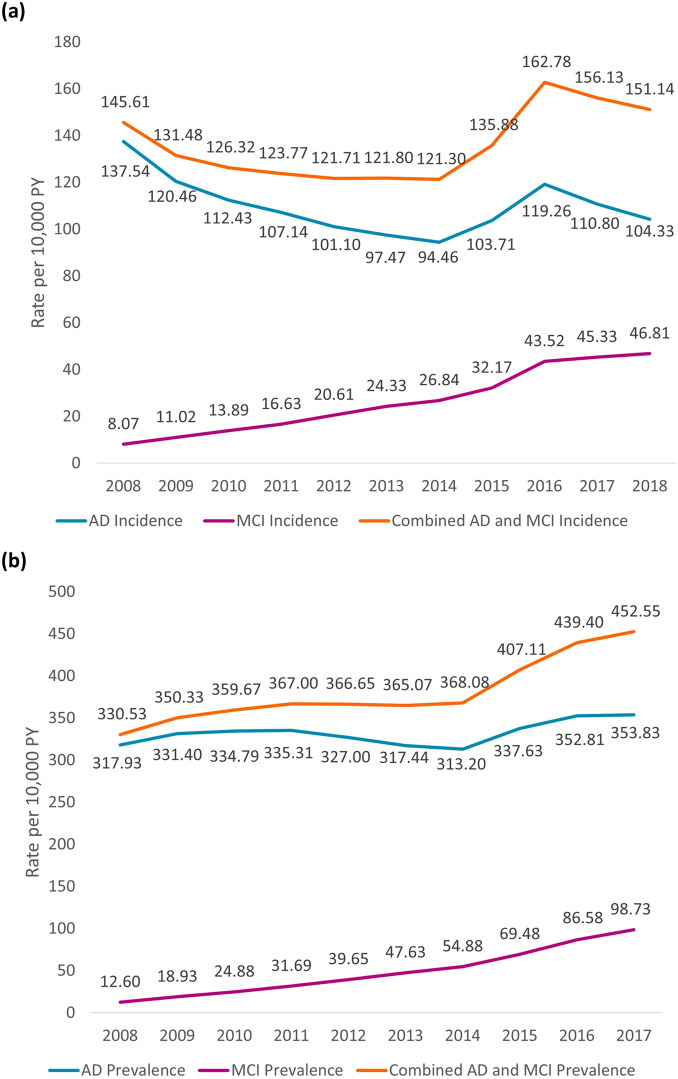
In the FFS population, the yearly incidence of AD per 10,000 person-years generally decreased from 137.5 to 104.3 over the analysis period from 2008 through 2018; however, the yearly incidence of MCI markedly increased from 8.1 to 46.8 per 10,000 person-years (Fig. 1a). The combined yearly incidence of AD + MCI per 10,000 person-years in the FFS population was relatively unchanged: 146.6 in 2008 to 151.1 in 2018 (Fig. 1a). In the MA population, the incidence rates of AD and MCI per 10,000 person-years in 2016 were 133.8 and 44.9, respectively.
Fig. 1.
Epidemiological trends in the FFS populationa: a yearly incidence of AD and MCI (2008–2018); b yearly prevalence of AD and MCI (2008–2017). AD Alzheimer’s disease, FFS fee-for-service, MCI mild cognitive impairment. aThe summed estimation has some overlap since patients could meet criteria for both MCI and AD within a single year
Prevalence Trends
In the FFS population, the yearly prevalence of AD per 10,000 person-years generally rose from 317.9 to 353.8 over the analysis period from 2008 through 2017, while the yearly prevalence of MCI increased from 12.6 to 98.7 (Fig. 1b). The combined yearly prevalence of AD + MCI per 10,000 person-years in the FFS population rose from 330.5 in 2008 to 452.6 in 2018 (Fig. 1b). In the MA population, the prevalence rates of AD and MCI per 10,000 person-years in 2016 were 320.5 and 82.3, respectively.
Incidence and Prevalence Trends Stratified by Sex or by Race Over Time (FFS)
The epidemiologic trends over time among FFS beneficiaries by sex and race subgroups over time were mostly consistent with the overall population trends (See Fig. S1 in supplementary material). Females had higher incidence and prevalence of AD and MCI than males in all years assessed; however, the gap between sexes was much wider for AD than for MCI. When stratified by race/ethnicity, Hispanic beneficiaries consistently had higher incidence and prevalence of AD relative to other racial/ethnic subgroups; however, the racial/ethnic distribution for MCI differed, with white beneficiaries having a higher incidence and prevalence of MCI relative to other subgroups.
Subgroup Analyses Findings (FFS, 2017)
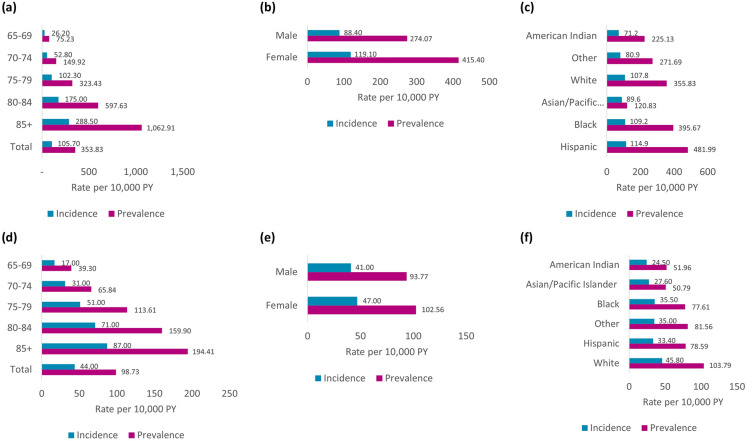
Age
The yearly incidence of AD per 10,000 person-years increased consistently from the youngest to oldest aged subgroup, from 26.2 in beneficiaries aged 65–69 years to 288.5 in those aged ≥ 85 years (Fig. 2a). The yearly prevalence of AD per 10,000 person-years also rose markedly by age, from 75.2 in the 65–69 years age subgroup to 1,062.9 in the ≥ 85 years age subgroup. The yearly incidence of MCI per 10,000 person-years increased from the youngest to oldest aged subgroup, from 17.0 among beneficiaries aged 65–69 years to 87.0 in those aged ≥ 85 years; the yearly prevalence of MCI per 10,000 person-years also rose from 39.3 in the 65–69 years subgroup to 194.4 in the ≥ 85 years subgroups (Fig. 2d).
Fig. 2.
Epidemiology of AD and MCI by demographic subgroups in the FFS population (2017): a AD by age; b AD by sex; c AD by race; d MCI by age; e MCI by sex; f MCI by race. AD Alzheimer’s disease, FFS fee-for-service, MCI mild cognitive impairment
Sex
In the FFS population, both the yearly incidence and prevalence of AD per 10,000 person-years were higher in women (119.1 and 415.4, respectively) than in men (88.4 and 274.1, respectively) (Fig. 2b). The yearly incidence and prevalence of MCI per 10,000 person-years were also both higher in women (47.0 and 102.6, respectively) than in men (41.0 and 93.8, respectively) (Fig. 2e).
Analyses of sex by age subgroup found that the incidence and prevalence of AD and MCI per 10,000 person-years in both males and females consistently increased with age (see Figure S2A–D in supplementary material). For AD, widest gap between females and males was observed in the ≥ 85 years subgroup, and this gap was particularly pronounced for the prevalence data. For MCI, the gap in incidence and prevalence between sexes was not pronounced and widening in the ≥ 85 years subgroup was not observed.
Race/Ethnicity
The yearly incidence of AD per 10,000 person-years did not greatly differ by ethnicity but was slightly higher among Hispanic (114.9), and Black (109.2) beneficiaries compared to white (107.8) beneficiaries. The prevalence of AD per 10,000 person-years was also highest in Hispanic (482.0), followed by Black (395.7), and white (355.8) beneficiaries (Fig. 2c). The yearly incidence of MCI per 10,000 person-years was highest among white beneficiaries (45.8) followed by Black (35.5) and Hispanic (33.4) beneficiaries; the yearly prevalence of MCI per 10,000 person-years was highest in white (103.8), followed by Hispanic (78.6) and Black (77.6) beneficiaries (Fig. 2f).
Analyses of race/ethnicity by age group found that the incidence and prevalence of both AD and MCI per 10,000 person-years generally increased with age for Black, Hispanic, and white beneficiaries (See Figure S2E–H in supplementary material). The incidence of AD was higher in Black beneficiaries relative to other race/ethnic groups in all age subgroups < 85 years; prevalence of AD in beneficiaries who were Hispanic was higher than those who were white and comparable to Black beneficiaries across all ages < 85 years; the gap in AD prevalence for Hispanic beneficiaries vs other racial/ethnic groups widened in the ≥ 85 years subgroup. The incidence and prevalence of MCI was higher in white beneficiaries relative to other races/ethnicities in all age subgroups ≥ 70 years, and the gap widened with increasing age.
Geographic Distribution
The prevalence of AD and MCI by US state in 2017 is depicted in Fig. 3. The prevalence of AD per 10,000 person-years was highest in Rhode Island (RI; 453.0) followed by Florida (FL; 437.5), Connecticut (427.5), Texas (414.4), and Hawaii (HI; 412.5). The prevalence of MCI per 10,000 person-years was highest in RI (155.7), FL (151.6), Massachusetts (144.3), Minnesota (138.2), and HI (134.2).
Fig. 3.
Prevalence by US Statesa in 2017: a AD in FFS population; b MCI in FFS population. AD Alzheimer’s disease, FFS fee-for-service, MCI mild cognitive impairment. Tables corresponding to each map list areasa with the 10 highest prevalence rates. aThe analysis included the 50 states in the United States of America and the District of Columbia
Sensitivity Analyses
In the FFS population, the cumulative prevalence of AD per 10,000 person-years rose from 318.4 in 2008 to 470.3 in 2017 while cumulative prevalence of MCI in this time period increased from 11.9 to 168.9 (See Figure S3 in supplementary material). Cumulative prevalence estimation increased the number of FFS beneficiaries with AD and MCI in 2017 to 1,208,398 and 434,127, respectively (for reference, FFS 2017 yearly estimates were 952,403 for AD and 265,752 for MCI). The analysis of symptomatic AD treatment use found that among 952,403 CMS (FFS + MA) beneficiaries with AD in 2016, 55% were using AChEIs or memantine; use of these drugs was also found in 27.2% of patients with MCI (47.1% of the AD + MCI patients combined). Addition of diagnostic codes F1-F4 in the AD prevalence estimation substantially increased the number of CMS beneficiaries with dementia in 2016 by 23% to 1,744,423; similarly, addition of R41.x led to an almost fourfold increase in the MCI prevalence estimate to 1,315,542 CMS beneficiaries in 2016.
Discussion
As the Alzheimer’s disease treatment paradigm changes, identification of patients who are eligible for new therapies is a timely and essential task. Our diagnostic code-based prevalence rates were 3.4% for AD and 0.85% for MCI in Medicare beneficiaries aged ≥ 65 years (2016). Higher dementia/MCI rates reported in the US Health and Retirement Study Harmonized Cognitive Assessment Protocol (10% for dementia and 22% for MCI) [23] and the Chicago Health and Aging Project (11.3% for AD and 22.7% for MCI) [2], are likely due to active sampling methodology. Surveys that include cognitive assessments can detect subtle changes/early stages of cognitive impairment; thus, in addition to capturing patients with known AD/MCI diagnoses through linked records, the prevalence rates can also include patients not yet seeking care for and/or diagnosed with AD/MCI within administrative databases. Both active sampling and diagnostic coding-based methodologies are valid; however, diagnostic coding-based analyses such as ours are representative of patients already within the healthcare system for AD/MCI, and thus may offer a realistic gauge for healthcare resource allocation/spending in the immediate future.
Health system preparedness for AAT is a clinical priority for anticipating/ensuring adequate resources to meet the needs of eligible patients [24] and for implementing/improving patient management processes [25]. Notably, in a recent cross-sectional analysis of 237 participants in a population-based study who had MCI or mild dementia (considered possible or probably Alzheimer’s disease), application of AAT clinical trial inclusion/exclusion criteria rendered < 10% of patients eligible for AAT [24].
We considered other relevant/relatively recent CMS-based analyses. A study of 3 MA plans reported the prevalence of AD and AD-related dementias (ADRD), based on one diagnostic code, was 5.6% (2014) and 6.5% (2016) among beneficiaries aged ≥ 65 years [15]. Another study, using a 5% national sample of FFS beneficiaries aged ≥ 65 years (2007–2019), reported > 20% ADRD prevalence rates [26]. A study examining dementia prevalence, reported rates of approximately 9% in FFS and 7% in MA (2016– 2017) [16]. Of interest, recent predictive model-based analyses of MCI in FFS and MA estimated that < 10% of expected MCI cases are diagnosed [17, 26]. Findings from prior CMS investigations are not directly comparable with our findings since relatively broad dementia codes were typically used for case identification; whereas, we used AD- and MCI-specific codes with strict criteria of 2 diagnostic codes ≥ 30 days apart. Our sensitivity analyses finding of higher prevalence with expanded coding underscores that estimations can vary based on differences in coding practice patterns. Thus, differences in study populations, case ascertainment, coding practices, sampling strategies, incidence/prevalence estimation methods, and other data analysis techniques can lead to disparities in prevalence estimates.
Of note, epidemiologic estimates among MA beneficiaries (available for 2016) were comparable to those in FFS beneficiaries. Baseline comorbidity profiles between beneficiaries with AD and MCI in 2016 (FFS and MA combined) were generally comparable: hypertension and hyperlipidemia were the most common medical conditions; depression and anxiety were the most common psychiatric comorbidities.
Our longitudinal analysis of FFS from 2008 to 2018 found that yearly AD incidence per 10,000 person-years decreased by almost 25% from 138 to 104, while MCI incidence increased over fivefold from 8 to 47; however, the sum of AD and MCI incidence from 2008 to 2018 was relatively stable (146–151). From 2008 to 2017, yearly AD prevalence per 10,000 person-years in FFS increased slightly from 318 to 354, while MCI prevalence rose over sevenfold from 13 to 99. Longitudinal trends for AD and MCI stratified by race and sex were consistent with the overall population trends; albeit with higher rates in women relative to men, especially for AD; higher rates of AD among Hispanic beneficiaries and slightly higher rates of MCI among white beneficiaries relative to other race/ethnic groups. The extent to which the trends in AD and MCI reflect changes in actual disease rates vs changes in AD/MCI diagnostic coding is uncertain. The relationship between AD and MCI is complex and understanding of both conditions is evolving [27]. We speculate that the rising trend in MCI cases relative to AD, as seen in Medicare data, likely reflects improvements in early/diagnosis due to advances in neuro-imaging and screening along with increased medical awareness. The upward trend in MCI may also be driven by aging populations, evolving diagnostic criteria, and an increasing focus on early-stage cognitive health/decline. While the rise in MCI diagnoses provides an opportunity for early intervention, it also raises concerns about over-diagnosis and the potential impact on patient experiences and treatment decisions. This highlights the need for more precise diagnostic criteria and tailored management strategies to improve care for individuals with MCI.
Additionally, cerebrospinal fluid biomarkers, such as amyloid beta, tau proteins, and phosphorylated tau, provide more objective and accurate measures of disease pathology and severity compared to traditional clinical diagnostic criteria, which often rely primarily on symptomatic assessments. These biomarkers have been increasingly recognized for their role in early detection and monitoring of AD, offering a more refined understanding of disease progression.
In 2017, increased age and female sex were associated with higher incidence/prevalence of both AD and MCI. Regardless of sex or race/ethnicity, AD/MCI increased with age. For AD, the widest gap between females and males was in beneficiaries aged 85+ years, possibly since women have greater longevity than men [28]; this gap was wider for prevalence than incidence. Hispanic, followed by Black beneficiaries had consistently higher incidence/prevalence of AD than white beneficiaries; however, white beneficiaries > 70 years had a higher incidence/prevalence of MCI, possibly reflecting more healthcare access or healthcare-seeking behavior for MCI in this race/ethnicity group. Our geographic analysis found that prevalence of both AD and MCI were highest in RI and FL. Our sensitivity analysis finding that 55% of beneficiaries with AD had AChEIs or memantine documented was consistent with another Medicare-based investigation that reported 56.7% of 9812 newly diagnosed patients (2008–2012) received such treatments after initial AD diagnosis [29].
Limitations
CMS claims-based estimates reflect recognized diagnoses submitted by any clinician providing care for beneficiaries, rather than true disease prevalence [15]. Underdiagnosis or failure to code for AD/MCI may occur if clinicians do not prioritize AD/MCI or hesitate to assign diagnostic codes due to perceived stigma [13]. In some cases, coding in administrative claims databases may reflect use of rule-out codes during a clinical work-up rather than final diagnoses. We required 2 diagnostic codes ≥ 30 days apart for ascertainment to be more likely to reflect clinically assessed AD/MCI. This conservative approach aligns with best practices for observational studies using claims data, reducing the potential for false positives due to misdiagnosis or administrative convenience that could result from reliance on single-code-based ascertainment [30, 31]. The ICD codes for dementia, including some of the AD-specific codes employed in our study, have been shown to reliably identify cases, with reported sensitivity ranging from 50–80% and specificity between 85–95% [32–34]. Medicare claims data remain a critical resource for identifying AD and dementia at the population level. The large sample size and longitudinal follow-up enable robust analyses of disease prevalence, healthcare utilization, and treatment patterns, offering valuable insights into real-world trends and outcomes despite inherent limitations.
A limitation of our sensitivity analysis using cumulative prevalence is that the prevalence estimates from the earlier years of the 10-year observation period were accumulated from fewer years relative to the later years of the period, which may cause artificial increase in prevalence in the later years relative to earlier years. Another important consideration is that available codes do not distinguish AD stages or MCI subtypes, thus the proportion of patients potentially eligible for AAT cannot be precisely determined. Since all-cause MCI was identified, it is likely that about half of these patients had MCI due Alzheimer’s disease pathology [1, 35, 36]; however, any patients younger than 65 with MCI would not be represented in the current analysis. Finally, many beneficiaries meeting AD/MCI case definitions in 2016 also had ≥ 1 non-specific dementia code recorded, possibly reflecting clinical work-up that was subsequently refined; however, it is not possible to know definitively.
Conclusions
This retrospective claims-based analysis provides estimates of the diagnostic code-based incidence/prevalence of AD and MCI among US Medicare beneficiaries. In 2016, 3.4% and 0.85% of FFS + MA beneficiaries had AD and MCI, respectively using our diagnostic code criteria. Sensitivity analyses with expanded coding increased these estimates; however, the added codes were not AD/MCI-specific. Available diagnostic code-based AD/MCI estimates in MA from 2016 were comparable to those in FFS in 2016. With the availability of AAT, we anticipate that the population with diagnosed MCI/AD within the CMS database may rise over time; therefore, periodic updates of incidence/prevalence estimates may provide support for timely healthcare decision-making.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Editorial assistance and medical writing support were provided by Kulvinder Katie Singh, PharmD of KK Singh, LLC (Branchburg, NJ, USA). Support for this assistance was funded by Eisai, Inc (Nutley, NJ, USA).
Author Contributions
All authors contributed to the study concept and design, analysis and interpretation of data, and manuscript preparation. Haixin Zhang, Amir Abbas Tahami Monfared, and Quanwu Zhang were involved in data acquisition.
Funding
This research and the journal’s Rapid Service Fee were funded by Eisai Inc., Nutley, NJ, USA.
Data Availability
The data supporting the findings of this study are openly available at the Centers for Medicare and Medicaid Services: www.ccwdata.org
Declarations
Conflict of Interest
Haixin Zhang and Quanwu Zhang are employees of Eisai, Inc. (Nutley, NJ, USA). Amir Abbas Tahami Monfared is an employee of Eisai Inc. (Nutley, NJ, USA) and holds the position of Adjunct Professor of Epidemiology and Biostatistics at McGill University. He also serves as an Associate Editor for the Journal of Alzheimer's Disease but does not receive honoraria for this role. Lawrence Honig has received funding for consultation from Biogen, Eisai, Genentech/Roche, Medscape, and Prevail/Lilly. Additionally, he has received institutional research funding from Abbvie, Acumen, Alector, AstraZeneca, Biogen, Bristol-Myer Squibb, Cognition, EIP, Eisai, Genentech/Roche, Janssen/Johnson & Johnson, Eli Lilly, Merck, Transposon, UCB, and Vaccinex.
Ethical Approval
This analysis conformed to CMS privacy requirements; an approval for the overarching database research protocol was obtained through a central IRB. The entire CMS beneficiary population over the selected time period was analyzed to assure diversity, equity, and inclusion. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patient data were deidentified, and due to the study's retrospective design, obtaining formal patient consent was not required. This study does not involve any new research with human participants or animals conducted by the authors.
References
- 1.Alzheimer’s Association. 2024 Alzheimer's disease facts and figures. Alzheimers Dement. 2024;20(5):3708–3821. [DOI] [PMC free article] [PubMed]
- 2.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17(12):1966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Census Bureau (2018) An aging nation: projected number of children and older adults. https://www.census.gov/library/visualizations/2018/comm/historic-first.html Last updated October 8, 2021. Accessed on March 26, 2024.
- 4.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration (2021) FDA grants accelerated approval for Alzheimer’s drug. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug, Last updated June 7, 2021. Accessed on March 26, 2024.
- 6.Lecanemab [prescribing information]. Eisai Inc., Nutley, NJ; July 2023.
- 7.Donanembab [prescribing information]. Eli Lilly and Company, Indianapolis, IN; July 2024.
- 8.Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, Dent G, Hansson O, Harrison K, von Hehn C, Iwatsubo T, Mallinckrodt C, Mummery CJ, Muralidharan KK, Nestorov I, Nisenbaum L, Rajagovindan R, Skordos L, Tian Y, van Dyck CH, Vellas B, Wu S, Zhu Y, Sandrock A. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. [DOI] [PubMed] [Google Scholar]
- 9.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 10.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM, TRAILBLAZER-ALZ 2 Investigators. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services (2021) 2022 Medicare Parts A & B Premiums and Deductibles/2022 Medicare Part D Income-Related Monthly Adjustment Amounts. https://www.cms.gov/newsroom/fact-sheets/2022-medicare-parts-b-premiums-and-deductibles2022-medicare-part-d-income-related-monthly-adjustment, Last updated November 12, 2021. Accessed on March 26, 2024.
- 12.United States Department of Health and Human Services (2022) Statement from HHS secretary Becerra: 2022 medicare part B premium increase attributable to Alzheimer’s drug aduhelm will be adjusted and incorporated into upcoming 2023 medicare premium determination. https://www.hhs.gov/about/news/2022/05/27/statement-hhs-secretary-becerra-2022-medicare-part-b-premium-increase-attributable-to-alzheimers-drug-aduhelm-will-be-adjusted-incorporated-into-upcoming-2023.html, Last updated May 27, 2022. Accessed on March 26, 2024.
- 13.Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2017;13(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thunell J, Ferido P, Zissimopoulos J. Measuring Alzheimer’s disease and other dementias in diverse populations using medicare claims data. J Alzheimers Dis. 2019;72(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jutkowitz E, Bynum JPW, Mitchell SL, Cocoros NM, Shapira O, Haynes K, Nair VP, McMahill-Walraven CN, Platt R, McCarthy EP. Diagnosed prevalence of Alzheimer’s disease and related dementias in Medicare Advantage plans. Alzheimers Dement (Amst). 2020;12(1): e12048. 10.1002/dad2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haye S, Thunell J, Joyce G, Ferido P, Tysinger B, Jacobson M, Zissimopoulos J. Estimates of diagnosed dementia prevalence and incidence among diverse beneficiaries in traditional medicare and medicare advantage. Alzheimers Dement (Amst). 2023;15(3): e12472. 10.1002/dad2.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the US Medicare population: observational analysis. Alzheimers Res Ther. 2023;15(1):128. 10.1186/s13195-023-01272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. What’s Medicare? Available at: https://www.medicare.gov/what-medicare-covers/your-medicare-coverage-choices/whats-medicare, Accessed on March 26, 2024.
- 19.Tarazi W, Welch WP, Nguyen N, Bosworth A, Sheingold S, De Lew N, Sommers BD. Medicare beneficiary enrollment trends and demographic characteristics (Issue Brief No. HP- 2022–08). Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. 2022. Available at: https://aspe.hhs.gov/sites/default/files/documents/b9ac26a13b4fdf30c16c24e79df0c99c/medicare-beneficiary-enrollment-ib.pdf Accessed on October 18, 2026.
- 20.Xu L, Welch WP, Sheingold S, De Lew N, Sommers BD. Medicare switching: patterns of enrollment growth in medicare advantage, 2006–22. Health Aff (Millwood). 2023;42(9):1203–11. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Health, National Cancer Institute Division of Cancer Control & Population Sciences. Comorbidity SAS Macro (2021 Version). https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2021.html, Last updated September 24, 2021. Accessed on March 26, 2024.
- 22.Williams B. National Center For Chronic Disease Prevention and Health Promotion regions. Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/27761, Last updated February 21, 2013. Accessed on March 26, 2024.
- 23.Manly JJ, Jones RN, Langa KM, Ryan LH, Levine DA, McCammon R, Heeringa SG, Weir D. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79(12):1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittock RR, Aakre JA, Castillo AM, Ramanan VK, Kremers WK, Jack CR Jr, Vemuri P, Lowe VJ, Knopman DS, Petersen RC, Graff-Radford J, Vassilaki M. Eligibility for anti-amyloid treatment in a population-based study of cognitive aging. Neurology. 2023;101(19):e1837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields LBE, Hust H, Cooley SD, Cooper GE, Hart RN, Dennis BC, Freeman SW, Cain JF, Shang WY, Wasz KM, Orr AT, Shields CB, Barve SS, Pugh KG. Initial experience with lecanemab and lessons learned in 71 patients in a regional medical center. J Prev Alz Dis. 2024. 10.14283/jpad.2024.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Jun H, Becker A, Wallick C, Mattke S. Detection rates of mild cognitive impairment in primary care for the United States medicare population. J Prev Alzheimers Dis. 2024;11(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J. Mild cognitive impairment in relation to Alzheimer’s disease: an investigation of principles, classifications, ethics, and problems. Neuroethics. 2023. 10.1007/s12152-023-09522-5. [Google Scholar]
- 28.Arias E, Tejada-Vera B, Kochanek KD, Ahmad FB. Provisional life expectancy estimates for 2021. Vital Statistics Rapid Release; no 23. Hyattsville, MD: National Center for Health Statistics. 2022. 10.15620/cdc:118999
- 29.Bent-Ennakhil N, Coste F, Xie L, Aigbogun MS, Wang Y, Kariburyo F, Hartry A, Baser O, Neumann P, Fillit H. A real-world analysis of treatment patterns for cholinesterase inhibitors and memantine among newly-diagnosed Alzheimer’s disease patients. Neurol Ther. 2017;6(1):131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simard M, Rahme E, Dubé M, Boiteau V, Talbot D, Sirois C. Multimorbidity prevalence and health outcome prediction: assessing the impact of lookback periods, disease count, and definition criteria in health administrative data at the population-based level. BMC Med Res Methodol. 2024;24(1):113. 10.1186/s12874-024-02243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Sauver JL, Chamberlain AM, Bobo WV, Boyd CM, Finney Rutten LJ, Jacobson DJ, McGree ME, Grossardt BR, Rocca WA. Implementing the US Department of Health and Human Services definition of multimorbidity: a comparison between billing codes and medical record review in a population-based sample of persons 40–84 years old. BMJ Open. 2021;11(4): e042870. 10.1136/bmjopen-2020-042870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grodstein F, Chang CH, Capuano AW, Power MC, Marquez DX, Barnes LL, Bennett DA, James BD, Bynum JPW. Identification of dementia in recent medicare claims data, compared with rigorous clinical assessments. J Gerontol A Biol Sci Med Sci. 2022;77(6):1272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura LMVR, Festa N, Price M, Volya M, Benson NM, Zafar S, Weiss M, Blacker D, Normand SL, Newhouse JP, Hsu J. Identifying medicare beneficiaries with dementia. J Am Geriatr Soc. 2021;69(8):2240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available at the Centers for Medicare and Medicaid Services: www.ccwdata.org