Abstract
Collagen, a major component of the extracellular matrix, is crucial for the structural integrity of the Caenorhabditis elegans cuticle. While several proteins involved in collagen biosynthesis have been identified, the complete regulatory network remains unclear. This study investigates the role of CALU-1, an ER-resident calcium-binding protein, in cuticle collagen formation and maintenance. We employed genetic analyses, including the generation of single and double mutants, scanning electron microscopy, and transcriptome profiling to characterize CALU-1 function. Our results demonstrate that CALU-1 is essential for proper cuticle structure, including annuli, furrows, and alae formation. Synthetic lethality was observed between calu-1 and dpy-18 (encoding a prolyl 4-hydroxylase subunit) mutations, while double mutants of calu-1 with peptidyl-prolyl cis–trans isomerase (PPIase) genes exhibited exacerbated phenotypes. CALU-1 deficiency led to altered collagen stability, increased cuticle permeability, and differential expression of stress response genes similar to collagen mutants. We conclude that CALU-1 plays a critical role in regulating collagen biosynthesis, possibly by modulating the ER environment to optimize the function of collagen-modifying enzymes. These findings provide new insights into the complex regulation of extracellular matrix formation in C. elegans, with potential implications for understanding related processes in other organisms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-025-05582-3.
Keywords: Nematode, Exoskeleton, Extracelluar matrix, Calumenin, Cuticle permeability, Stress
Introduction
The extracellular matrix (ECM) is a complex network of secreted proteins and carbohydrates that provides structural and biochemical support to surrounding cells [1, 2]. In multicellular organisms, the ECM plays crucial roles in cell adhesion, cell-to-cell communication, and differentiation [3, 4]. Caenorhabditis elegans, a small free-living nematode, has emerged as a powerful model organism for studying ECM biology due to its genetic tractability and transparent body [5, 6]. The C. elegans exoskeleton, known as the cuticle, is a specialized form of ECM that is synthesized by the underlying hypodermis [7, 8]. This cuticle is composed primarily of cross-linked collagens, cuticulins, and glycoproteins, forming a complex, multi-layered structure that is essential for the animal's shape, movement, and protection against environmental stresses [9–11]. The cuticle is synthesized five times during C. elegans development: once in the embryo and subsequently at the end of each of the four larval stages, making it an excellent system for studying the dynamic processes of ECM formation and remodeling [12, 13]. Recent advances in genetic and molecular techniques have further enhanced our understanding of cuticle formation and its regulation in C. elegans, providing insights that may be applicable to ECM biology across species [13–15].
Collagen is a major component of the cuticle of C. elegans [7] as well as human tissues including skin and bone [7, 16]. The C. elegans genome has 181 genes encoding collagens with a conserved characteristic structure of short interrupted blocks of Gly-X–Y motif flanked by conserved cysteine residues [7, 9]. Although 173 out of the 181 collagen genes are unique to nematodes and are predicted to encode cuticular collagens [13], the principles of collagen synthesis, folding, and modification are largely conserved across species. The molecular properties of collagen-based ECM are largely determined by the intracellular processes of collagen, such as folding, modification and quality control, which are controlled by various molecules in the endoplasmic reticulum (ER). Prolyl 4-hydroxylation is the major modification of procollagen that occurs in the ER, allowing it to fold properly into a thermally stable form [7]. This process is controlled by prolyl 4-hydroxylase (P4H) to convert prolines to 4-hydroxyprolines and this hydroxylation is known to enhance the conformational stability of the triple helix of procollagen [17–20]. The major form of P4H in wild type C. elegans is known to be a mixed tetramer (DPY-18 (PHY-1)/PHY-2/(PDI-2)2) with two catalytic α-subunit isoforms (DPY-18 (PHY-1) and PHY-2) and two protein disulfide isomerases (PDI-2) serving as the β-subunits of P4H [21]. Disruption of the genes encoding the P4H enzyme by mutation or RNAi has shown that this enzyme is crucial for survival through cuticle collagen biogenesis in C. elegans [22–24]. The procollagen trimerization is mediated by ER-resident peptidyl prolyl cis–trans isomerases (PPIases) [25, 26]. Comparative genome analysis has identified C. elegans orthologs of PPIases resident in ER [27] and the enzymatic activity has been confirmed [28]. They include FKB-3/FKBP9, FKB-4/FKBP9 (FKBP10), FKB-5/FKBP9 (FKBP10) and FKB-7/FKPB14 (FKBP7) belonging to FK506-binding protein (FKBP) family of PPIase. Three of the C. elegans FKBPs (FKB-3, 4 and 5) have double PPIase domains whereas FKB-7 has a single PPIase domain and Ca2+-binding EF-hand motif [27]. Previous studies have shown that single loss of FKBPs is dispensable for normal development and viability in yeast [29] and worm [28]. However, loss of multiple FKBPs has disrupted the structural integrity of the cuticular ECM in C. elegans, resulting in cold-sensitive lethality [28].
As collagens represent essential structural proteins, reduced production or structural stability due to imperfect collagen structure caused by missense mutations in collagen genes or nonsense mutations in human FKBP14 can lead to rare genetic disorders such as osteogenesis imperfecta (OI) and Ehlers-Danlos syndrome (EDS) [30, 31]. Conversely, interfering with collagen biosynthesis by inhibiting human P4H may be beneficial in the treatment of fibrotic diseases in which normal collagen is either overproduced or produced in a way that exacerbates the pathological process [32–35]. In C. elegans, cuticular collagens are synthesized and secreted from the hypodermis during each molting cycle [7]. Therefore, incomplete collagen production can threaten the survival of C. elegans by increasing the permeability of cuticle, making it much more sensitive to various environmental insults [36–38]. Despite the importance of collagen, our understanding of collagen production is still limited, especially how the many ER proteins regulate this process. Here we propose a novel key regulator of collagen production, calumenin, an ER-resident Ca2+ binding protein.
The number of proteins belong to the CREC family (Cab45, Reticulocalbin, ERC-45, and Calumenin), which contain multiple EF-hand Ca2+ -binding motifs and localize to the ER secretory pathway [39]. Calumenin contains five and six EF-hand motifs for C. elegans and Homo sapiens, respectively, with relatively low affinity for Ca2+ [40, 41]. Vertebrate calumenin has been involved in various malignant diseases such as colorectal cancer [42], breast cancer [43], glioma [44], and bladder cancer [45] with yet unknown regulatory roles. CeCALU-1, a single calumenin homolog of C. elegans, has been implicated in the regulation of fertility, pharyngeal pumping (eating behavior), defecation, and the formation of dauer, a form of developmental adaptation to the environment in nematodes [40, 46]. It has also been proposed as a drug target for human-infective nematodes because of its involvement in cuticle development [47]. In C. elegans, functional loss of calumenin (calu-1) leads to cuticle defects, resulting in the semi-dumpy (Dpy) and/or semi-roller (Rol) morphology of the worm, which is also seen in the functional loss of collagens [7, 40]. The cuticle defect in the calu-1 mutant (calu-1(tm1783)) also includes abnormal cuticle substructures with irregular annuli and furrows, and deformed alae on the surface of the worm's outer layer [40]. This abnormal cuticle formation in the calu-1(tm1783) mutant completely blocks the mutant from forming a dauer, which involves a global reorganization of the cuticle structure [10, 46, 48]. Since some of the steps of collagen biosynthesis (prolyl 4-hydroxylation and trimerization) take place in the ER and the calu-1(tm1783) mutant has cuticle defects, the possible role of CALU-1 in the formation of cuticle collagens was investigated in this study. We found that the dysfunction of CALU-1 led to the lethality of worms when P4H was knocked out at the same time. Dysfunction of CALU-1 disrupted the normal annular structures of PPIase mutants as well as some collagen mutants, resulting in increased cuticle permeability. In addition, CALU-1 dysfunction induced differential expression of several stress response genes as did collagen mutations. Taken together, our results suggest that CALU-1 is an indispensable player in the regulation of collagen production.
Material and methods
C. elegans strains and maintenance
The strains used in this study were obtained from the Caenorhabditis Genetics Center (CGC): Bristol N2 (wild-type), CB1166 dpy-4(e1166) IV, CB61 dpy-5(e61) I, CB184 dpy-13(e184) IV, CB386 dpy-18(e364) III, and XW18042 qxIs722[dpy-7p::dpy-7::sfGFP] and the National BioResource Project, Japan: calu-1(tm1783) X, fkb-3(tm348) V, fkb-4(tm10219) V, fkb-5(tm475) I, fkb-7(tm6752) I, and fkb-7(tm6972) I. All mutant strains were outcrossed more than six times to get rid of other mutations. Worms were grown and maintained using Nematode Growth Media (NGM) seeded with Escherichia coli OP50 as a food source according to standard procedure [49].
The following strains were generated in this study:
WKS3025 fkb-3(tm348);calu-1(tm1783).
WKS3026 fkb-4(tm10219);calu-1(tm1783).
WKS3027 fkb-5(tm475);calu-1(tm1783).
WKS3028 fkb-7(tm6752);calu-1(tm1783).
WKS3029 fkb-7(tm6972);calu-1(tm1783).
WKS3031 fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783).
WKS3032 fkb-5(tm475);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783).
WKS3033 fkb-5(tm475) fkb-7(tm6972);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783).
WKS3081 dpy-4(e1166);calu-1(tm1783).
WKS3082 dpy-5(e61);calu-1(tm1783).
WKS3083 dpy-13(e184);calu-1(tm1783).
WKS3084 fkb-5(tm475);fkb-3(tm348) fkb-4(tm10219).
WKS3085 fkb-5(tm475) fkb-7(tm6972);fkb-3(tm348) fkb-4(tm10219).
WKS3086 calu-1(tm1783);qxIs722[dpy-7p::dpy-7::sfGFP].
Genotyping of deletion mutant alleles
Genomic deletions in calu-1, fkb-3, fkb-4, fkb-5, fkb-7 and dpy-13 mutant strains were analyzed by genotyping PCR (Table S1) of the corresponding genomic region. In brief, single worm from mutant strains or wild type N2 was lysed and subsequently used for PCR using AccuPower PCR Premix (Bioneer, #K-2016).
Sequencing of mutant alleles
Point mutations in dpy-4, dpy-5 and dpy-18 mutant strains were analyzed by PCR amplification (Table S2) of the corresponding genomic region followed by sequencing. Briefly, a single worm from mutant strains or wild type N2 was lysed and subsequently used for PCR using Phusion Plus DNA Polymerase (Thermo Scientific, #F630S). Purified PCR product was cloned into TA-Blunt vector (TOPcloner TA-Blunt core kit, Enzynomics, #EZ017S) and resultant clones were analyzed by Sanger sequencing.
Transcript analysis of mutant alleles by RT-PCR
Transcripts of fkb-3(tm348), fkb-4(10219), fkb-7(tm6752) and fkb-7(tm6972) mutant strains were analyzed by RT-PCR (Table S3). Briefly, total RNA was prepared from each mutant strains or wild type N2 using NucleoSpin RNA plus kit (Macherey-Nagel, #740984.50) and used for cDNA synthesis (Transcriptor First strand cDNA Synthesis kit, Roche, #04896866001). The subsequent amplification was performed using SYBR green QPCR master mix (Brilliant III Ultra-Fast SYBR Green qPCR Master Mix, Agilent, #600882) in Agilent AriaMx Real-time PCR machine. The resultant PCR product was cloned into TA vector (pGEM-T easy vector, Promega, #A1360) followed by sequencing.
Analysis of cuticle structure by scanning electron microscopy (SEM)
SEM samples were prepared as described with modification [50, 51]. Briefly, synchronized population of 1-day old adults were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2) at 4 °C overnight. Animals were rinsed several times in 0.05 M sodium cacodylate buffer (pH 7.2) and fixed again in 1% osmium tetroxide in 0.05 M sodium cacodylate buffer (pH 7.2) at 4 °C for 1.5 h. Animals were rinsed several times in distilled water at RT for 10 min and then dehydration was done at RT for each 10 min in 30, 50, 70, 80, 90 and 100% (3 times) ethanol. Animal samples were chemically dried using HMDS (hexamethyldisilazane) for 15 min and allowed to dry at RT overnight in a clean bench after transferred to multiple cover slides. Animals were then mounted on metal stubs and coated with gold (plasma sputter coater, VG microtech) before imaging. Imaging was done using a Bio Scanning Electron Microscope (S-3000N, Hitachi, Tokyo, Japan) installed in the Center for University-wide Research Facilities (CURF) at Jeonbuk National University and FE-SEM (S-4800, Hitachi, Tokyo, Japan) at the Core Facility for Supporting Analysis and Imaging of Biomedical Materials in Wonkwang University, supported by the National Research Facilities and Equipment Center.
Measurement of body length
Body length was measured as described with modification [52]. Briefly, 2-day old adults (synchronized from L4 (the fourth larval) stage) were immobilized on a 2% agarose pad with 0.25–0.5 mM levamisole, photographed under Nomarski microscope (Axioskop2, Carl Zeiss, Oberkochen, Germany), and then the length was measured from head to tail. The measurement was repeated at least three times.
Body morphology, cuticle permeability assay and microscopy
To observe body morphology of animals, 2-day old adults (synchronized from L4 (the fourth larval) stage) were immobilized on a 2% agarose pad with 0.25–0.5 mM levamisole, photographed under Nomarski microscope (Axioskop2, Carl Zeiss, Oberkochen, Germany).
Cuticle permeability was assessed by using Hoechst 33258 stain. Whole animals were stained with the dye as described [37, 53]. Briefly, synchronized 1-day old adults were stained with 10 μg/ml Hoechst 33258 (Sigma-Aldrich, #94403) for 30 min at RT. Excessive dye was removed by washing with M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 liter. Sterilize by autoclaving). Animals were immobilized on 2% agarose pad by levamisole and then visualized under the DAPI filter using a super-resolution confocal microscope (LSM980, Carl Zeiss, Oberkochen, Germany) at the Core Facility for Supporting Analysis and Imaging of Biomedical Materials in Wonkwang University, supported by the National Research Facilities and Equipment Center. 7–15 animals were observed and scored as permeable when at least 10 stained nuclei in the head (pharynx) were observed.
Whole transcriptome RNA sequencing
The mixed population of N2 and calu-1(tm1783) mutants were harvested from three plates and RNA was extracted from three replicates per strain. Total RNA was sent to Macrogen Inc. (Seoul, Republic of Korea). Total RNA concentration was calculated by Quant-IT RiboGreen (Invitrogen, #R11490). Total RNA integrity was checked using TapeStation RNA screentape (Agilent, #5067–5576). Only high-quality RNA preparations, with RIN greater than 7.0, were used for RNA library construction. A library was independently prepared with 1 μg of total RNA for each sample by Illumina TruSeq Stranded mRNA Sample Prep Kit (Illumina, Inc., San Diego, CA, USA, #RS-122–2101). The resultant cDNA libraries were quantified using KAPA Library Quantification kits for Illumina Sequencing platforms according to the qPCR Quantification Protocol Guide (KAPA BIOSYSTEMS, #KK4854) and qualified using the TapeStation D1000 ScreenTape (Agilent Technologies, # 5067–5582). Indexed libraries were then submitted to an Illumina NovaSeq (Illumina, Inc., San Diego, CA, USA), and the paired-end (2 × 100 bp) sequencing was performed.
The raw sequences were processed to remove low quality and adapter sequence before analysis and aligned to the reference genome sequence of Caenorhabditis elegans (WBcel235) using HISAT v2.1.0 [54]. After alignment, StringTie v2.1.3b was used to assemble aligned reads into transcripts and to estimate their abundance [55, 56]. It provides the relative abundance estimates as Read Count values of transcript and gene expressed in each sample. Statistical analysis was performed to find differentially expressed genes (DEGs) using the estimates of abundances for each gene in samples. Genes with one more than zeroed Read Count values in the samples were excluded. To facilitate log2 transformation, 1 was added to each Read Count value of filtered genes. Filtered data were log2-transformed and subjected to RLE normalization. Statistical significance of the differential expression data was determined using nbinomWaldTest using DESeq2 and fold change in which the null hypothesis was that no difference exists among groups. False discovery rate (FDR) was controlled by adjusting p value using Benjamini–Hochberg algorithm. For DEG set, hierarchical clustering analysis was performed using complete linkage and Euclidean distance as a measure of similarity. Gene-enrichment and functional annotation analysis and pathway analysis for significant gene list were performed based on gProfiler (https://biit.cs.ut.ee/gprofiler/orth) and KEGG pathway (http://www.genome.jp/kegg/pathway.html).
To compare transcriptional changes of stress response genes with the dpy-7(e88) transcriptome (GSE107704), the genes with ≥ threefold change and p-value ≤ 0.05 were extracted and the log2 fold change was then compared to the calu-1(tm1783) transcriptome. Heat map was generated by Morpheus software (https://software.broadinstitute.org/morpheus).
Cold-sensitive assay
The cold-sensitive assay was performed as previously described with minor modification [28]. L4 larvae of strains were placed on seeded plates and grown at 12 °C. To minimize the effect of different brood sizes between strains, 10 animals were placed on the medium for mutants with brood sizes similar to wild type N2, such as fkb single mutants and 20 animals were placed on the medium for mutants with brood sizes similar to the calu-1 mutant, such as fkb;calu-1 double mutants. This is because the mutants including the calu-1 mutation had approximately half the brood size of the wild type N2 and fkb single mutants (data not shown). Although quadruple (fkb-5;fkb-3 fkb-4;calu-1) and quintuple mutants (fkb-5 fkb-7;fkb-3 fkb-4;calu-1) had much lower brood size, this does not affect the interpretation of the results because they were not able to survive. After 2 weeks, the population density was observed and photographed under a stereomicroscope. Assay was repeated at least three times.
Statistical analysis
Graphpad Prism (Version 5.00 for windows, GraphPad Software, San Diego, CA, USA) was used to produce the graphs and to perform the statistical analysis. The p-values were calculated using paired t-test with 95% confidence interval and one-way ANOVA (analysis of variance).
Results
Both P4H and CALU-1 are required for survival of nematodes
The dpy-18(e364);calu-1(tm1783) double mutant was initially generated to investigate the role of CALU-1 in prolyl 4-hyroxylation, which is the major post-translational modification of procollagen. We have extensively investigated the mutant characteristics of the genes encoding C. elegans P4H. Consistent with its essential role in exoskeleton formation and maintenance, functional loss of PDI-2, a single β-subunit of P4H, led to embryonic lethality of the worms [24]. On the other hand, functional loss of PHY-2, one of the catalytic α-subunits of P4H, showed no visible morphological effect, but complete loss of DPY-18, the other α-subunit of P4H, resulted in dumpy phenotype in the worms [22–24]. In addition, dpy-18 knockout mutant has been shown to have abnormal branched annuli and alae structure [57]. Therefore, the dpy-18 knockout mutant was used in this study to investigate the functional relevance of CALU-1 and P4H. The dpy-18(e364) null mutant has a point mutation resulting in the production of immature protein by premature stop [23] (Fig. S1A and B). This leads mutant animals to be dumpy. The calu-1(tm1783) loss-of-function mutant has a deletion spanning from the 5’ upstream region to the second exon, resulting in semi-dumpy and semi-roller morphology of mutant animals [40]. Double mutant between dpy-18(e364) and calu-1(tm1783) was generated and surprisingly, it could not survive. It was embryonic lethal and/or larval lethal. The lethal embryos and larvae were collected from independent parental mutants, which is homozygous for the dpy-18(e364) allele and heterozygous for the calu-1(tm1783) allele (dpy-18(e364);calu-1(tm1783/ +)). Compared to normally developed embryos of wild type N2 (Fig. S1C), the embryonic development was arrested, resulting in malformed and lethal embryos in dpy-18(e364);calu-1(tm1783) (Fig. S1D). Larval development was also arrested early, presumably shortly after hatching, with malformed body morphology (Fig. S1E). These microscopically confirmed F2 lethal larvae (Fig. S1E) were collected and genotyped for calu-1 and dpy-18 mutations (Fig. S1F). The larval lethal progeny were also collected from parental mutants, which is heterozygous for the dpy-18(e364) allele and homozygous for the calu-1(tm1783) allele (dpy-18(e364/ +);calu-1(tm1783)). The genotype was then confirmed by PCR and sequencing in two different generations, the second (F2) and fourth (F4) filarial generations (Fig. S1G). Given that the dpy-18;phy-2 double mutant, in which functional redundancy of α-subunit was completely removed, resulted in embryonic lethality [24], the synthetic lethality of the dpy-18;calu-1 double mutant suggests that CALU-1 may be necessary for the proper functioning of P4H or that it has an independent but equally critical role in collagen modification.
The calu-1 mutation disrupts normal cuticle structures in FKBP single mutants
Trimerization of procollagen is another important step in collagen folding and this procedure is mediated by ER-resident peptidyl prolyl cis–trans isomerases (PPIases) [25, 26]. C. elegans orthologs of ER-resident PPIases include FKB-3, FKB-4, FKB-5 and FKB-7 belonging to FKBPs (FK506-binding proteins) family of PPIase [27]. In order to investigate the genetic relationship between CALU-1 and FKBPs, deletion mutants of FKBPs were first obtained and RT-PCR was used to identify the mutant characteristics. The fkb-3(tm348) mutant has 1,487 bp-long deletion covering 5’-UTR (untranslated region) and the first exon as shown in Fig. S2A. The in-frame deletion in fkb-3(tm348) mutant resulted in FKB-3 protein only having C-terminal PPIase domain (Figs. S2A and S3A).The fkb-4(tm10219) mutant contains 114 bp-long deletion including a part of last intron and exon, producing a loss of most C-terminal PPIase domain and ER retention signal in FKB-4 protein (Figs. S2B and S3B). The fkb-5(tm475) mutant retains 446 bp-long in-frame deletion and this resulted in FKB-5 protein losing the half C-terminal PPIase domain and ER retention signal (Figs. S2C and S3C). The fkb-7(tm6752) mutant loses 292 bp-long genomic fragment including entire third exon and fkb-7(tm6972) mutant has 499 bp-long deletion covering entire third and fourth exons. Each deletion is in-frame deletion making a partial PPIase domain in FKB-7 protein (Figs. S2D and S3D). ER retention signals are only intact in fkb-3(tm348), fkb-7(tm6752) and fkb-7(tm6972) mutants (shown in blue in Fig. S2).
Then, double mutants between calu-1 and PPIases mutants were generated and the cuticle structure of them were examined by SEM (scanning electron microscope). The most visible surface structures of cuticle are specifically observed; the annuli and the alae. The annuli are organized circumferentially around the cylindrical body of worms with narrow intervals making annular furrows [7]. The alae are lateral and longitudinal ridges overlying the seam cells [58]. As reported [40], every calu-1(tm1783) mutants examined displayed irregular annuli and furrows (Fig. 1B) compared to wild type N2, which all showed regularly organized structures (Fig. 1A). In contrast, each individual mutant of FKBPs examined exhibited 100% normal annuli and furrows similar to wild type N2, and the width of the annuli of the each FKBPs mutants appeared to be comparable to N2 (Fig. 1C, E, G, I and K). However, all double mutants examined showed 100% abnormal annuli and furrows similar to calu-1(tm1783) mutant (Fig. 1D, F, H, J and L).
Fig. 1.
CALU-1 maintains the annular structure of cuticle. Scanning electron micrograph of A wild type, B calu-1(tm1783), C fkb-3(tm348), D fkb-3(tm348);calu-1(tm1783), E fkb-4(tm10219), F fkb-4(tm10219);calu-1(tm1783), G fkb-5(tm475), H fkb-5(tm475);calu-1(tm1783), I fkb-7(tm6752), J fkb-7(tm6752);calu-1(tm1783), K fkb-7(tm6972), and L fkb-7(tm6972);calu-1(tm1783). The n numbers are shown on the scale bars; total observed n in black, n of normal annuli and furrow in yellow, n of irregular annuli and furrows in red. Scale bars, 5 μm
The investigation of alae structure was followed. Wild type N2 displayed three distinct ridges of alae that are notable morphological feature of adult stage (Fig. 2A), whereas the calu-1(tm1783) mutant exhibited deformed alae as previously reported [40] (Fig. 2B). The alae phenotypes were categorized for the quantification of defects as follows; normal, fused, discontinued, and fused and discontinued. The statistical analysis showed that the alae defects of calu-1(tm1783) mutant are significant compared to that of wild type N2 (Fig. 2C). The alae were normally formed in each single mutant of FKBPs (Fig. 2D, G, J, M and P), showing no significant differences to wild type N2 (Fig. 2F, I, L, O and R). Except for fkb-4(tm10219);calu-1(tm1783), all double mutants examined were significantly different from each corresponding single mutant of FKBPs (Fig. 2F, L, O and R). On the other hand, they were not statistically different from the calu-1(tm1783) mutant (Fig. 2F, O, L and R). The fkb-4(tm10219);calu-1(tm1783) double mutant displayed alae phenotypes similar to fkb-4(tm10219) mutant rather than calu-1(tm1783) mutant (Fig. 2I).
Fig. 2.
CALU-1 maintains the alae structure of cuticle. Scanning electron micrograph of A wild type, B calu-1(tm1783), D fkb-3(tm348), E fkb-3(tm348);calu-1(tm1783), G fkb-4(tm10219), H fkb-4(tm10219);calu-1(tm1783), J fkb-5(tm475), K fkb-5(tm475);calu-1(tm1783), M fkb-7(tm6752), N fkb-7(tm6752);calu-1(tm1783), P fkb-7(tm6972), and Q fkb-7(tm6972);calu-1(tm1783). C, F, I, L, O and R Alae phenotypes were categorized and quantified. n numbers are shown in the figures. Scale bars, 10 μm. ***, p < 0.0001; ns, not significant
Taken together, our results reveals that CALU-1 is essential for maintaining the structural integrity of the cuticle in C. elegans. FKBPs alone do not disrupt cuticle integrity, but double mutants with calu-1 show consistent abnormalities, reinforcing the dominant role of CALU-1 in cuticle development. Interestingly, the fkb-4(tm10219);calu-1(tm1783) double mutant displays alae characteristics similar to fkb-4(tm10219), suggesting that FKB-4 may partially compensate for CALU-1 loss. Overall, these findings highlight the vital role of CALU-1 and the complex interactions with FKBP proteins in regulating cuticle morphology.
CALU-1 and PPIase interact to regulate body length and cuticle integrity in C. elegans
It has been shown that calu-1(tm1783) mutant displayed shorter body length due to defective cuticle development [40]. In order to test whether dysfunction of PPIase could cause the same defect, fkb single mutants and fkb;calu-1 double mutants were examined for body length. As results, every fkb single mutants (Fig. 3C, E, G, I and K, gray bar in each graph) showed comparable body length to wild type N2 (Fig. 3A; black bar in the graphs) with no statistical significance. As expected, the body length of calu-1(tm1783) mutant (Fig. 3B; green bar in the graphs) was significantly shorter than N2. Meanwhile, fkb mutations exacerbated the short body length of calu-1(tm1783) mutant. Every fkb;calu-1 double mutants showed even shorter body length than calu-1(tm1783) single mutant with statistical significance (Fig. 3D, F, H, J and L; purple bar in each graph). As C. elegans has multiple FKBP genes resident in the ER, multiple mutants were generated among fkbs and calu-1 mutant to get rid of functional redundancy of PPIases. A quadruple mutants (fkb-5(tm475);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783)) was generated to remove functional redundancy of FKBPs containing dual PPIases (FKB-3, FKB-4 and FKB-5). A quintuple mutant (fkb-5(tm475) fkb-7(tm6972);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783)) was also generated to finally exclude the last FKBP (FKB-7) which have a single PPIase domain and EF hand motif. The fkb-7(tm6972) mutant was used to obtain a quintuple mutant because it has a larger deletion than fkb-7(tm6752) mutant, expecting more significant loss of function. Both multiple mutants (Fig. 3O and P; orange and blue bars in the graph) were also shorter than calu-1(tm1783) mutant (Fig. 3N; green bar in the graph). To clarify the functional roles and relationships of FKBPs and CALU-1 in body length regulation, each double mutant was compared with each other and also with multiple mutants. The body lengths have been converted to a percentage of the body length of the wild type (100%) to correct for the variation that occurs in each measurement (Fig. 3Q and R). As shown in Fig. 3Q, there was an additive reduction in body length as more FKBPs were mutated. Particularly, FKB-5 appears to have a stronger effect than either FKB-3 or FKB-4 when mutated with CALU-1, despite their domain similarity. However, the additional FKB-7 mutation slightly restored the shortened body length of the quadruple mutant, rather than worsening its body length (Fig. 3R), suggesting another role for FKB-7, such as unbalanced Ca2+-dependent tethering of other proteins, which is somewhat beneficial for body length regulation, since it has an EF hand motif. Our results indicate that CALU-1 is essential for proper cuticle formation, and PPIases play a significant role in modulating the severity of defects associated with its loss, highlighting an important regulatory network in maintaining body shape and integrity.
Fig. 3.
fkb mutations exacerbate the small body length of calu-1(tm1783) mutant. Micrograph and body length of A and M wild type, B and N calu-1(tm1783), C fkb-3(tm348), D fkb-3(tm348);calu-1(tm1783), E fkb-4(tm10219), F fkb-4(tm10219);calu-1(tm1783), G fkb-5(tm475), H fkb-5(tm475);calu-1(tm1783), I fkb-7(tm6752), J fkb-7(tm6752);calu-1(tm1783), K fkb-7(tm6972), L fkb-7(tm6972);calu-1(tm1783), O fkb-5(tm475);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783), P fkb-5(tm475) fkb-7(tm6972);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783). Body length (Mean ± SEM) and n numbers are shown in the bar graphs. Scale bars, 50 μm. Q and R Comparison of body length among double, quadruple, and quintuple mutants. Body length was normalized by that of wild type N2. *, p < 0.05; **, p < 0.01, ***, p < 0.0001; ns, not significant
CALU-1 and PPIase interact to adapt to cold stress by regulating cuticle integrity in C. elegans.
Previously, the role of FKBPs has been addressed using single and multiple mutants in budding yeast Saccharomyces cerevisiae [29] and free-living nematode C. elegans [28]. Studies have indicated that they are dispensable for normal development and viability in those organisms. Similar results were observed in our study, in that all mutants we characterized showed normal development and viability under standard growth conditions although the mutant alleles of FKBPs such as fkb-4(tm10219), fkb-7(tm6752) and fkb-7(tm6972) have been characterized for the first time in this study. Combined triple (fkb-5;fkb-3 fkb-4) and fkb-4;fkb-5 double mutants have been however found to arrest at lower temperature (12 °C) by affecting molting, cuticle collagen expression, and structural integrity of the cuticular ECM, suggesting their absolute requirement at subphysiological temperature [28]. We also investigated the cold-sensitive effects of FKBP mutants at 12 °C, focusing on CALU-1's involvement (Fig. 4). While wild type N2 and most FKBP single mutants grew normally (Fig. 4A, C, E, I and K), the calu-1(tm1783) mutant showed slower growth and reduced reproduction (Fig. 4B). Notably, the fkb-5(tm475) single mutant exhibited slower growth and reduced fertility compared to wild type (Fig. 4G), contrasting with previous reports [28]. This phenotype was exacerbated in the fkb-5;calu-1 double mutant, which showed smaller body size and very slow growth (Fig. 4H). Other fkb;calu-1 double mutants largely resembled the calu-1 single mutant (Fig. 4D, F and J), except for fkb-7(tm6972);calu-1, which showed mild defects (Fig. 4L). Strikingly, multiple mutants carrying the fkb-5 mutation (fkb-5;fkb-3 fkb-4;calu-1 and fkb-5 fkb-7;fkb-3 fkb-4;calu-1) were lethal, failing to survive at 12 °C (Fig. 4N and O). These findings suggest that FKB-5 plays a central role in cold-sensitivity responses and that CALU-1 appears to contribute to this response, possibly through its role in maintaining normal cuticle development.
Fig. 4.
CALU-1 and FKBPs are required for cold-sensitivity adaptation. Micrograph of A wild type, B calu-1(tm1783), C fkb-3(tm348), D fkb-3(tm348);calu-1(tm1783), E fkb-4(tm10219), F fkb-4(tm10219);calu-1(tm1783), G fkb-5(tm475), H fkb-5(tm475);calu-1(tm1783), I fkb-7(tm6752), J fkb-7(tm6752);calu-1(tm1783), K fkb-7(tm6972), L fkb-7(tm6972);calu-1(tm1783), M fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783), N fkb-5(tm475);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783), and O fkb-5(tm475) fkb-7(tm6972);fkb-3(tm348) fkb-4(tm10219);calu-1(tm1783). Scale bars, 1 mm
Dysfunction of CALU-1 results in defects in the formation and stability of collagen
In order to test whether dysfunction of CALU-1 eventually resulted in defects in the formation and/or stability of collagens, the genetic interaction between calu-1 and collagen genes were examined. Firstly, the localization of DPY-7 was analyzed in the calu-1(tm1783) mutant background. Early expressed DPY-7 collagen is essential for the presence or persistence of the annular furrows of cuticle [59]. Thus, immunostaining of DPY-7 has shown the continuous DPY-7 bands in wild type but small disjointed fragments in dpy-7(e88) loss-of-function mutant [59]. In this study, DPY-7::GFP was used to investigate the localization of DPY-7 collagen because it appeared on the cuticle as circumferential bands in wild type similar to the pattern observed by immunostaining [60]. In addition, this is possible to observe DPY-7 localization in a live worm without the fixation step required for immunostaining. As results, the cuticle DPY-7 bands appeared normal in every wild type N2 examined (Fig. 5A) but became disorganized in all examined calu-1(tm1783) mutant (Fig. 5B). The impaired localization patterns of DPY-7 by calu-1 mutation were highly similar to those by dpy-7 mutation as well as the mutation of genes such as dpy-2, dpy-3, dpy-8 and dpy-10, which are early expressed together with dpy-7 and all components of the same structure in the annular furrows [59]. Consistent with the role of DPY-7 in annular furrow assembly, the fragmented pattern of DPY-7 on the cuticle surface of calu-1(tm1783) mutant suggests that CALU-1 is required for the formation and/or assembly of DPY-7 collagen.
Fig. 5.
CALU-1 is required for assembly and/or localization of furrow collagen. The localization of DPY-7 collagen is shown in A wild type and B calu-1(tm1783) background. The n numbers are shown on the scale bars; total observed n in white, n of normal pattern in yellow, n of disorganized pattern in red. Scale bars, 10 μm
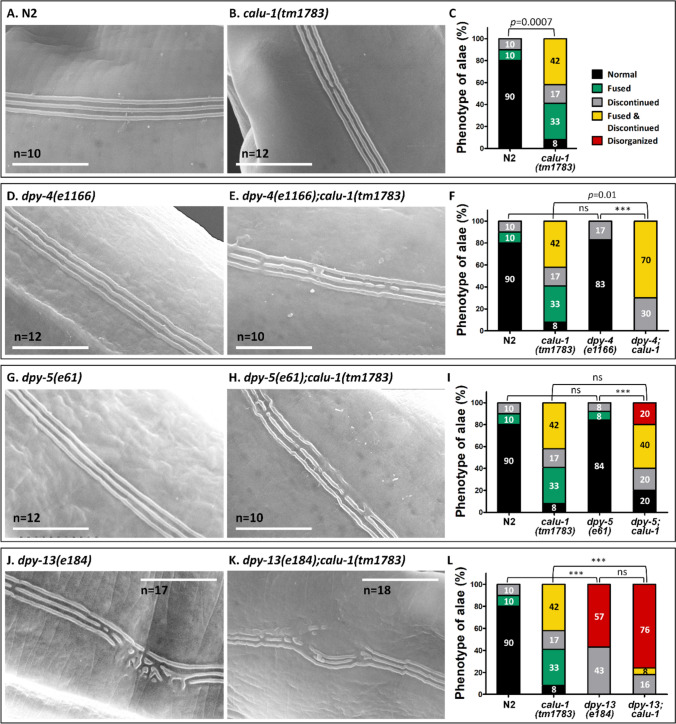
Next, double mutants were generated between calu-1 and some of collagen mutants. Three collagen mutants, dpy-4(e1166), dpy-5(e61) and dpy-13(e184), were chosen because annuli and annular furrows are normally formed in these mutants, suggesting that these genes are not required for the presence of the annular furrows on the cuticle [37, 59]. In addition, since the normal annular structure of PPIase mutants was completely disrupted by calu-1 mutation, the annular structure could be a standard to judge the effect of CALU-1 on collagen stability. SEM analysis again showed the normal annular structure in wild type N2 (Fig. 6A) and a disorganized annular structure in the calu-1(tm1783) mutant (Fig. 6B). As reported, dpy-4(e1166), dpy-5(e61) and dpy-13(e184) mutants exhibited 100% normal annular structure (Fig. 6C, E and G). In addition, both dpy-5(e61) and dpy-13(e184) mutants showed narrow annuli compared to wild type N2, as previously reported [59]. Surprisingly, all double mutants which examined (Fig. 6D, F and H) showed severely disorganized annular structure similar to that of calu-1(tm1783) mutant, suggesting that CALU-1 is necessary to produce and/or stabilize these annuli collagens.
Fig. 6.
CALU-1 maintains the annular structure of cuticle by regulating annuli collagens. Scanning electron micrograph of A wild type, B calu-1(tm1783), C dpy-4(e1166), D dpy-4(e1166);calu-1(tm1783), E dpy-5(e61), F dpy-5(e61);calu-1(tm1783), G dpy-13(e184), and H dpy-13(e184);calu-1(tm1783). The n numbers are shown on the scale bars; total observed n in black, n of normal annuli and furrow in yellow, n of irregular annuli and furrows in red. Scale bars, 5 μm
Meanwhile, the alae structure was also observed in adult stage (Fig. 7). Alae phenotypes of wild type N2 (Fig. 7A) and the calu-1(tm1783) mutant (Fig. 7B) were reproduced similarly to Fig. 2 (Fig. 7C). Both dpy-4(e1166) and dpy-5(e61) mutants were found to be normal in alae structure (Fig. 7D and G) showing no significant differences to wild type N2 (Fig. 7F and I). However, the calu-1 mutation caused alae defects in dpy-4(e1166) and dpy-5(e61) mutants as in the calu-1(tm1783) mutant (Fig. 7E, F, H and I). Interestingly, dpy-13(e184) mutant displayed severely deformed alae structure such as frequently discontinued and/or totally disorganized ridges compared to wild type N2 as shown in Fig. 7J with statistical significance (Fig. 7L). The alae defects in this dpy-13(e184) mutant seems to be exacerbated by the calu-1 mutation. Most of the dpy-13(e184);calu-1(tm1783) double mutants (Fig. 7K) exhibited much more severely disrupted alae ridges (Fig. 7L), although the statistical significance was not observed between dpy-13(e184) and dpy-13(e184);calu-1(tm1783) double mutant. These results suggest that CALU-1 is important component to form normal alae structure with DPY-13 collagen.
Fig. 7.
CALU-1 maintains the alae structure of cuticle by regulating annuli collagens. Scanning electron micrograph of A wild type, B calu-1(tm1783), D dpy-4(e1166), E dpy-4(e1166);calu-1(tm1783), G dpy-5(e61), H dpy-5(e61);calu-1(tm1783), J dpy-13(e184), and K dpy-13(e184);calu-1(tm1783). C, F, I and L Alae phenotypes were categorized and quantified. The n numbers are shown in the figures. Scale bars, 10 μm. ***, p < 0.0001; ns, not significant
The completely deformed cuticle structure caused by both calu-1 and collagen mutation eventually led to the destruction of the body’s integrity. As shown in Fig. 8, compared to wild type N2 (Fig. 8A, black bar in each graph), the calu-1(tm1783) mutant displayed slightly Dpy phenotype (Fig. 8B, green bar in each graph). The dpy-4(e1166) mutant showed a similar Dpy phenotype to the calu-1(tm1783) mutant (Fig. 8C, gray bar in the graph). Both dpy-5(e61) and dpy-13(e184) mutants showed a more severe Dpy phenotype than the calu-1(tm1783) mutant (Fig. 8E and G, gray bars in each graph). Interestingly, all double mutants (Fig. 8D, F and H, purple bar in each graph) have a far more severe Dpy phenotype than do the single dpy mutants and the calu-1 mutant with significant differences, suggesting that CALU-1 is essential for maintaining an animal’s body shape and integrity by regulating annuli collagens to form proper cuticle structure and plays a synergistic role with dpy genes in maintaining cuticle integrity.
Fig. 8.
All dpy;calu-1 double mutants exhibit far more severe dumpy phenotype. Micrograph of A wild type, B calu-1(tm1783), C dpy-4(e1166), D dpy-4(e1166);calu-1(tm1783), E dpy-5(e61), F dpy-5(e61);calu-1(tm1783), G dpy-13(e184), and H dpy-13(e184);calu-1(tm1783). Body length (Mean ± SEM) and n numbers are shown in the bar graphs. Scale bars, 50 μm. ***, p < 0.0001
CALU-1 is necessary to maintain the cuticle permeability barrier by regulating collagen
One of the biological functions of collagen-enriched cuticle is that they serve a highly impervious barrier between the animal and its environment. Thus, when specific collagens are missing, the cuticle permeability is increasing and this leads to the staining of cell-permeable nucleic acid dye in the head or hypodermis, which does not occur in normal wild type C. elegans [37]. A. Sandhu et al. has screened 4 cuticular collagens (DPY-7, DPY-8, DPY-9 and DPY-10) which were required to maintain the barrier and showed staining of head nuclei with Hoechst 33258 upon RNAi. Since the above 4 collagen mutants exhibited irregular annuli and furrows, the cuticle permeability of calu-1(tm1783) mutant was also examined in this study. As expected, every calu-1(tm1783) mutant we examined showed staining of head nuclei with Hoechst 33258 but every wild type N2 did not (Fig.9A and B). The stained head nuclei were counted to test the statistical significance and the permeability defect of the calu-1(tm1783) mutant was significant compared to wild type N2 (Fig. 9C). As already known [37], dpy-4(e1166), dpy-5(e61) and dpy-13(e184) mutants had no Hoechst 33258 permeability defect (Fig. 9D, G, and J) showing no significant difference to wild type N2 (Fig. 9F, I and L). However, all double mutants examined (Fig. 9E, H and K) showed similar (Fig. 9F and I) or stronger staining patterns (Fig. 9L) of head nuclei with Hoechst 33258 compared to calu-1(tm1783) single mutant. These results strongly suggest that CALU-1 is necessary to maintain the cuticle permeability by regulating annuli collagens and thus to protect animals from various environmental stimuli.
Fig. 9.
CALU-1 is required to maintain cuticle barrier function. Micrograph showing Hoechst 33258 staining-based permeability results of A wild type, B calu-1(tm1783), D dpy-4(e1166), F dpy-4(e1166);calu-1(tm1783), G dpy-5(e61), H dpy-5(e61);calu-1(tm1783), J dpy-13(e184), and K dpy-13(e184);calu-1(tm1783). A dotted line indicates the area of the head to be monitored. The number of stained nuclei was quantified and shown in C, F, I and L. The n numbers are shown on the scale bars. Scale bars, 20 μm. ***, p < 0.0001; ns, not significant
Stress response genes are differentially expressed in the calu-1(tm1783) mutant
It has been shown that disruption of annular furrows coactivates detoxification, hyperosmotic, and antimicrobial response genes. Mutation or silencing of six collagen genes (dpy-2, 3, 7, 8, 9, and 10) has severely disrupted annular furrows without any alae defect and activated detoxification, antimicrobial, and osmotic stress responses [36, 37, 57, 59, 61–67]. Thus, whole transcriptome RNA sequencing was performed using calu-1(tm1783) mutant and the differential gene expression (DEG) was analyzed. If CALU-1 is required for producing stable collagens, the transcriptional changes by calu-1 mutation may be similar to those by collagen mutations such as dpy-7 or dpy-10. As expected, the calu-1 mutation induced differential expression of stress response genes (Fig. 10); antimicrobial peptide genes (Fig. 10A), osmotic response genes (Fig. 10B), and detoxification genes (Fig. 10C). The transcriptional changes of genes observed in the calu-1(tm1783) transcriptome were compared to those of collagen mutant showing disrupted annular furrows. The transcriptome data of dpy-7(e88) [36] were extracted and the log fold change of stress response genes were compared (Fig. S4). Similar to the dpy-7 data, calu-1 mutation activated genes of antimicrobial, osmotic, and detoxification responses. All nlp-29 cluster (nlp-27, nlp-28, nlp-29, nlp-31 and nlp-34) [66, 68] and four (cnc-2, cnc-3, cnc-4 and cnc-11) of cnc-2 cluster (cnc-1, cnc-2, cnc-3, cnc-4, cnc-5 and cnc-11) genes [66, 68] were upregulated in the calu-1(tm1783) mutant (Figs. 10A and S4A). Genes encoding C-type lectin domain-containing proteins (clec) involved in the innate immune response [69] were also differentially expressed in the mutant (Figs. 10A and S4A). An extremely high expression of gpdh-1 gene encoding the rate limiting enzyme GPDH (glycerol-3-phosphate dehydrogenase) was observed in the calu-1(tm1783) mutant (Figs. 10B and S4B; (130 of log2 fold compared to wild type). Along with gpdh-1, the transporters hmit-1.1 and hmit-1.2, which are responsible for osmolyte accumulation, were also increased in calu-1 mutant. The hmit-1.1, which has been reported to play a major role in the osmotic response [70], was upregulated significantly more than hmit-1.2 in the calu-1 mutant. The calu-1 mutation also activated and/or deactivated many genes encoding detoxification enzymes [71] for phase I (CYPs, cytochrome P450s) and II (UGTs, UDP-glucuronosyl or glycosyl transferease and GSTs, glutathione S transferases) metabolism (Figs. 10C and S4C). In addition, several genes involved in antioxidant responses were upregulated in the calu-1 mutant, including sod-3 (superoxide dismutase), gpx-3, and gpx-5 (glutathione peroxidase) (data not shown), just as they were in the dpy-9 and dpy-10 collagen mutants [37]. These results show that CALU-1 is essential for maintaining cuticle collagens, which act as a cuticle-associated damage sensor that coordinates environmental stress responses such as antimicrobial, osmotic, and detoxification response.
Fig. 10.
Stress response genes are differentially expressed in calu-1(tm1783) mutant. Heat maps of differentially expressed genes associated with A antimicrobial peptides, B osmotic stress response, and C detoxification. Three replica were analyzed for wild type N2 (N2_1, N2_2, and N2_3) and calu-1(tm1783) mutant (calu-1_1, calu-1_2, and calu-1_3)
Discussion
CALU-1: A novel regulator of cuticle integrity and collagen stability in C. elegans
The C. elegans cuticle is organized into a series of circumferential bands, the annuli, which are separated by indentations called furrows and this circumferential annular pattern is interrupted over the lateral seam epidermis, which instead contains longitudinal alae ridge [7]. Based on the phenotypic characteristics of the calu-1(tm1783) mutant in C. elegans, the role of CALU-1 in proper cuticle development was questioned. Our findings reveal CALU-1 as a critical player in maintaining cuticle integrity and collagen stability in C. elegans. This study provides compelling evidence for CALU-1's role in collagen biosynthesis, cuticle formation, and stress response regulation, offering new insights into the complex process of extracellular matrix formation in nematodes.
Essential role of CALU-1 in collagen modification and nematode viability
The synthetic lethality observed in dpy-18(e364);calu-1(tm1783) double mutants underscores the essential roles of both CALU-1 and P4H in nematode development and survival. This unexpected finding suggests that CALU-1 may play an essential role in P4H function or operate in a parallel pathway equally vital for collagen modification. The lethality of these double mutants, reminiscent of the effects seen when both α-subunits of P4H are removed, underscores the fundamental importance of proper collagen processing in nematode development and viability. Interestingly, P4H genes (dpy-18 and phy-2) and FKBP gene (fkb-3) were found to be upregulated in the calu-1(tm1783) mutant, similar to the furrow defective dpy-7(e88) mutant (data not shown). This may be a compensatory response to cuticle defects, especially furrow defects, because furrow defects lead to increased cuticle permeability, making worms vulnerable to environmental stress. This may also be a compensatory response to restore non-functional collagens that are not properly modified by the malfunction of CALU-1.
CALU-1 and FKBPs: a complex interplay in cuticle development
Based on our results, all of the FKBP mutants we have characterized appear to be loss-of-function mutants, but protein levels need to be evaluated. While individual FKBP mutants show normal development under standard conditions, the introduction of calu-1 mutations alongside FKBP mutations dramatically alters this landscape. The resulting defects in growth, reproduction, and body morphology underscore CALU-1's critical importance in these physiological processes. This suggests that CALU-1 may act as a master regulator, potentially orchestrating or enhancing the activities of FKBPs.
Our cold-sensitivity experiments provided further insights into the complex interplay between CALU-1 and FKBPs. While most FKBP single mutants grew normally at subphysiological temperature (12 °C), the fkb-5(tm475) mutant showed slower growth and reduced fertility. Intriguingly, this phenotype was exacerbated by the calu-1 mutation, with fkb-5;calu-1 double mutants exhibiting severe growth defects. In addition, multiple mutants carrying the fkb-5 mutation failed to survive at 12 °C. These results suggest that FKB-5 plays a central role in cold stress adaptation, similar to body length regulation, possibly due to its abundant expression throughout the post-embryonic life cycle and its predominance in the hypodermis [25]. CALU-1 appears to contribute to this response, potentially through its role in maintaining normal cuticle development. These observations open up new avenues for investigating how CALU-1 and FKBPs collaborate in stress adaptation mechanisms, particularly in response to temperature changes.
CALU-1's critical role in collagen formation and stability
C. elegans mutations in cuticular collagen genes have been identified to result in a variety of body shape defects, including phenotypes such as dumpy (Dpy), roller (Rol), blister (Bli), squat (Sqt), ray abnormal (Ram), small (Sma), and long (Lon) [7]. Here, we have covered several dpy cuticle collagen genes: dpy-2, dpy-3, dpy-4, dpy-5, dpy-7, dpy-8, dpy-9, dpy-10 and dpy-13. These dpy genes are expressed at different time points during development, with dpy-2, 3, 7, 8, 9 and 10 genes being expressed 4 h before molting (called early-expressed collagen genes), and dpy-5 and dpy-13 genes being expressed 2 h before molting (called intermediately-expressed collagen genes). In terms of substructure, the former (DPY-2, 3, 7, 8, 9 and 10) is localized to the furrow; called furrow collagens and the latter (DPY-4, 5 and 13) marks only annuli; called annuli collagens [15]. Previous work has shown, using fluorescent labeling and immunostaining techniques, that these annuli collagens and furrow collagens are interdependent to form a specific substructure that independently exhibits specific stripe and band patterns [59, 72]. There are over 20 genes that, when mutated, cause the dumpy phenotype in adults, but the loss of just six dpy genes (dpy-2, 3, 7, 8, 9 and 10) is known to disrupt furrows [36, 37].
Our investigations into collagen localization and cuticle structure in various mutants provide strong evidence that CALU-1 is essential for the proper formation and maintenance of both early- and intermediately-expressed collagens independent of furrow and annulus localization. The disruption of annular furrows in calu-1 mutants, similar to furrow-defective dpy mutants, and the ability of calu-1 mutations to cause furrow defects in dpy-4, 5 and 13 mutants that originally lacked such defects, highlight CALU-1's importance in both furrow and annuli collagen regulation. The synergistic effects observed in dpy;calu-1 double mutants, resulting in more severe dumpy phenotypes. Considering the previous report that the loss of any two collagens from within the same group results in a phenotype similar to loss of either collagen alone, whereas loss of one collagen from each group results in a compounding effect because both substructures would be absent from the cuticle [59], the synergistic compounding effect further supports the notion that CALU-1 acts as an upstream common regulator of furrow and annuli collagen formation.
Consistent with the upregulation of collagen modifying enzyme genes (dpy-18, phy-2 and fkb-3) in the calu-1(tm1783) mutant, many collagen genes (bli, col, dpy, rol and sqt), including annuli collagens (dpy-4, -5 and -13), were also found to be upregulated in the mutant (data not shown). Further studies, such as multi-omics analysis between transcriptome and proteome profiles for the calu-1(tm1783) mutant, may elucidate the precise and specific mechanical function of CALU-1 in the cuticle development.
CALU-1's influence on cuticle permeability and stress responses
The annular furrow is closely correlated with sensing various environmental stimuli, and when mutated, only six dpy genes (dpy-2, 3, 7, 8, 9 and 10) lead to furrow loss, which increases cuticle permeability and activates detoxification, osmotic, and antimicrobial response genes [36–38]. The calu-1 mutation leads to defects in annular furrows, increased cuticle permeability, and differential expression of detoxification, osmotic, and antimicrobial response genes. The striking upregulation of gpdh-1 and other stress response genes in calu-1 mutant mirrors patterns seen in furrow-defective dpy mutants, suggesting a conserved mechanism linking cuticle integrity to stress responses.
Several transcription factors are known to act downstream of furrow loss to induce stress responses, such as SKN-1/Nrf, ELT-3/GATA, STA-2/Stat and BLMP-1/Blimp-1 [36, 37, 73] and knockdown of these transcription factors results in altered expression of specific sets of collagen genes [74–76]. The identification of ChIP-proven binding sites for key transcription factors like SKN-1/Nrf, ELT-3/GATA and BLMP-1/Blimp-1 in the calu-1 promoter region [77] opens up intriguing possibilities for the regulation of calu-1 expression and its role in developmental programs, including dauer formation [46].
CALU-1's role in alae formation
Our study provides the quantitative analysis of alae defects in calu-1 mutant, revealing CALU-1's importance in alae structure formation and novel insights into alae formation, complementing and extending previous research on dpy mutants. Our SEM analysis revealed complex alae phenotypes in various dpy mutants, sometimes differing from previous reports. Notably, while some dpy mutants with furrow loss (dpy-2, 3, 7, 8, 9 and 10) were reported to have normal alae [36, 57, 59, 61], we found that dpy-13(e184) exhibits severely defective alae structure, in contrast to the normal alae reported for dpy-13(e458) [57, 59]. Allele-specific effects may cause different mutations in the same gene to affect protein function in distinct ways. The dpy-13(e184) mutant has a small 36 bp in-frame deletion near the middle of the gene, making this allele semi-dominant, while the dpy-13(e458) mutant has a larger 723 bp deletion removing all conserved Gly-X–Y repeats [78]. Our SEM analysis revealed that the dpy-4(e1166) mutant showed normal alae structure, consistent with previous reports [57]. For dpy-5(e61), we observed predominantly normal alae structure in most animals (10 out of 12), with occasional fused or discontinued alae. This contrasts with earlier reports of absent alae in dpy-5 RNAi-treated animals [36], highlighting the sensitivity of different analysis methods (SEM, RNAi in COL-19::GFP expressing animals and immunostaining). However, it is crucial to note that the possibility of insufficient sample sizes cannot be ruled out. Importantly, our results demonstrate that DPY-13, in conjunction with CALU-1, plays a crucial role in adult alae formation. The dpy-13(e184);calu-1(tm1783) double mutant showed synergistic defects in alae formation, body length, and permeability with statistical significance. This underscores the importance of these proteins in maintaining cuticle integrity.
Meanwhile, various other proteins are known to contribute to alae formation including alae ridge marking collagens (BLI-6 and COL-12) [79, 80], epicuticlins [81], cuticlins, which are stage-specific ZP (Zona Pellucida) proteins [82, 83], extracellular matrix nidogen domain protein such as DEX-1 [84, 85], ZP-rich provisional matrix proteins such as LET-653, NOAH-1 and FBN-1 [86–88], putative lipid transporters such as lipocalin [86], and cytoskeleton proteins such as actin, actomyosin, and non-muscle myosin [88]. Future studies should explore potential functional relationships between CALU-1 and these molecules to further elucidate the mechanisms of alae development.
Mechanistic insight of CALU-1 in cuticle development
CALU-1, with its low affinity for calcium, likely functions as a calcium sensor rather than a buffer in the ER [39, 89]. While collagen P4H itself is not directly regulated by calcium [90] and not all PPIases are calcium-dependent, CALU-1 might influence collagen synthesis through subtle modulation of the ER environment. It could respond to small changes in ER calcium levels, potentially affecting the activity of calcium-dependent chaperones involved in P4H assembly or modulating calcium-sensitive PPIases [91, 92]. CALU-1 might also play a role in coordinating the activities of various ER-resident proteins involved in collagen folding, including P4H, PPIases, and other chaperones [93, 94]. In fact, CALU-1 has been shown to interact with and modulate the activity of SERCA (Sarco/Endoplasmic Reticulum Ca2+-ATPase) pumps, which are crucial for maintaining ER calcium homeostasis [95]. Additionally, CALU-1 could act as a sensor for ER stress conditions that impact overall collagen biosynthesis, influencing both P4H function and PPIase activity. Future studies should focus on how CALU-1's calcium-sensing properties contribute to maintaining optimal conditions for P4H and PPIase function in collagen synthesis, possibly by investigating its interactions with other ER-resident proteins involved in protein folding and quality control [96].
The calu-1 has been reported to disrupt molting when inactivated by RNAi [12], although we haven't yet tested this using the calu-1(tm1783) mutant. In addition, the calu-1 and P4H genes (dpy-18 and phy-2) transcripts but not the PPIase genes (fkb-3, -4, -5 and -7) transcripts have been reported to be downregulated during L3 (the third larval stage) molting and be upregulated during L4 (the fourth larval stage) molting [97]. Interestingly, genes related to molting such as molting-related protease (nas-36) and protease inhibitor (bli-5) were found to be upregulated in the calu-1(tm1783) mutant (data not shown). Since cuticular collagens are synthesized and secreted from the hypodermis during each molting cycle [7], and several protease (NAS-36 and NAS-37) and protease inhibitors (BLI-5 and MLT-11) are required for this molting process [15], this suggests that CALU-1 may contribute to proper molting by affecting collagen biosynthesis and related enzymatic functions. Further studies, including multi-omics analysis, may elucidate the exact relationships with CALU-1.
Meanwhile, recent proteomics studies have provided direct evidence for the interaction between calumenin and collagen I [98]. DiChiara et al. identified calumenin as a new interactor of collagen I using human cell-based selective immunoprecipitation integrated with quantitative mass spectrometry-based proteomics. While our study in C. elegans provides valuable insights into the role of CALU-1 in collagen biosynthesis, it is important to note that the nematode cuticle collagens differ significantly from vertebrate fibrillar collagens. C. elegans lacks a direct homolog of mammalian collagen I [13]. However, the principles of collagen synthesis, folding, and modification are largely conserved across species.
Our findings suggest that CALU-1 plays a crucial role in the biosynthesis and stability of C. elegans cuticle collagens. This role may involve interactions with collagen-modifying enzymes like P4H and PPIases, or direct interactions with collagen molecules themselves. While we cannot draw direct parallels to specific mammalian collagens, the involvement of a calcium-binding protein like CALU-1 in collagen biosynthesis highlights the importance of calcium homeostasis and sensing in this process.
Future studies should focus on identifying the specific C. elegans collagens that might interact with CALU-1, and investigating how these interactions are influenced by calcium levels in the ER. Additionally, research could explore whether the role of CALU-1 in collagen biosynthesis extends to other invertebrate or vertebrate systems, keeping in mind the differences in collagen types and structures across species. While we cannot directly extrapolate our findings to mammalian collagen I, the fundamental principles uncovered in our C. elegans studies may still provide valuable insights into the general mechanisms of collagen biosynthesis and the role of calcium-binding proteins in this process.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate Dr. Joohong Ahnn in Hanyang University and Dr. Shoei Mitani for providing strains. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Author Contributions
K.E.L. and J.H.C. performed experiments, analyzed the data and wrote the original draft. H.O.S. conceived and performed experiments, wrote and edited the manuscript, and secured funding.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A2C1013033) and a grant from the Yuhan Innovation Program (YIP) of Yuhan Corporation.
Data availability
Most data generated or analyzed during this study are included in this article and its supplementary information file. The dataset obtained from whole transcriptome RNA sequencing is available from the corresponding author upon reasonable request.
Declarations
Conflict of interests
The authors declare no conflict of interest.
Consent for publication
All authors consented to publish the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kyung Eun Lee and Jeong Hoon Cho have contributed equally to this work.
References
- 1.Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123(Pt 24):4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326(5957):1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley WP, Peters SB, Larsen M (2008) Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121(Pt 3):255–264 [DOI] [PubMed] [Google Scholar]
- 4.Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15(12):802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsi AK, Wightman B, Chalfie M (2015) A transparent window into biology: a primer on Caenorhabditis elegans. Genetics 200(2):387–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenyon C (1988) The nematode Caenorhabditis elegans. Science 240(4858):1448–1453 [DOI] [PubMed] [Google Scholar]
- 7.Page AP, Johnstone IL: The cuticle. WormBook 2007:1–15. [DOI] [PMC free article] [PubMed]
- 8.Chisholm AD, Hsiao TI (2012) The Caenorhabditis elegans epidermis as a model skin. I: development, patterning, and growth. WIRE Dev Biol, 1(6):861–878. [DOI] [PMC free article] [PubMed]
- 9.Johnstone IL (2000) Cuticle collagen genes. Exp Caenorhabditis Elegans Trends Genet 16(1):21–27 [DOI] [PubMed] [Google Scholar]
- 10.Kramer JM: Extracellular Matrix. 1997.
- 11.Cox GN, Kusch M, Edgar RS (1981) Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol 90(1):7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frand AR, Russel S, Ruvkun G (2005) Functional genomic analysis of C. elegans molting. PLoS Biol 3(10):e312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teuscher AC, Jongsma E, Davis MN, Statzer C, Gebauer JM, Naba A, Ewald CY (2019) The in-silico characterization of the Caenorhabditis elegans matrisome and proposal of a novel collagen classification. Matrix Biol Plus 1:100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewald CY, Landis JN, Porter Abate J, Murphy CT, Blackwell TK (2015) Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519(7541):97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaram MV, Pujol N (2024) The Caenorhabditis elegans cuticle and precuticle: a model for studying dynamic apical extracellular matrices in vivo. Genetics. 10.1093/genetics/iyae072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78:929–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myllyharju J, Kivirikko KI (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 20(1):33–43 [DOI] [PubMed] [Google Scholar]
- 18.Gorres KL, Raines RT (2010) Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol 45(2):106–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg RA, Prockop DJ (1973) The thermal transition of a non-hydroxylated form of collagen. evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun 52(1):115–120 [DOI] [PubMed] [Google Scholar]
- 20.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT (2001) Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc 123(4):777–778 [DOI] [PubMed] [Google Scholar]
- 21.Myllyharju J, Kukkola L, Winter AD, Page AP (2002) The exoskeleton collagens in Caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J Biol Chem 277(32):29187–29196 [DOI] [PubMed] [Google Scholar]
- 22.Friedman L, Higgin JJ, Moulder G, Barstead R, Raines RT, Kimble J (2000) Prolyl 4-hydroxylase is required for viability and morphogenesis in Caenorhabditis elegans. Proc Natl Acad Sci U S A 97(9):4736–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill KL, Harfe BD, Dobbins CA, L’Hernault SW (2000) dpy-18 encodes an alpha-subunit of prolyl-4-hydroxylase in caenorhabditis elegans. Genetics 155(3):1139–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter AD, Page AP (2000) Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol Cell Biol 20(11):4084–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachinger HP (1987) The influence of peptidyl-prolyl cis-trans isomerase on the in vitro folding of type III collagen. J Biol Chem 262(35):17144–17148 [PubMed] [Google Scholar]
- 26.Steinmann B, Bruckner P, Superti-Furga A (1991) Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem 266(2):1299–1303 [PubMed] [Google Scholar]
- 27.Pemberton TJ, Kay JE (2005) Identification and comparative analysis of the peptidyl-prolyl cis/trans isomerase repertoires of H. sapiens, D. melanogaster, C. elegans, S. cerevisiae and Sz, pombe. Comp Funct Genomics 6(5–6):277–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter AD, Eschenlauer SC, McCormack G, Page AP (2007) Loss of secretory pathway FK506-binding proteins results in cold-sensitive lethality and associate extracellular matrix defects in the nematode Caenorhabditis elegans. J Biol Chem 282(17):12813–12821 [DOI] [PubMed] [Google Scholar]
- 29.Dolinski K, Muir S, Cardenas M, Heitman J (1997) All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 94(24):13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myllyharju J, Kivirikko KI (2001) Collagens and collagen-related diseases. Ann Med 33(1):7–21 [DOI] [PubMed] [Google Scholar]
- 31.Baumann M, Giunta C, Krabichler B, Ruschendorf F, Zoppi N, Colombi M, Bittner RE, Quijano-Roy S, Muntoni F, Cirak S et al (2012) Mutations in FKBP14 cause a variant of Ehlers-Danlos syndrome with progressive kyphoscoliosis, myopathy, and hearing loss. Am J Hum Genet 90(2):201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanauske-Abel HM (1991) Prolyl 4-hydroxylase, a target enzyme for drug development. design of suppressive agents and the in vitro effects of inhibitors and proinhibitors. J Hepatol 13:8–15 [DOI] [PubMed] [Google Scholar]
- 33.Myllyharju J (2008) Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med 40(6):402–417 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbloom J, Castro SV, Jimenez SA (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152(3):159–166 [DOI] [PubMed] [Google Scholar]
- 35.Friedman SL, Sheppard D, Duffield JS, Violette S (2013) Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5(167):167sr161 [DOI] [PubMed] [Google Scholar]
- 36.Dodd W, Tang L, Lone JC, Wimberly K, Wu CW, Consalvo C, Wright JE, Pujol N, Choe KP (2018) A damage sensor associated with the cuticle coordinates three core environmental stress responses in Caenorhabditis elegans. Genetics 208(4):1467–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandhu A, Badal D, Sheokand R, Tyagi S, Singh V (2021) Specific collagens maintain the cuticle permeability barrier in Caenorhabditis elegans. Genetics. 10.1093/genetics/iyaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandler LM, Choe KP (2022) Extracellular matrix regulation of stress response genes during larval development in Caenorhabditis elegans. G3 Bethesda. 10.1093/g3journal/jkac221 [DOI] [PMC free article] [PubMed]
- 39.Honore B (2009) The rapidly expanding CREC protein family: members, localization, function, and role in disease. BioEssays 31(3):262–277 [DOI] [PubMed] [Google Scholar]
- 40.Cho JH, Song HO, Singaravelu G, Sung H, Oh WC, Kwon S, Kim DH, Ahnn J (2009) Pleiotropic roles of calumenin (calu-1), a calcium-binding ER luminal protein. Caenorhabditis elegans FEBS Lett 583(18):3050–3056 [DOI] [PubMed] [Google Scholar]
- 41.Yabe D, Nakamura T, Kanazawa N, Tashiro K, Honjo T (1997) Calumenin, a Ca2+-binding protein retained in the endoplasmic reticulum with a novel carboxyl-terminal sequence. HDEF J Biol Chem 272(29):18232–18239 [DOI] [PubMed] [Google Scholar]
- 42.Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, Pena C, Lopez-Lucendo M, Villar-Vazquez R, de Herreros AG et al (2013) Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res 19(21):6006–6019 [DOI] [PubMed] [Google Scholar]
- 43.Kurpinska A, Suraj J, Bonar E, Zakrzewska A, Stojak M, Sternak M, Jasztal A, Walczak M (2019) Proteomic characterization of early lung response to breast cancer metastasis in mice. Exp Mol Pathol 107:129–140 [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Wang J, Xu S, Shi F, Shan A (2021) Calumenin contributes to epithelial-mesenchymal transition and predicts poor survival in glioma. Transl Neurosci 12(1):67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du Y, Miao W, Jiang X, Cao J, Wang B, Wang Y, Yu J, Wang X, Liu H (2021) The epithelial to mesenchymal transition related gene calumenin is an adverse prognostic factor of bladder cancer correlated with tumor microenvironment remodeling, gene mutation, and ferroptosis. Front Oncol 11:683951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KE, Cho JH, Song HO (2023) Calumenin, a Ca2+ binding protein is required for dauer formation in caenorhabditis elegans. Biol (Basel). 10.3390/biology12030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi TW, Cho JH, Ahnn J, Song HO (2018) Novel findings of anti-filarial drug target and structure-based virtual screening for drug discovery. Int J Mol Sci 19(11):3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassada RC, Russell RL (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol 46(2):326–342 [DOI] [PubMed] [Google Scholar]
- 49.Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall DH (1995) Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol 48:395–436 [DOI] [PubMed] [Google Scholar]
- 51.Hall DH, Hartwieg E, Nguyen KC (2012) Modern electron microscopy methods for C. elegans. Methods Cell Biol 107:93–149 [DOI] [PubMed] [Google Scholar]
- 52.Ji YJ, Nam S, Jin YH, Cha EJ, Lee KS, Choi KY, Song HO, Lee J, Bae SC, Ahnn J (2004) RNT-1 the C elegans homologue of mammalian RUNX transcription factors, regulates body size and male tail development. Dev Biol 274(2):402–412 [DOI] [PubMed] [Google Scholar]
- 53.Moribe H, Yochem J, Yamada H, Tabuse Y, Fujimoto T, Mekada E (2004) Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J Cell Sci 117(Pt 22):5209–5220 [DOI] [PubMed] [Google Scholar]
- 54.Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33(3):290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL (2016) Transcript-level expression analysis of RNA-seq experiments with HISAT. StringTie and Ballgown Nat Protoc 11(9):1650–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thein MC, McCormack G, Winter AD, Johnstone IL, Shoemaker CB, Page AP (2003) Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev Dyn 226(3):523–539 [DOI] [PubMed] [Google Scholar]
- 58.Cox GN, Staprans S, Edgar RS (1981) The cuticle of Caenorhabditis elegans, II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev Biol 86(2):456–470 [DOI] [PubMed] [Google Scholar]
- 59.McMahon L, Muriel JM, Roberts B, Quinn M, Johnstone IL (2003) Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol Biol Cell 14(4):1366–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao R, Li M, Zhang Q, Yang C, Wang X (2020) An ECM-to-Nucleus signaling pathway activates lysosomes for C. elegans larval development. Dev Cell 52(1):21–37 [DOI] [PubMed] [Google Scholar]
- 61.Cox GN, Laufer JS, Kusch M, Edgar RS (1980) Genetic and phenotypic characterization of roller mutants of Caenorhaditis elegans. Genetics 95(2):317–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wheeler JM, Thomas JH (2006) Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics 174(3):1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamitina T, Huang CG, Strange K (2006) Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci U S A 103(32):12173–12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zugasti O, Bose N, Squiban B, Belougne J, Kurz CL, Schroeder FC, Pujol N, Ewbank JJ (2014) Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat Immunol 15(9):833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zugasti O, Thakur N, Belougne J, Squiban B, Kurz CL, Soule J, Omi S, Tichit L, Pujol N, Ewbank JJ (2016) A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes. BMC Biol. 10.1186/s12915-016-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H (2008) Ewbank JJ (2008) Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog 4(7):e1000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohlfing AK, Miteva Y, Hannenhalli S, Lamitina T (2010) Genetic and physiological activation of osmosensitive gene expression mimics transcriptional signatures of pathogen infection in C elegans. PLoS One 5(2):e9010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zugasti O, Ewbank JJ (2009) Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 10(3):249–256 [DOI] [PubMed] [Google Scholar]
- 69.Pan W, Huang X, Guo Z, Nagarajan R, Mylonakis E (2021) Identification and functional analysis of cytokine-like protein CLEC-47 in caenorhabditis elegans. Bio 12(5):e0257921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kage-Nakadai E, Uehara T, Mitani S (2011) H+/myo-inositol transporter genes, hmit-1.1 and hmit-1.2, have roles in the osmoprotective response in Caenorhabditis elegans. Biochem Biophys Res Commun 410(3):471–477 [DOI] [PubMed] [Google Scholar]
- 71.Lindblom TH, Dodd AK (2006) Xenobiotic detoxification in the nematode Caenorhabditis elegans. J Exp Zool A Comp Exp Biol 305(9):720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y, Li W, Dong Y, Xia C (2023) C. elegans hemidesmosomes sense collagen damage to trigger innate immune response in the epidermis. Cells 12(18):2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesbahi H, Pho KB, Tench AJ, Leon Guerrero VL, MacNeil LT (2020) Cuticle collagen expression is regulated in response to environmental stimuli by the GATA transcription factor ELT-3 in Caenorhabditis elegans. Genetics 215(2):483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operana TN, Esmaillie R, Blackwell TK: Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife. 10.7554/eLife.07836 [DOI] [PMC free article] [PubMed]
- 75.Yin J, Madaan U, Park A, Aftab N, Savage-Dunn C (2015) Multiple cis elements and GATA factors regulate a cuticle collagen gene in Caenorhabditis elegans. Genesis 53(3–4):278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hyun M, Kim J, Dumur C, Schroeder FC, You YJ (2016) BLIMP-1/BLMP-1 and metastasis-associated protein regulate stress resistant development in caenorhabditis elegans. Genetics 203(4):1721–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyle AP, Araya CL, Brdlik C, Cayting P, Cheng C, Cheng Y, Gardner K, Hillier LW, Janette J, Jiang L et al (2014) Comparative analysis of regulatory information and circuits across distant species. Nature 512(7515):453–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou X, Xu F, Mao H, Ji J, Yin M, Feng X, Guang S (2014) Nuclear RNAi contributes to the silencing of off-target genes and repetitive sequences in Caenorhabditis elegans. Genetics 197(1):121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams JRG, Pooranachithra M, Jyo EM, Zheng SL, Goncharov A, Crew JR, Kramer JM, Jin Y, Ernst AM, Chisholm AD (2023) Nanoscale patterning of collagens in Celegans apical extracellular matrix. Nat Commun 14(1):7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y, Kang J, Zhen R, Zhang L, Chen C (2023) A genome-wide CRISPR screen identifies the CCT chaperonin as a critical regulator of vesicle trafficking. FASEB J 37(2):e22757 [DOI] [PubMed] [Google Scholar]
- 81.Pooranachithra M, Jyo EM, Brouilly N, Pujol N, Ernst AM, Chisholm AD (2024) C. elegans epicuticlins define specific compartments in the apical extracellular matrix and function in wound repair. Development. 10.1242/dev.204330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muriel JM, Brannan M, Taylor K, Johnstone IL, Lithgow GJ, Tuckwell D (2003) M142.2 (cut-6), a novel Caenorhabditis elegans matrix gene important for dauer body shape. Dev Biol 260(2):339–351 [DOI] [PubMed] [Google Scholar]
- 83.Sapio MR, Hilliard MA, Cermola M, Favre R, Bazzicalupo P (2005) The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev Biol 282(1):231–245 [DOI] [PubMed] [Google Scholar]
- 84.Flatt KM, Beshers C, Unal C, Cohen JD, Sundaram MV, Schroeder NE (2019) Epidermal remodeling in Caenorhabditis elegans dauers requires the nidogen domain protein DEX-1. Genetics 211(1):169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen JD, Flatt KM, Schroeder NE, Sundaram MV (2018) Epithelial shaping by diverse apical extracellular matrices requires the nidogen domain protein DEX-1 in Caenorhabditis elegans. Genetics 211(1):185–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forman-Rubinsky R, Cohen JD, Sundaram MV (2017) Lipocalins are required for apical extracellular matrix organization and remodeling in Caenorhabditis elegans. Genetics 207(2):625–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gill HK, Cohen JD, Ayala-Figueroa J, Forman-Rubinsky R, Poggioli C, Bickard K, Parry JM, Pu P, Hall DH, Sundaram MV (2016) Integrity of narrow epithelial tubes in the C. elegans excretory system requires a transient luminal matrix. PLoS Genet 12(8):e1006205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katz SS, Barker TJ, Maul-Newby HM, Sparacio AP, Nguyen KCQ, Maybrun CL, Belfi A, Cohen JD, Hall DH, Sundaram MV et al: A transient apical extracellular matrix relays cytoskeletal patterns to shape permanent acellular ridges on the surface of adult C. elegans. PLoS Genet 2022, 18(8):e1010348. [DOI] [PMC free article] [PubMed]
- 89.Blank B, von Blume J (2017) Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur J Cell Biol 96(5):383–390 [DOI] [PubMed] [Google Scholar]
- 90.Myllyharju J (2003) Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol 22(1):15–24 [DOI] [PubMed] [Google Scholar]
- 91.Wang P, Heitman J (2005) The cyclophilins. Genome Biol 6(7):226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dunyak BM, Gestwicki JE (2016) Peptidyl-Proline isomerases (PPIases): targets for natural products and natural product-inspired compounds. J Med Chem 59(21):9622–9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ellgaard L, Ruddock LW (2005) The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 6(1):28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vincenz-Donnelly L, Hipp MS (2017) The endoplasmic reticulum: A hub of protein quality control in health and disease. Free Radic Biol Med 108:383–393 [DOI] [PubMed] [Google Scholar]
- 95.Sahoo SK, Kim DH (2008) Calumenin interacts with SERCA2 in rat cardiac sarcoplasmic reticulum. Mol Cells 26(3):265–269 [PubMed] [Google Scholar]
- 96.Sicari D, Delaunay-Moisan A, Combettes L, Chevet E, Igbaria A (2020) A guide to assessing endoplasmic reticulum homeostasis and stress in mammalian systems. FEBS J 287(1):27–42 [DOI] [PubMed] [Google Scholar]
- 97.Turek M, Bringmann H (2014) Gene expression changes of Caenorhabditis elegans larvae during molting and sleep-like lethargus. PLoS ONE 9(11):e113269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DiChiara AS, Taylor RJ, Wong MY, Doan ND, Rosario AM, Shoulders MD (2016) Mapping and exploring the collagen-i proteostasis Network. ACS Chem Biol 11(5):1408–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most data generated or analyzed during this study are included in this article and its supplementary information file. The dataset obtained from whole transcriptome RNA sequencing is available from the corresponding author upon reasonable request.