Abstract
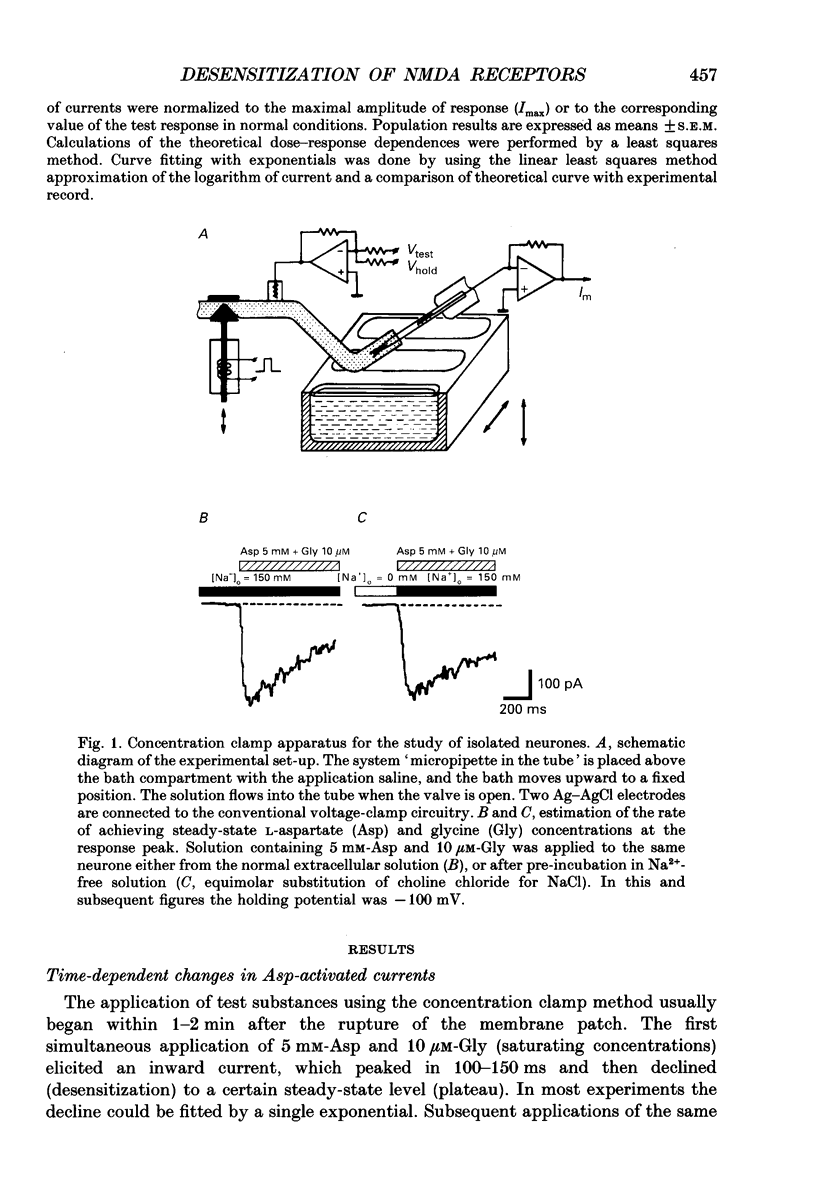
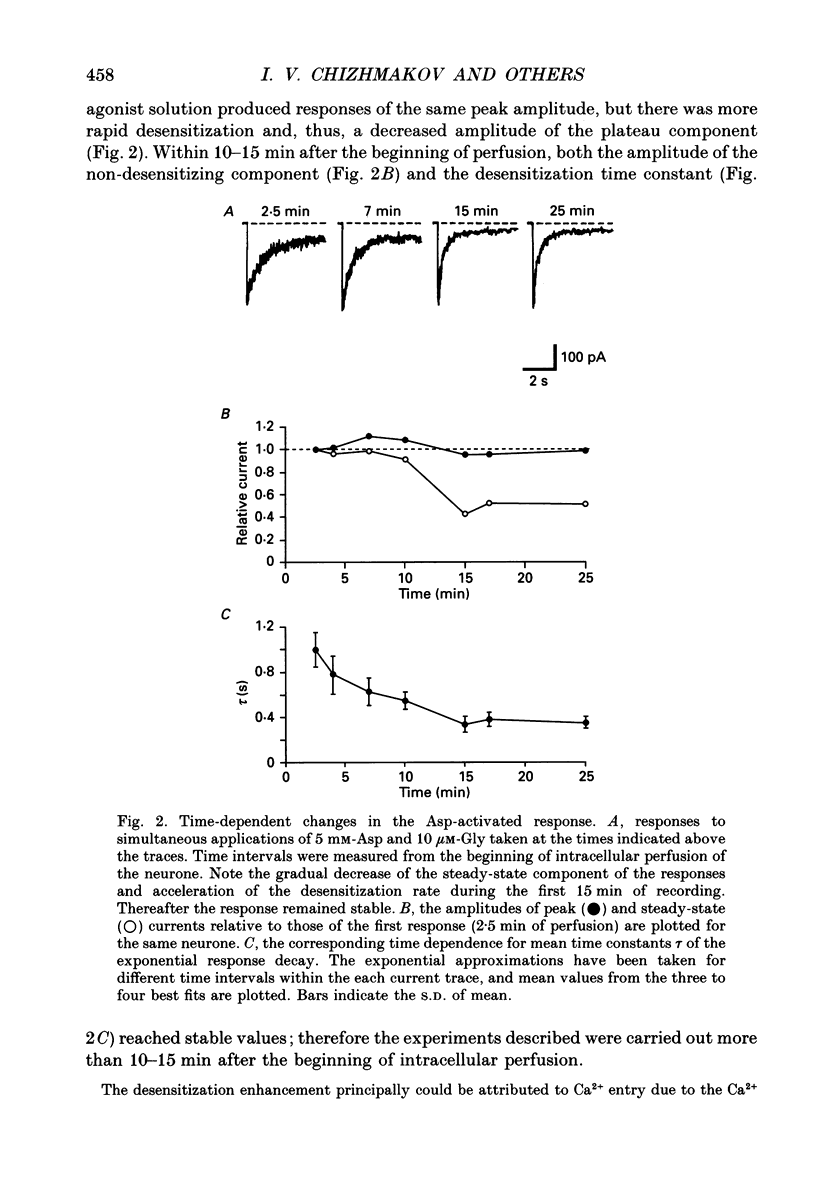
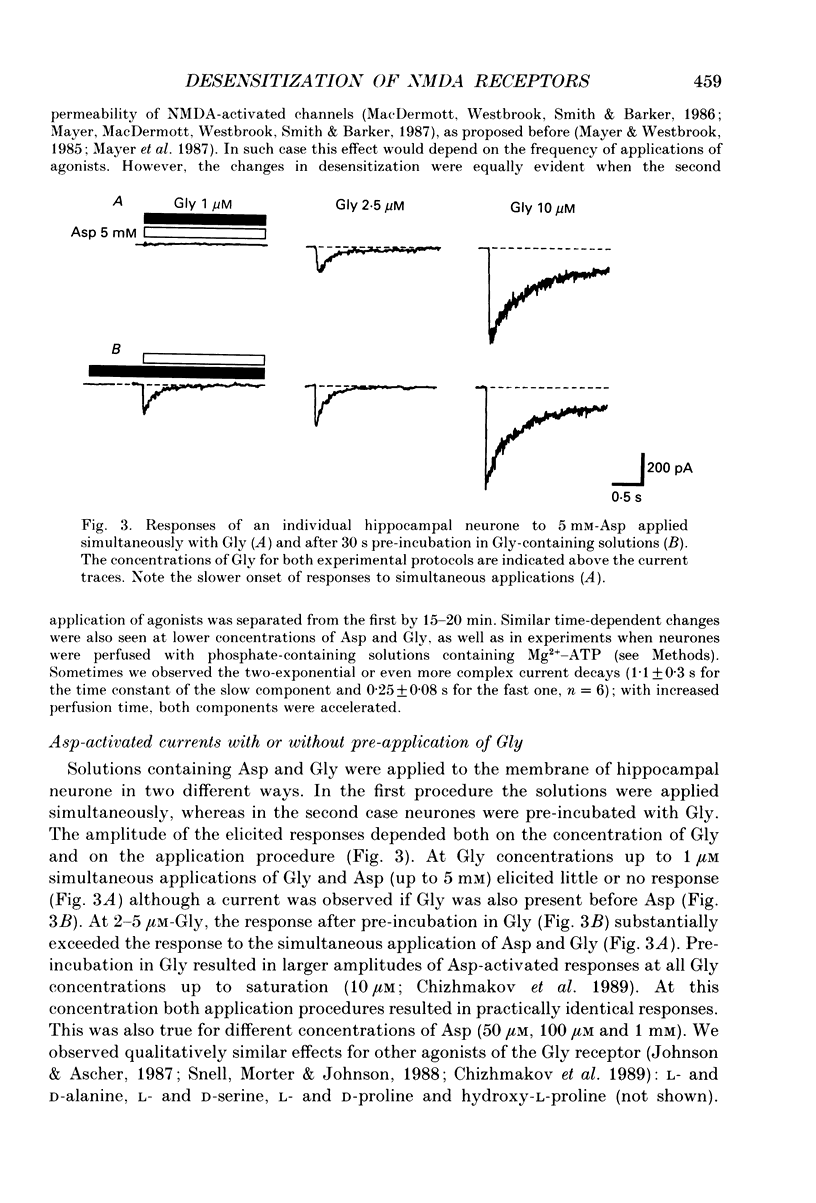
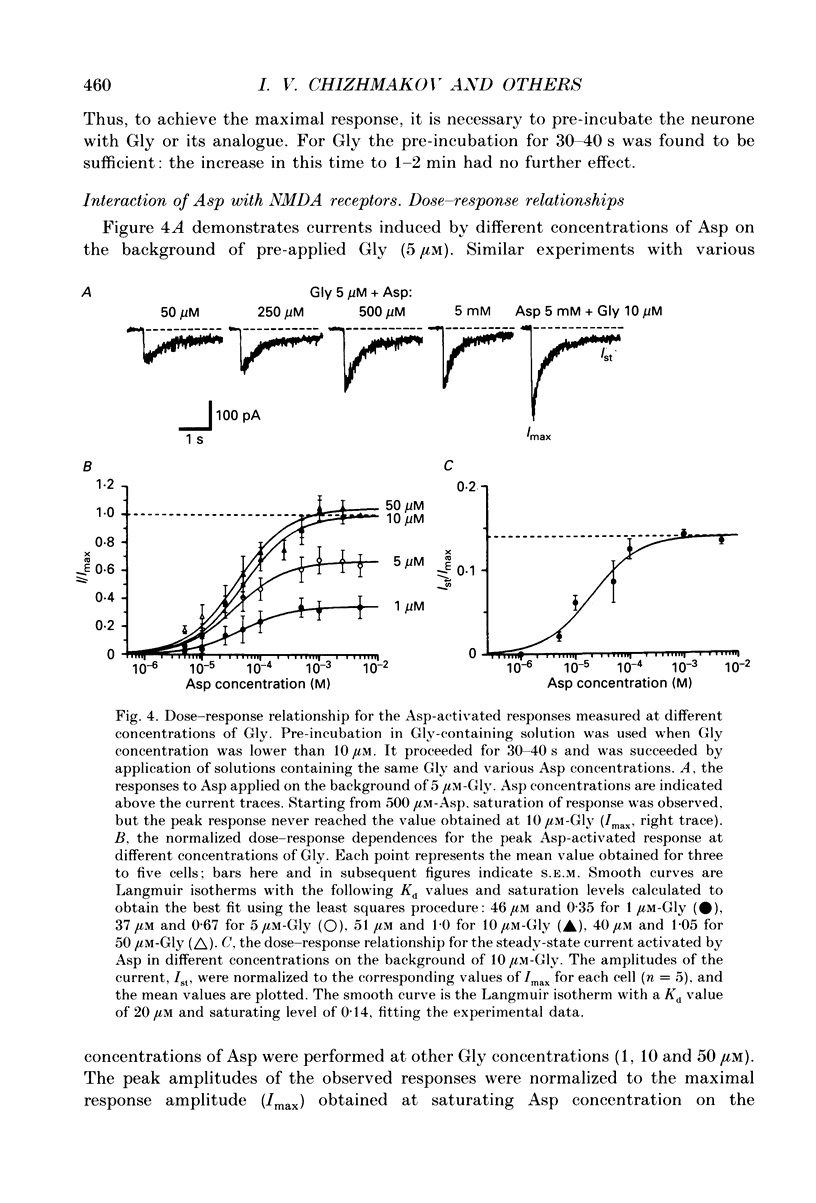
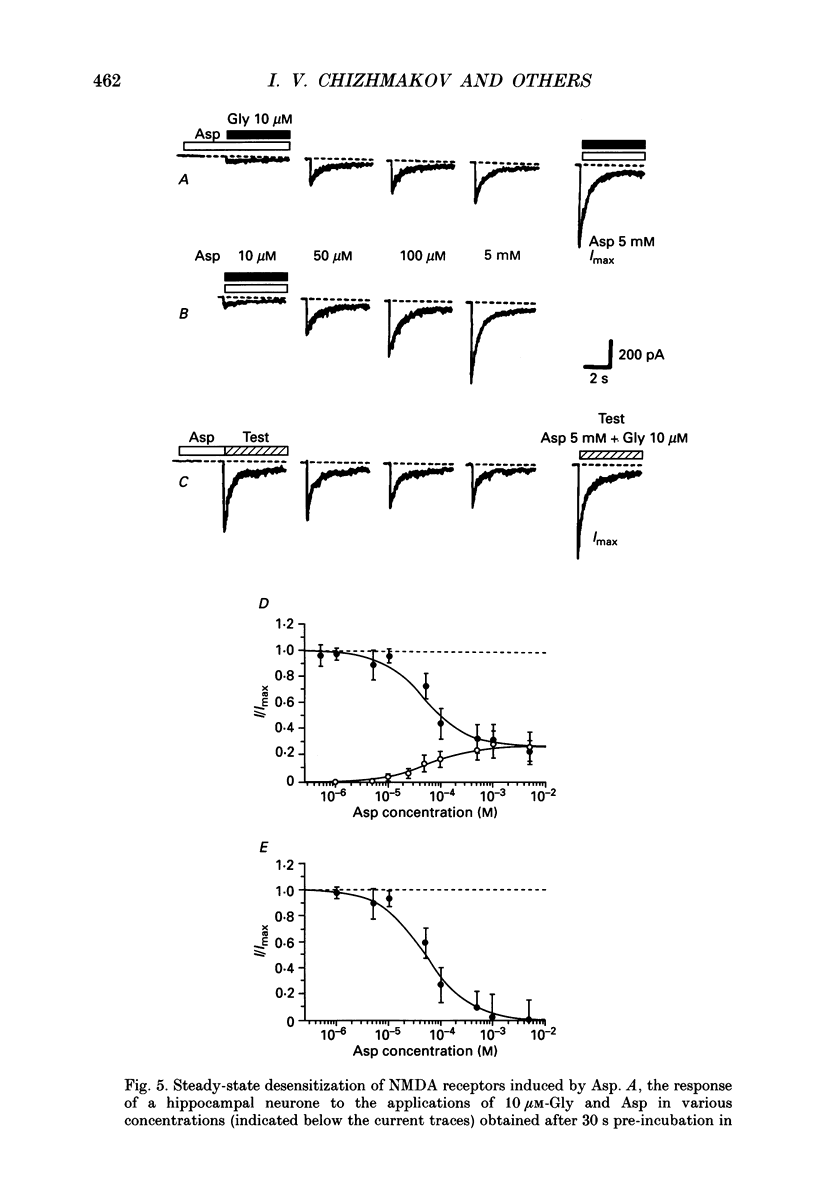
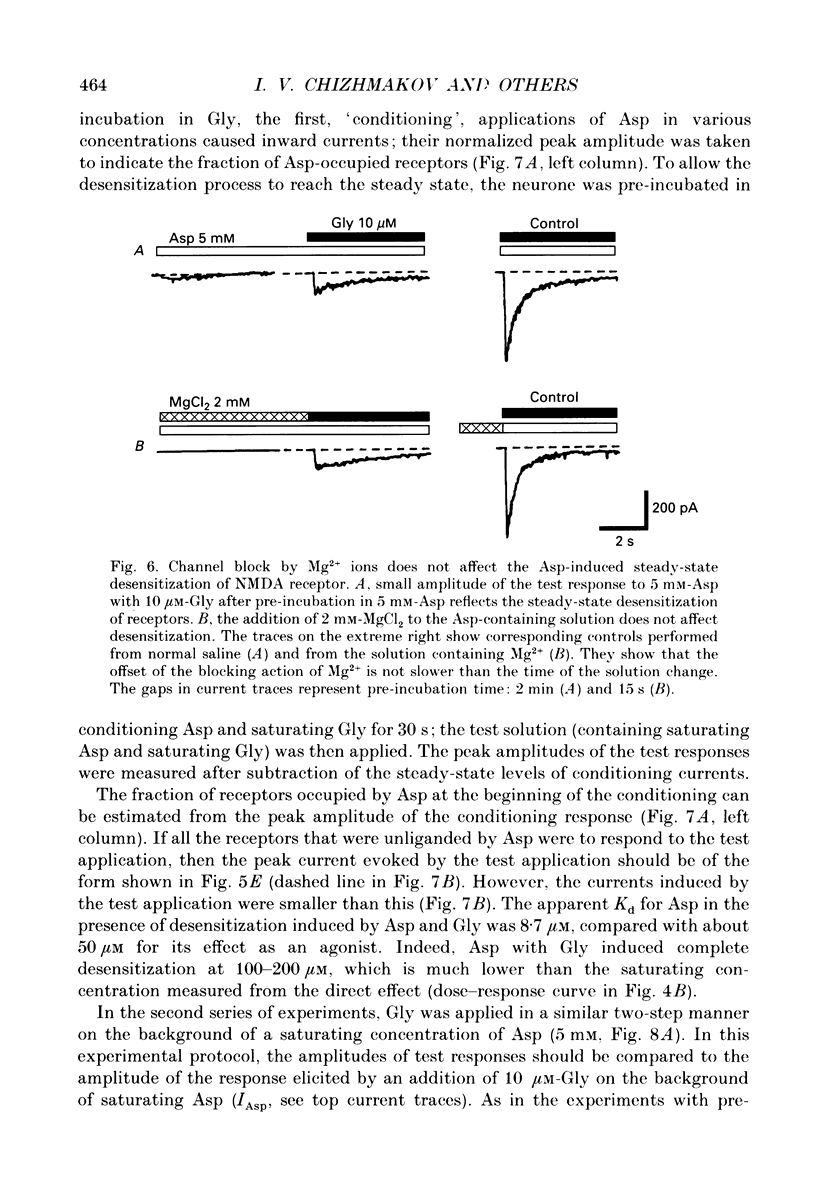
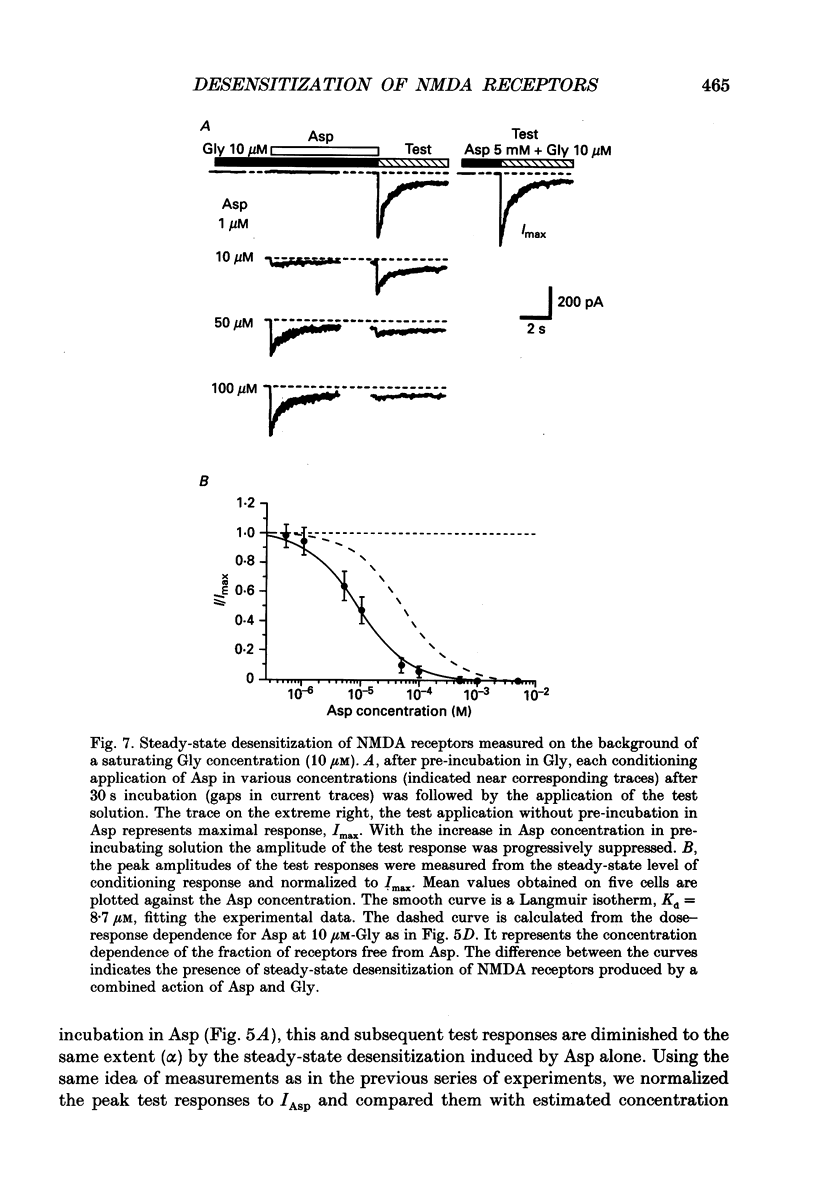
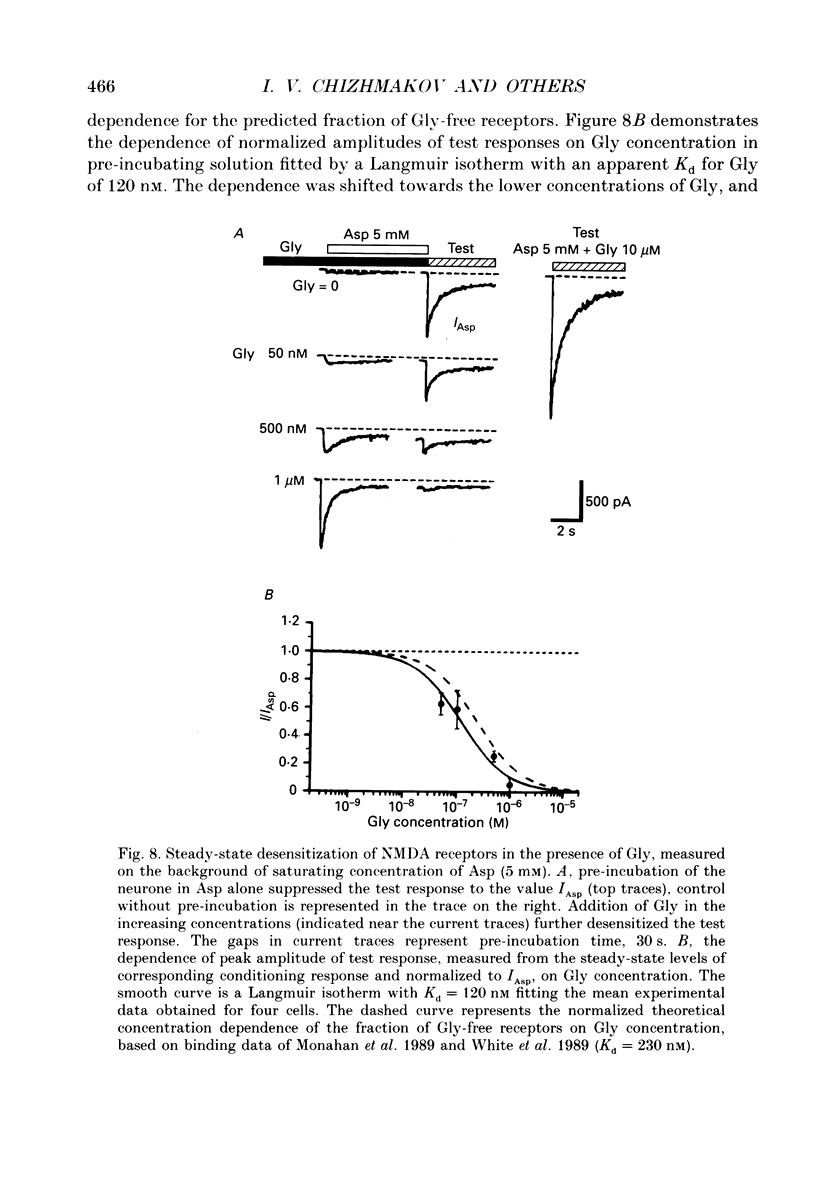
1. Whole-cell voltage-clamp recordings were made from rat isolated hippocampal neurones. Aspartate (Asp) and/or glycine (Gly) were applied by a method in which the external solution could be changed within 30 ms and thereafter held constant. 2. Asp and Gly applied together at maximal concentrations (5 mM and 10 microM, respectively) evoked an inward current due to activation of N-methyl-D-aspartate (NMDA) receptors. The current peaked and then declined to a steady state during the application. The time constant of desensitization (tau) was about 1 s when the agonists were applied soon after the onset of whole-cell recording. The desensitization became more rapid (tau = 0.3 s) and more complete during the first 15 min of recording, and thereafter remained stable; the amplitude of the peak response did not change throughout. In solutions containing 10 microM-Gly, Asp had an apparent Kd of 51 microM at the peak of response and 20 microM measured at the steady state. The steady-state current was 14% of the peak current. 3. Asp was applied after a conditioning exposure of the cell of Gly (from 1 to 50 microM), together with the same Gly concentration. The maximum current evoked by the application of Asp was increased while increasing Gly in the conditioning solution, with no change in the apparent Kd for Asp at the peak of Asp-activated response. 4. Various concentrations of Asp (plus 10 microM-Gly) were applied after a conditioning exposure to Asp (which alone was without effect). The maximum current induced by Asp applications was only 28% of that observed without conditioning Asp application, but the apparent Kd was unchanged (about 57 microM). 5. Test solution containing maximal concentrations of Asp and Gly was applied after conditioning exposure to both Asp (varying concentrations) and Gly (10 microM). Complete desensitization was caused by 200 microM-Asp. The apparent Kd for Asp to induce desensitization (8.7 microM) was less than the Kd as an agonist (51 microM). 6. Test solution containing maximal concentrations of Asp and Gly was applied after conditioning exposure to both Gly (varying concentrations) and Asp (5 mM). Complete desensitization was caused by 1 microM-Gly. The apparent Kd for Gly to induce desensitization (120 nM) was less than the Kd as a co-agonist (about 1 microM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Sep 1;154(1):85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Bonhaus D. W., Yeh G. C., Skaryak L., McNamara J. O. Glycine regulation of the N-methyl-D-aspartate receptor-gated ion channel in hippocampal membranes. Mol Pharmacol. 1989 Aug;36(2):273–279. [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Chizhmakov I. V., Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Glycine action on N-methyl-D-aspartate receptors in rat hippocampal neurons. Neurosci Lett. 1989 Apr 24;99(1-2):131–136. doi: 10.1016/0304-3940(89)90277-2. [DOI] [PubMed] [Google Scholar]
- Chizhmakov I. V., Kiskin N. I., Tsyndrenko AYa, Krishtal O. A. Desensitization of NMDA receptors does not proceed in the presence of kynurenate. Neurosci Lett. 1990 Jan 1;108(1-2):88–92. doi: 10.1016/0304-3940(90)90711-h. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Monaghan D. T., Ganong A. H. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Kemp J. A. Neurobiology. Glycine maintains excitement. Nature. 1989 Mar 30;338(6214):377–378. doi: 10.1038/338377a0. [DOI] [PubMed] [Google Scholar]
- Henderson G., Johnson J. W., Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990 Nov;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E. Indole-2-carboxylic acid: a competitive antagonist of potentiation by glycine at the NMDA receptor. Science. 1989 Mar 24;243(4898):1611–1613. doi: 10.1126/science.2467381. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Osipchuk YuV, Vrublevsky S. V. Properties of glycine-activated conductances in rat brain neurones. Neurosci Lett. 1988 Feb 3;84(3):271–276. doi: 10.1016/0304-3940(88)90519-8. [DOI] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Zukin R. S., Bennett M. V. N-methyl-D-aspartate activates different channels than do kainate and quisqualate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2083–2087. doi: 10.1073/pnas.86.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Mody I., Salter M. W., MacDonald J. F. Whole-cell voltage-clamp recordings in granule cells acutely isolated from hippocampal slices of adult or aged rats. Neurosci Lett. 1989 Jan 2;96(1):70–75. doi: 10.1016/0304-3940(89)90245-0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Monahan J. B., Corpus V. M., Hood W. F., Thomas J. W., Compton R. P. Characterization of a [3H]glycine recognition site as a modulatory site of the N-methyl-D-aspartate receptor complex. J Neurochem. 1989 Aug;53(2):370–375. doi: 10.1111/j.1471-4159.1989.tb07344.x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Snell L. D., Morter R. S., Johnson K. M. Structural requirements for activation of the glycine receptor that modulates the N-methyl-D-aspartate operated ion channel. Eur J Pharmacol. 1988 Oct 26;156(1):105–110. doi: 10.1016/0014-2999(88)90152-5. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- White W. F., Brown K. L., Frank D. M. Glycine binding to rat cortex and spinal cord: binding characteristics and pharmacology reveal distinct populations of sites. J Neurochem. 1989 Aug;53(2):503–512. doi: 10.1111/j.1471-4159.1989.tb07362.x. [DOI] [PubMed] [Google Scholar]
- Williams T. L., Stone T. W., Burton N. R., Smith D. A. Kynurenic acid and AP5 distinguish between NMDA receptor agonists. Exp Neurol. 1988 Dec;102(3):366–367. doi: 10.1016/0014-4886(88)90232-4. [DOI] [PubMed] [Google Scholar]


