Abstract
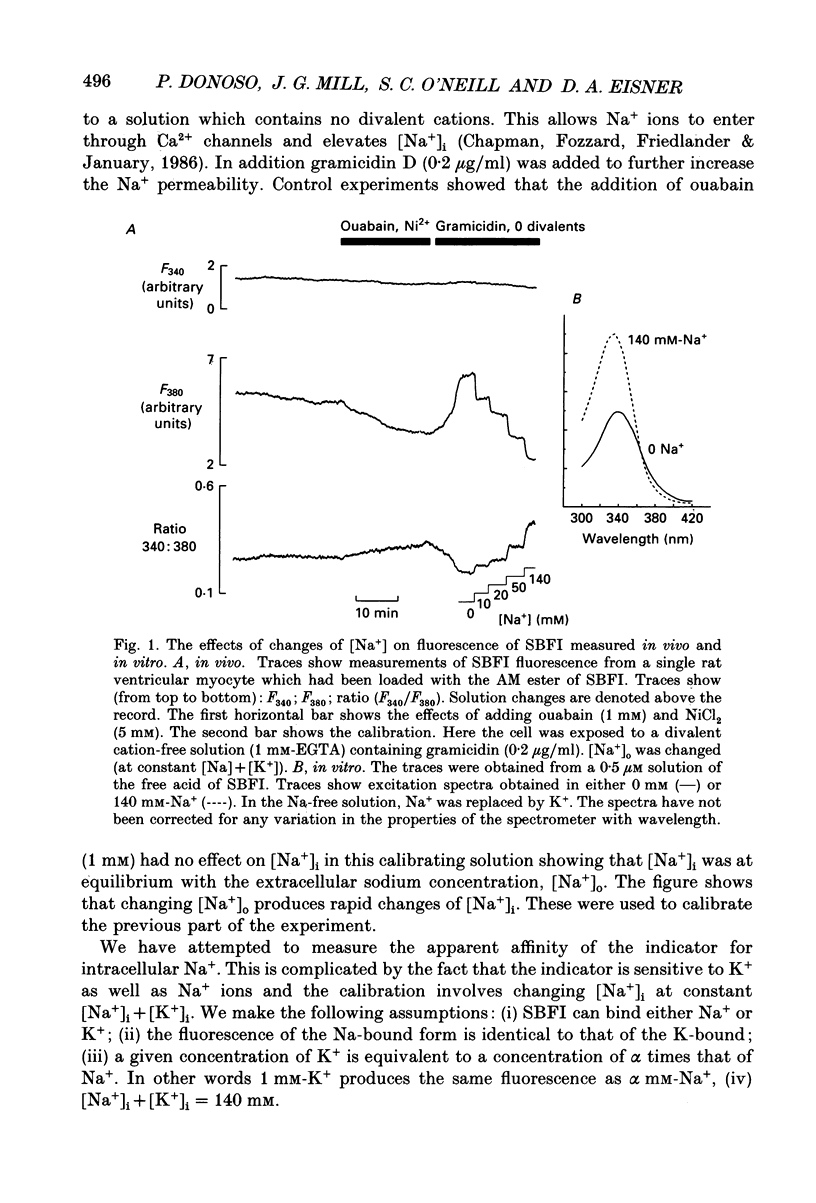
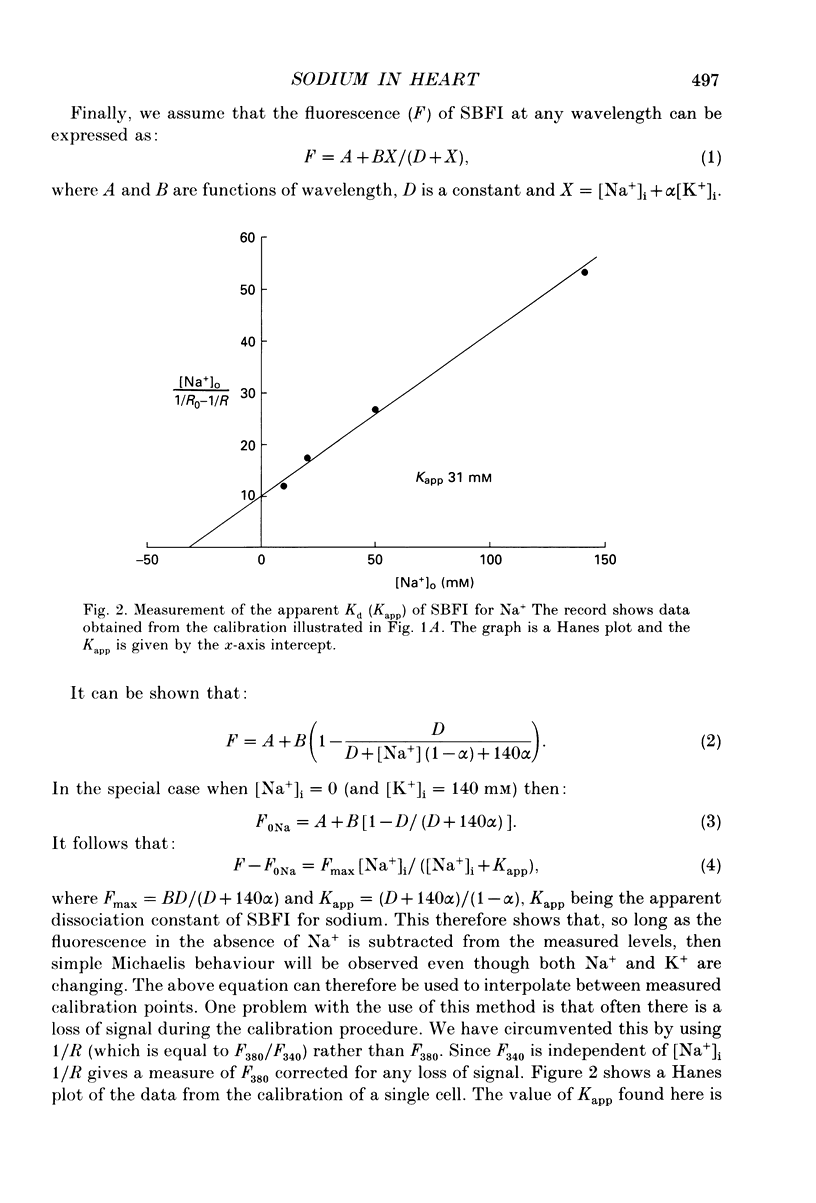
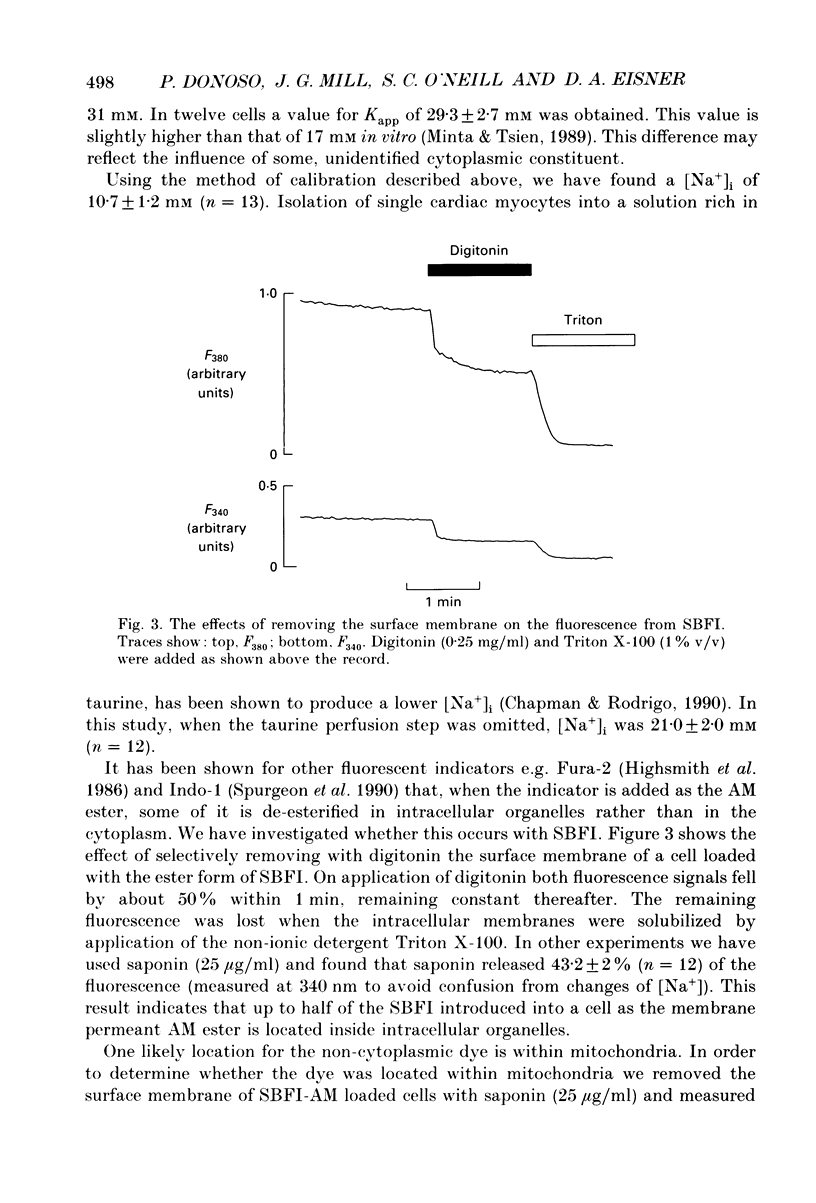
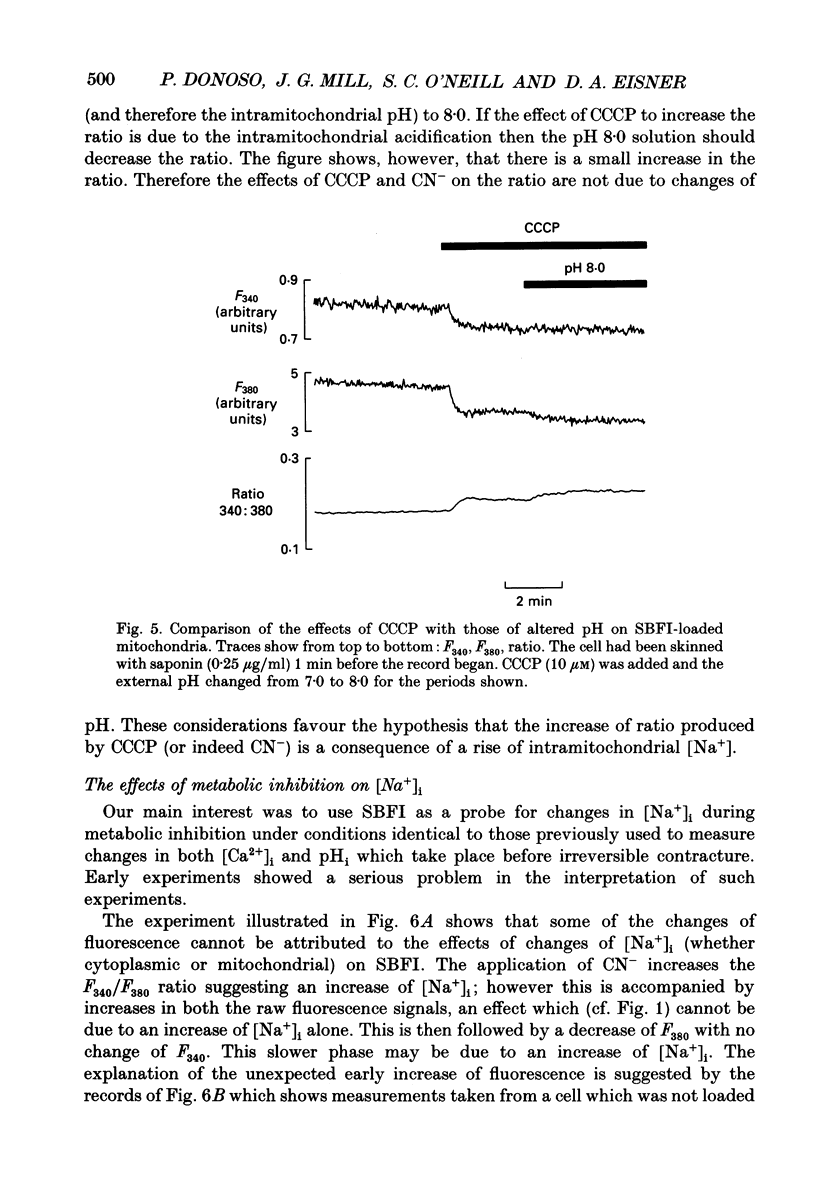
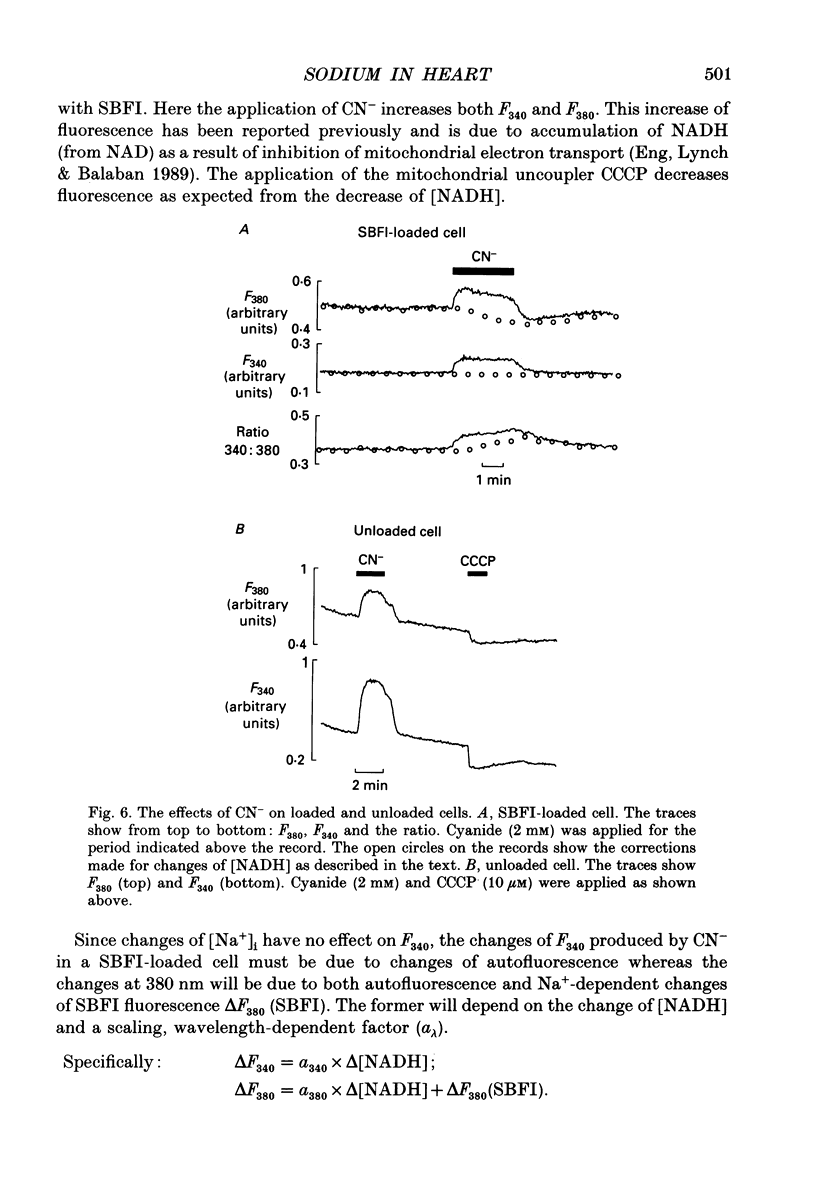
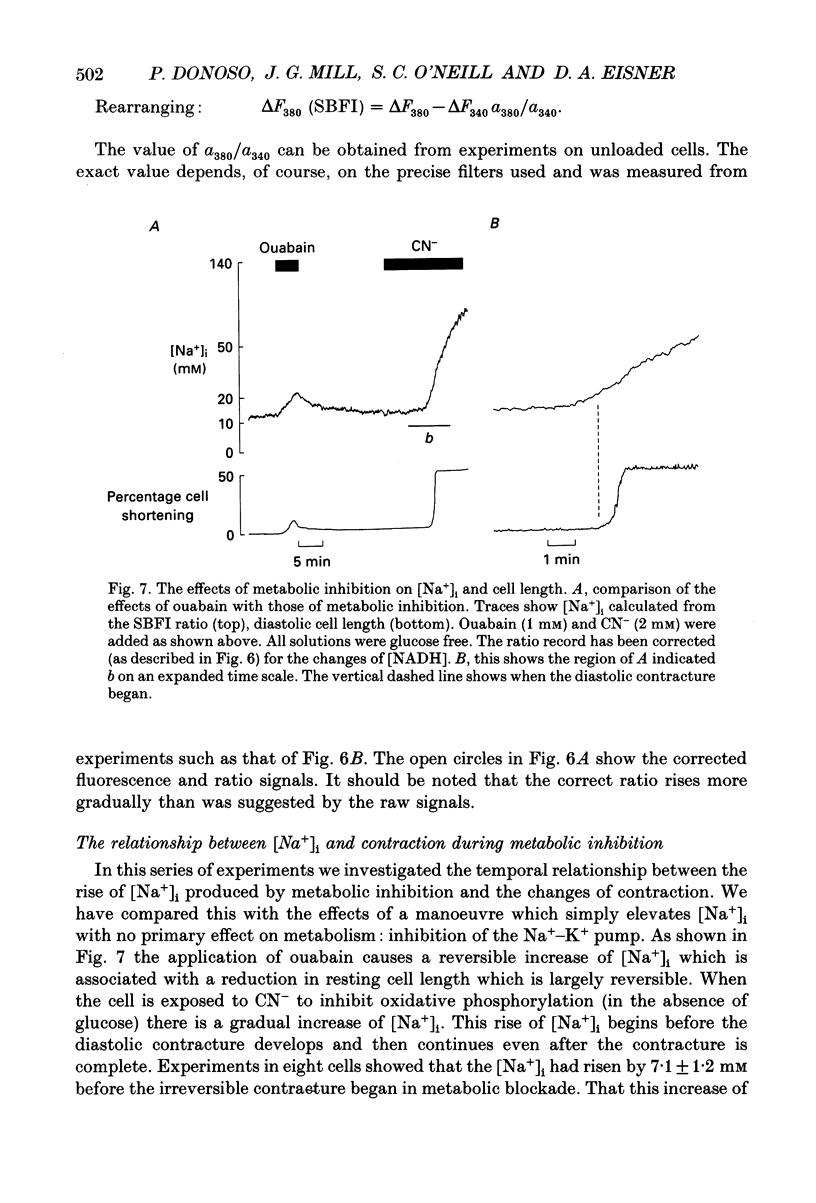
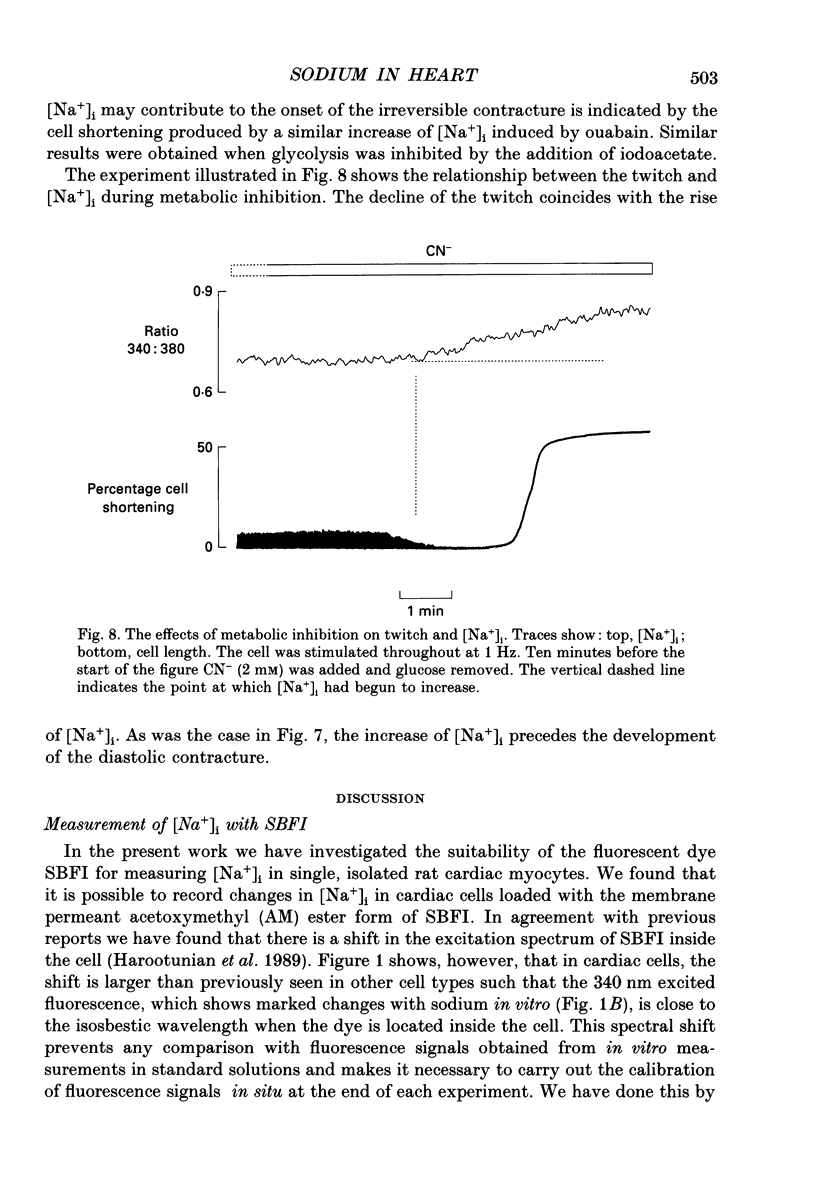
1. The fluorescent Na+ indicator SBFI was incorporated into isolated ventricular myocytes using the acetoxymethyl (AM) ester. 2. The excitation spectrum was found to be shifted about 20 nm in the cell compared to in vitro. In the cell, an increase of [Na+] decreased fluorescence at 380 nm (F380) and had no effect at 340 nm (F340). The ratio (R = F340/F380) was used as a measure of [Na+]i. 3. In vivo calibration of SBFI for [Na+]i was obtained by equilibrating [Na+] across the plasma membrane with a divalent-free solution in the presence of gramicidin D. 4. Selective removal of the surface membrane with saponin or digitonin released only about 50% of the indicator. Following saponin treatment, cyanide or carbonylcyanide m-chlorphenylhydrazone (CCCP) increased the apparent [Na+] measured by the remaining (presumably mitochondrial) SBFI. It is suggested that mitochondrial [Na+] is normally less than cytoplasmic. 5. Attempts to examine the effects of metabolic inhibition on [Na+]i were hampered by changes of autofluorescence due to changes of [NADH]. It is shown that this effect can be corrected for using the isosbestic signal (excited at 340 nm). 6. Inhibition of both aerobic metabolism (with CN-) and glycolysis (glucose removal or iodoacetate) produced a gradual increase of [Na+]i. This began before the resting contracture developed and may (via Na(+)-Ca2+ exchange) account for some of the rise of diastolic [Ca2+]i seen in previous work. The rise of [Na+]i began at about the same time as the decrease of systolic contraction and therefore at a time when [ATP]i had begun to fall.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Allshire A., Piper H. M., Cuthbertson K. S., Cobbold P. H. Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J. 1987 Jun 1;244(2):381–385. doi: 10.1042/bj2440381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Brierley G. P., Davis M. H., Cragoe E. J., Jr, Jung D. W. Kinetic properties of the Na+/H+ antiport of heart mitochondria. Biochemistry. 1989 May 16;28(10):4347–4354. doi: 10.1021/bi00436a034. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Richards D. E. Inhibition of the sodium pump by inorganic phosphate in resealed red cell ghosts. J Physiol. 1982 May;326:1–10. doi: 10.1113/jphysiol.1982.sp014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Eng J., Lynch R. M., Balaban R. S. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989 Apr;55(4):621–630. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. ATP hydrolysis associated with an uncoupled sodium flux through the sodium pump: evidence for allosteric effects of intracellular ATP and extracellular sodium. J Physiol. 1976 Apr;256(2):465–496. doi: 10.1113/jphysiol.1976.sp011333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri T. Intracellular sodium-calcium dissociation in early contractile failure in hypoxic ferret papillary muscles. J Physiol. 1987 Jul;388:449–465. doi: 10.1113/jphysiol.1987.sp016624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Highsmith S., Bloebaum P., Snowdowne K. W. Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1153–1162. doi: 10.1016/s0006-291x(86)80403-x. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Jung D. W., Davis M. H., Brierley G. P. Estimation of matrix pH in isolated heart mitochondria using a fluorescent probe. Anal Biochem. 1989 May 1;178(2):348–354. doi: 10.1016/0003-2697(89)90651-9. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G., Smith G. L. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989 Jun;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K. T. Effects of hypoxia and metabolic inhibition on the intracellular sodium activity of mammalian ventricular muscle. J Physiol. 1989 Sep;416:455–468. doi: 10.1113/jphysiol.1989.sp017771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister L. P., Page E. Effects of thyroxin on ultrastructure of rat myocardial cells: a stereological study. J Ultrastruct Res. 1973 Jan;42(1):136–155. doi: 10.1016/s0022-5320(73)80012-7. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Browne H. M., Dawes N. J. Studies on mitochondrial Ca2+-transport and matrix Ca2+ using fura-2-loaded rat heart mitochondria. Biochim Biophys Acta. 1989 Mar 23;973(3):420–427. doi: 10.1016/s0005-2728(89)80384-6. [DOI] [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reers M., Kelly R. A., Smith T. W. Calcium and proton activities in rat cardiac mitochondria. Effect of matrix environment on behaviour of fluorescent probes. Biochem J. 1989 Jan 1;257(1):131–142. doi: 10.1042/bj2570131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock M. J., Bers D. M. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989 Apr;256(4 Pt 1):C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Allen D. G. Effects of metabolic blockade on intracellular calcium concentration in isolated ferret ventricular muscle. Circ Res. 1988 Jun;62(6):1223–1236. doi: 10.1161/01.res.62.6.1223. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Silverman H. S., Houser S. R., Josephson R. A., Capogrossi M. C., Nichols C. G., Lederer W. J., Lakatta E. G. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. X-ray microanalysis of single cardiac myocytes frozen under voltage-clamp conditions. Am J Physiol. 1989 Feb;256(2 Pt 2):H574–H583. doi: 10.1152/ajpheart.1989.256.2.H574. [DOI] [PubMed] [Google Scholar]


