Abstract
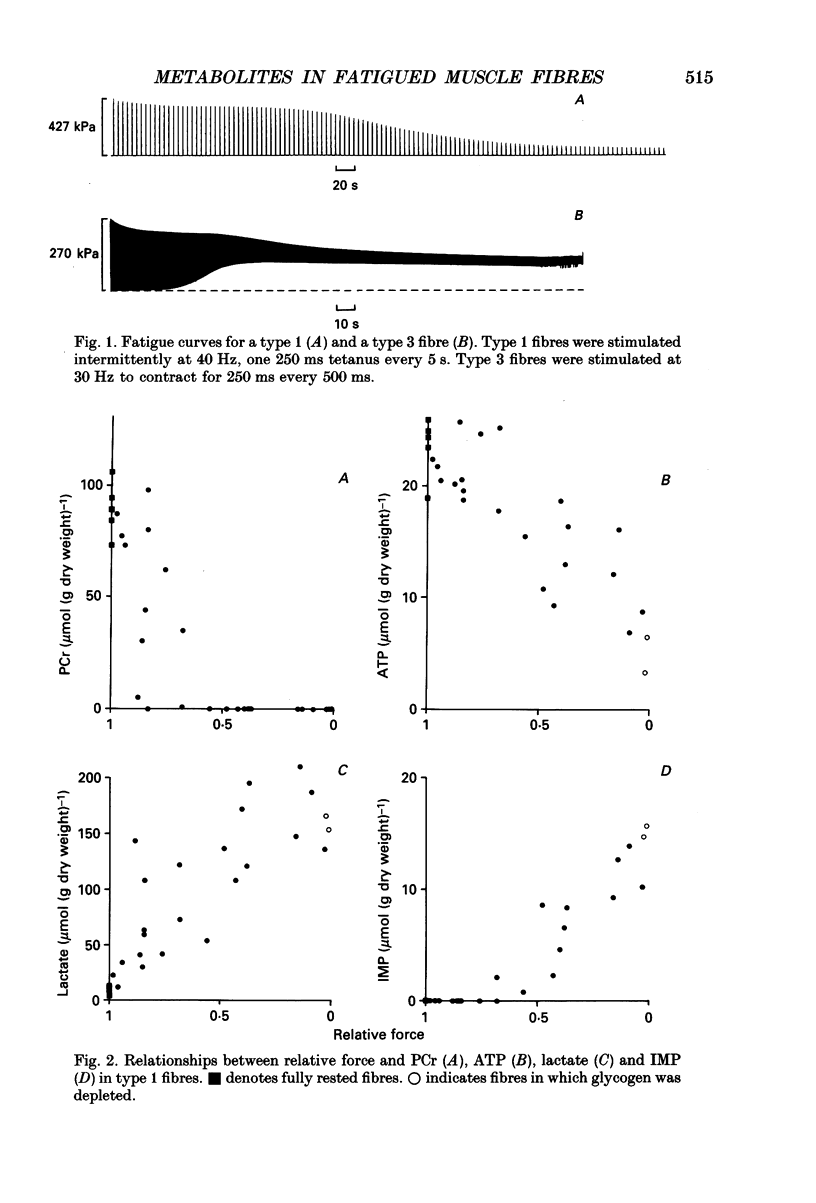
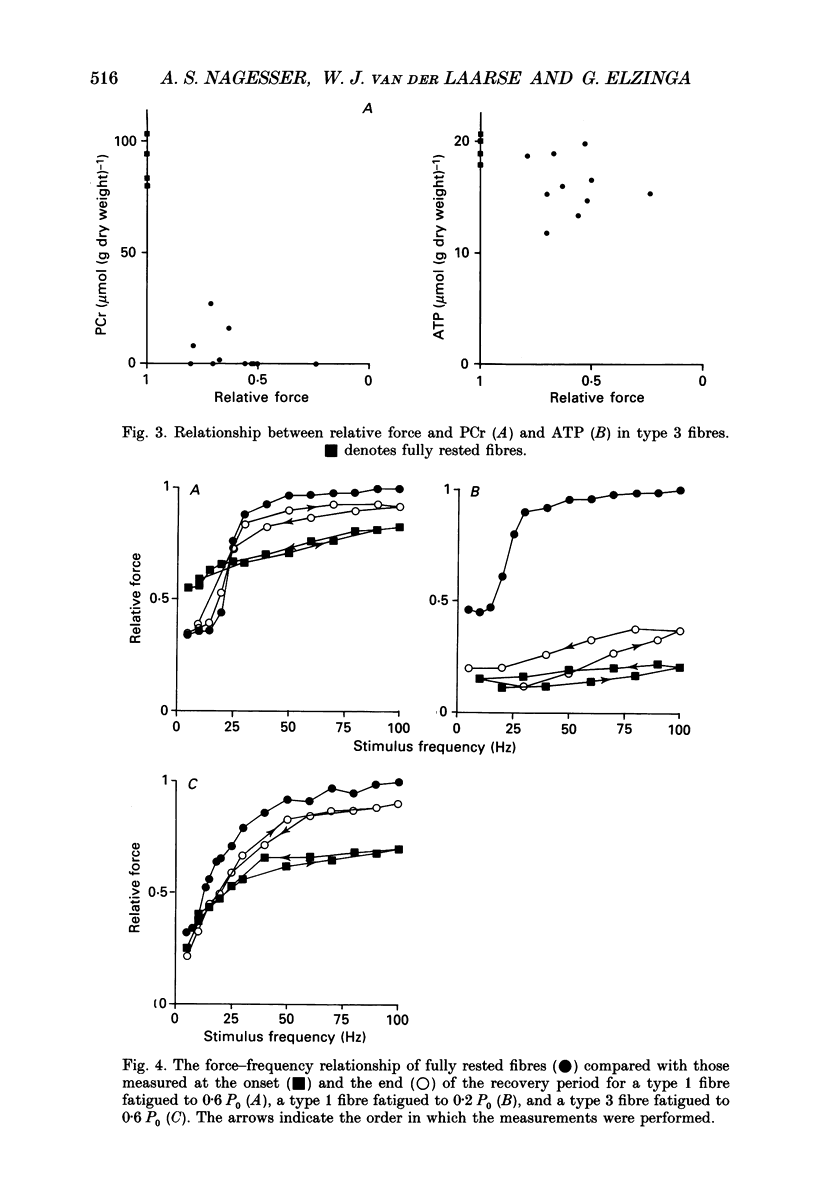
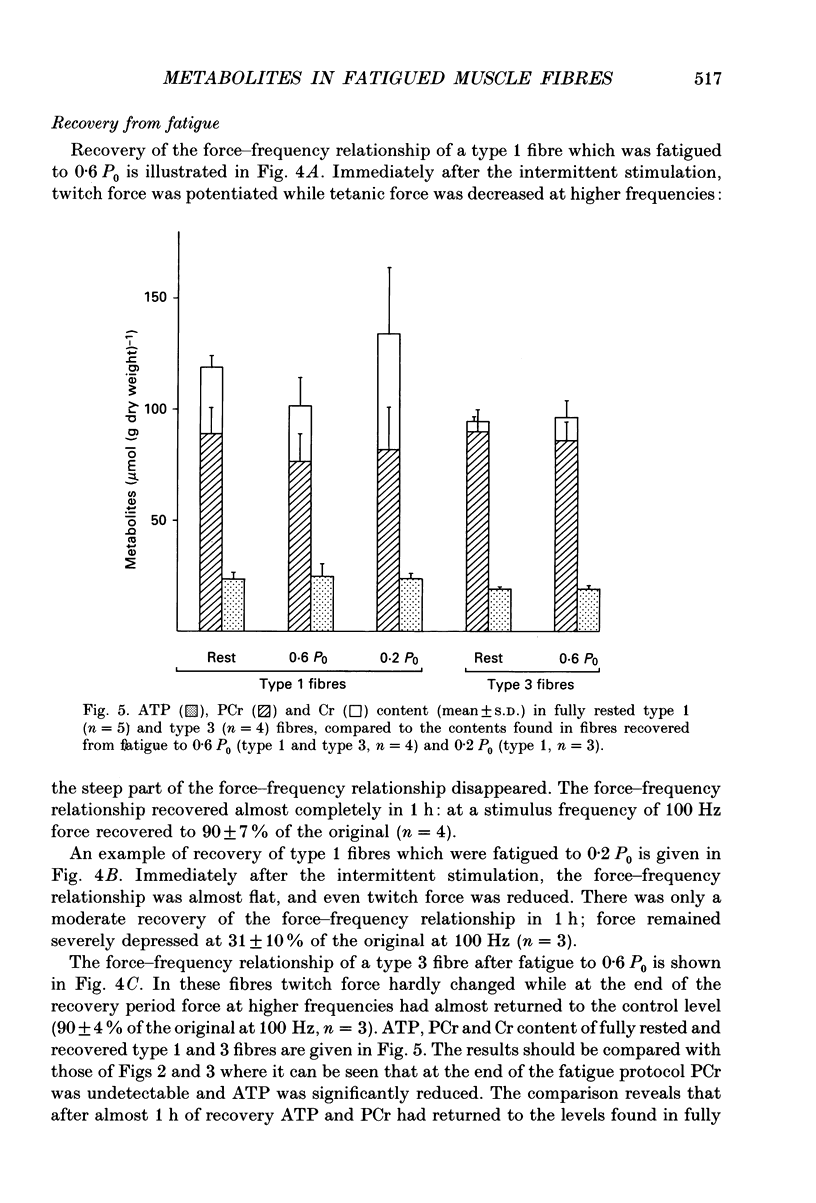
1. Peak isometric force of single fast (type 1) and slow (type 3) muscle fibres of Xenopus decreased when fibres were stimulated intermittently above their predicted sustainable duty cycle at 20 degrees C. Type 1 fibres could be fatigued to zero force. In most type 3 fibres force did not decrease below 50% of the original (P0) before activation failure, as indicated by irregular contractions. 2. Fibres were rapidly frozen at different force levels and analysed by high-performance liquid chromatography (HPLC) for ATP, IMP, phosphocreatine (PCr) and creatine (Cr). Lactate was determined enzymatically in type 1 fibres only. The relationships between force and PCr, and between force and ATP during fatigue were, apart from the range of values obtained, the same for both fibre types. When force had fallen to about 60-80% of original, PCr was fully reduced. At lower force levels, the ATP content-decreased, and a concomitant rise of IMP content was found. At zero force, ATP had fallen to about 25% of its value in rested type 1 fibres, and up to 200 mumol lactate (g dry weight)-1 had accumulated. 3. Recovery from fatigue was studied in fibres where force had fallen to 0.6 P0 (both fibre types) and 0.2 P0 (type 1 only). After 1 h of recovery ATP had in all cases returned to the level measured in rested fibres. In fibres fatigued to 0.6 P0, force almost returned to its original value. However, in type 1 fibres fatigued to 0.2 P0, it returned to only 0.3 P0. After 1 h of recovery the PCr/Cr ratio in type 1 fibres was lower (probability, P less than 0.05) than in control fibres, whereas in type 3 fibres it was not significantly different from controls. 4. The relationship between peak force and stimulus frequency, which had a sigmoid shape in fully rested fibres, was drastically changed by fatiguing stimulation. Immediately after fatiguing stimulation of type 1 fibres, force hardly increased with stimulus frequency, corresponding to the observation that calcium efflux from the sarcoplasmic reticulum was decreased at high stimulus frequencies. The force-frequency relationship of type 3 fibres was the same before and after intermittent stimulation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy changes and muscular contraction. Physiol Rev. 1978 Jul;58(3):690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Lännergren J., Stienen G. J. Stable maintenance heat rate and contractile properties of different single muscle fibres from Xenopus laevis at 20 degrees C. J Physiol. 1987 Dec;393:399–412. doi: 10.1113/jphysiol.1987.sp016829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Somlyo A. V., McClellan G., Shuman H., Borrero L. M., Somlyo A. P. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: electron probe analysis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Juengling E., Kammermeier H. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Anal Biochem. 1980 Mar 1;102(2):358–361. doi: 10.1016/0003-2697(80)90167-0. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H., Flock B. Transient appearance of vacuoles in fatigued Xenopus muscle fibres. Acta Physiol Scand. 1990 Nov;140(3):437–445. doi: 10.1111/j.1748-1716.1990.tb09019.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Maximum tension and force-velocity properties of fatigued, single Xenopus muscle fibres studied by caffeine and high K+. J Physiol. 1989 Feb;409:473–490. doi: 10.1113/jphysiol.1989.sp017508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Rapoport S. I. Fatigue and metabolism of frog muscle fibers during stimulation and in response to caffeine. Am J Physiol. 1981 Sep;241(3):C160–C166. doi: 10.1152/ajpcell.1981.241.3.C160. [DOI] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Vergara J. L., Rapoport S. I. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978 Nov;72(5):593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. S., Ovalle W. K., Jr Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat. 1973 Oct;116(Pt 1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Spurway N. C., Rowlerson A. M. Quantitative analysis of histochemical and immunohistochemical reactions in skeletal muscle fibres of Rana and Xenopus. Histochem J. 1989 Aug;21(8):461–476. doi: 10.1007/BF01845796. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Styles P., Matthews P. M., Arnold D. A., Gadian D. G., Bore P., Radda G. K. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986 Feb;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]
- Wilkie D. R. Muscular fatigue: effects of hydrogen ions and inorganic phosphate. Fed Proc. 1986 Dec;45(13):2921–2923. [PubMed] [Google Scholar]
- van der Laarse W. J., Diegenbach P. C., Elzinga G. Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil. 1989 Jun;10(3):221–228. doi: 10.1007/BF01739812. [DOI] [PubMed] [Google Scholar]
- van der Laarse W. J., Diegenbach P. C., Elzinga G. The duty cycle of single muscle fibers from Xenopus laevis. Prog Clin Biol Res. 1989;315:87–97. [PubMed] [Google Scholar]
- van der Laarse W. J., Lännergren J., Diegenbach P. C. Resistance to fatigue of single muscle fibres from Xenopus related to succinate dehydrogenase and myofibrillar ATPase activities. Exp Physiol. 1991 Jul;76(4):589–596. doi: 10.1113/expphysiol.1991.sp003526. [DOI] [PubMed] [Google Scholar]


