Abstract
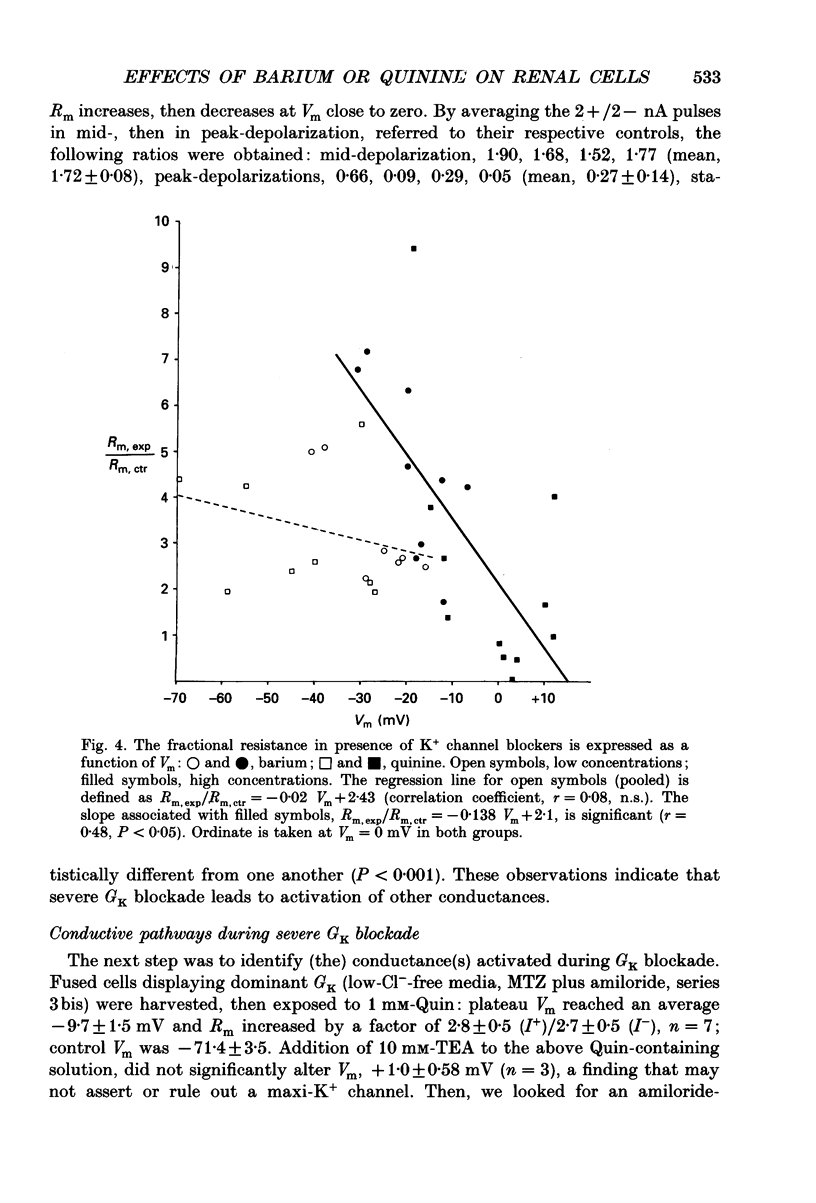
1. Frog proximal tubular cells were fused into giant cells. We measured membrane potential (Vm), its changes (delta Vm), and current-induced voltage changes (delta psi) in single cells, during control and experimental states. Each cell served as its own control. 2. In the presence of a physiological Ringer solution, the transference number for potassium (tK) was 0.50. Barium (3 mM) reduced membrane conductance (Gm) by 50%; low-Cl- solutions and low-Na+ solutions also diminished Gm, by 52 and 30%, respectively. The association of barium and low-NaCl solutions decreased Gm to approximately 38% of control, indicating that the impermeant substitute of a physiological ion may interact with other pathways; alternatively, blockade of steady-state conductances may activate physiologically silent processes. 3. In an attempt to enhance the contribution of the partial K+ conductance (GK) to Gm, fused cells were exposed to low-Cl- solutions, containing in addition 0.1 mM-methazolamide, to inhibit the rheogenic Na(+)-HCO3-symport, and 1 microM-amiloride, to block Na+ conductance (GNa). tK went up to 0.83. 4. The high tK preparation was challenged with barium (3 mM) or quinine (Quin, 1 mM). These blockers produced large depolarizations (approximately 60 mV), however, although Gm decreased along early- and mid-depolarization, Gm plateaued and eventually it increased with larger and larger depolarization. 5. Depolarization-associated increase in Gm reflects activation of other conductances. These are Na+, cationic, and K+ conductance(s) poorly sensitive to quinine or barium. In the presence of Ba(2+)- or Quin-induced depolarization, injection of depolarizing current produces delayed increase in conductance. 6. Depolarization-induced activation of cationic conductance (Gcat) and GNa results in enlargement of the K+ electrochemical potential difference, to about 70 mV; this difference allows recycling of K+ ions outwards, since a GK is still detected and may contribute up to 38% of the total conductance.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos T. Biionic potentials in the proximal tubule of Necturus kidney. J Physiol. 1973 Sep;233(2):375–394. doi: 10.1113/jphysiol.1973.sp010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos T., Planelles G. Organic anion permeation at the proximal tubule of necturus: an electrophysiological study of the peritubular membrane. Pflugers Arch. 1979 Sep;381(3):231–239. doi: 10.1007/BF00583254. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos T., Teulon J., Edelman A. Conductive properties of the proximal tubule in Necturus kidney. J Gen Physiol. 1980 May;75(5):553–587. doi: 10.1085/jgp.75.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouachour G., Planelles G., Anagnostopoulos T. Fusion of amphibian proximal convoluted cells into giant cells. Pflugers Arch. 1988 Feb;411(2):220–222. doi: 10.1007/BF00582319. [DOI] [PubMed] [Google Scholar]
- Cox T. C., Helman S. I. Na+ and K+ transport at basolateral membranes of epithelial cells. II. K+ efflux and stoichiometry of the Na,K-ATPase. J Gen Physiol. 1986 Mar;87(3):485–502. doi: 10.1085/jgp.87.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietl P., Wang W., Oberleithner H. Fused cells of frog proximal tubule: I. Basic membrane properties. J Membr Biol. 1987;100(1):43–51. doi: 10.1007/BF02209139. [DOI] [PubMed] [Google Scholar]
- Edelman A., Anagnostopoulos T. Further studies on ion permeation in proximal tubule of necturus kidney. Am J Physiol. 1978 Aug;235(2):F89–F95. doi: 10.1152/ajprenal.1978.235.2.F89. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Boulpaep E. L., Giebisch G. Electrical properties of chloride transport across the necturus proximal tubule. J Membr Biol. 1982;65(3):185–196. doi: 10.1007/BF01869962. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. L., Eaton D. C. Single-channel recordings from amiloride-sensitive epithelial sodium channel. Am J Physiol. 1985 Sep;249(3 Pt 1):C200–C207. doi: 10.1152/ajpcell.1985.249.3.C200. [DOI] [PubMed] [Google Scholar]
- Kawahara K. Ba2+-sensitive potassium permeability of the apical membrane in newt kidney proximal tubule. J Membr Biol. 1985;88(3):283–292. doi: 10.1007/BF01871092. [DOI] [PubMed] [Google Scholar]
- Kone B. C., Brady H. R., Gullans S. R. Coordinated regulation of intracellular K+ in the proximal tubule: Ba2+ blockade down-regulates the Na+,K+-ATPase and up-regulates two K+ permeability pathways. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6431–6435. doi: 10.1073/pnas.86.16.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M., Rector F. C., Jr, Berry C. A. SITS-sensitive basolateral anion current in rabbit proximal convoluted tubules. Am J Physiol. 1988 Jun;254(6 Pt 2):F828–F836. doi: 10.1152/ajprenal.1988.254.6.F828. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Laprade R., Cardinal J. Characterization of the apical membrane ionic permeability of the rabbit proximal convoluted tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F339–F347. doi: 10.1152/ajprenal.1986.250.2.F339. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Schmidt B., Dietl P. Fusion of renal epithelial cells: a model for studying cellular mechanisms of ion transport. Proc Natl Acad Sci U S A. 1986 May;83(10):3547–3551. doi: 10.1073/pnas.83.10.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Corthesy-Theulaz I., Gaeggeler H. P., Kraehenbuhl J. P., Rossier B. Expression of epithelial Na channels in Xenopus oocytes. J Gen Physiol. 1990 Jul;96(1):23–46. doi: 10.1085/jgp.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles G., Teulon J., Anagnostopoulos T. The effects of barium on the electrical properties of the basolateral membrane in proximal tubule. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):135–141. doi: 10.1007/BF00508838. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Effects of luminal hyperosmolality on electrical pathways of Necturas gallbladder. Am J Physiol. 1977 Mar;232(3):C99–108. doi: 10.1152/ajpcell.1977.232.3.C99. [DOI] [PubMed] [Google Scholar]
- Schwegler J. S., Steigner W., Heuner A., Silbernagl S. pHi-dependent membrane conductance of proximal tubule cells in culture (OK): differential effects on K(+)- and Na(+)-conductive channels. J Membr Biol. 1990 Sep;117(3):243–251. doi: 10.1007/BF01868454. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Hypotonic shock evokes opening of Ca2+-activated K channels in opossum kidney cells. Pflugers Arch. 1988 Oct;412(5):551–553. doi: 10.1007/BF00582547. [DOI] [PubMed] [Google Scholar]


