Abstract
1. Changes in ventilation and cardiovascular variables which occur during exercise may be partly due to 'radiation' of activity in central neurones innervating exercising muscles to the respiratory and cardiovascular control areas. To test this hypothesis, we compared ventilatory and cardiovascular responses to two levels of steady-state exercise with each leg separately, in subjects with painless unilateral leg weakness. We assumed that exercise with a weak leg would require more central neural drive than the same level of exercise with the normal leg. 2. Ventilation during exercise with the weak leg was greater than with the normal leg (P less than 0.02). This was a result of greater tidal volume (Vt; P less than 0.005). There was a greater increase in heart rate (P less than 0.005), and systolic (P = 0.001) and diastolic (P less than 0.02) blood pressures during exercise with the weak leg compared to exercise with the normal leg. The increases in stroke volume and cardiac output during exercise were not different with the two legs. 3. These results support the hypothesis that ventilation, blood pressure and heart rate are influenced by the central neural drive to exercise.
Full text
PDF

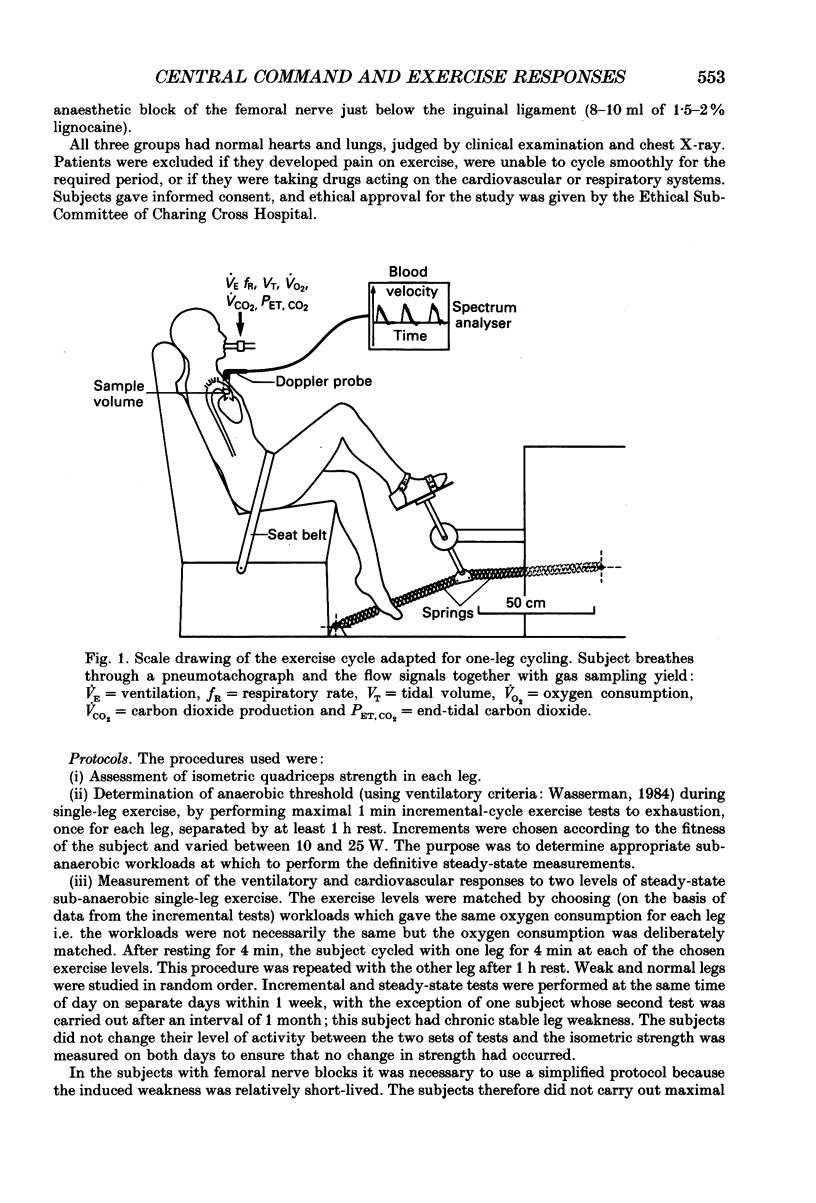

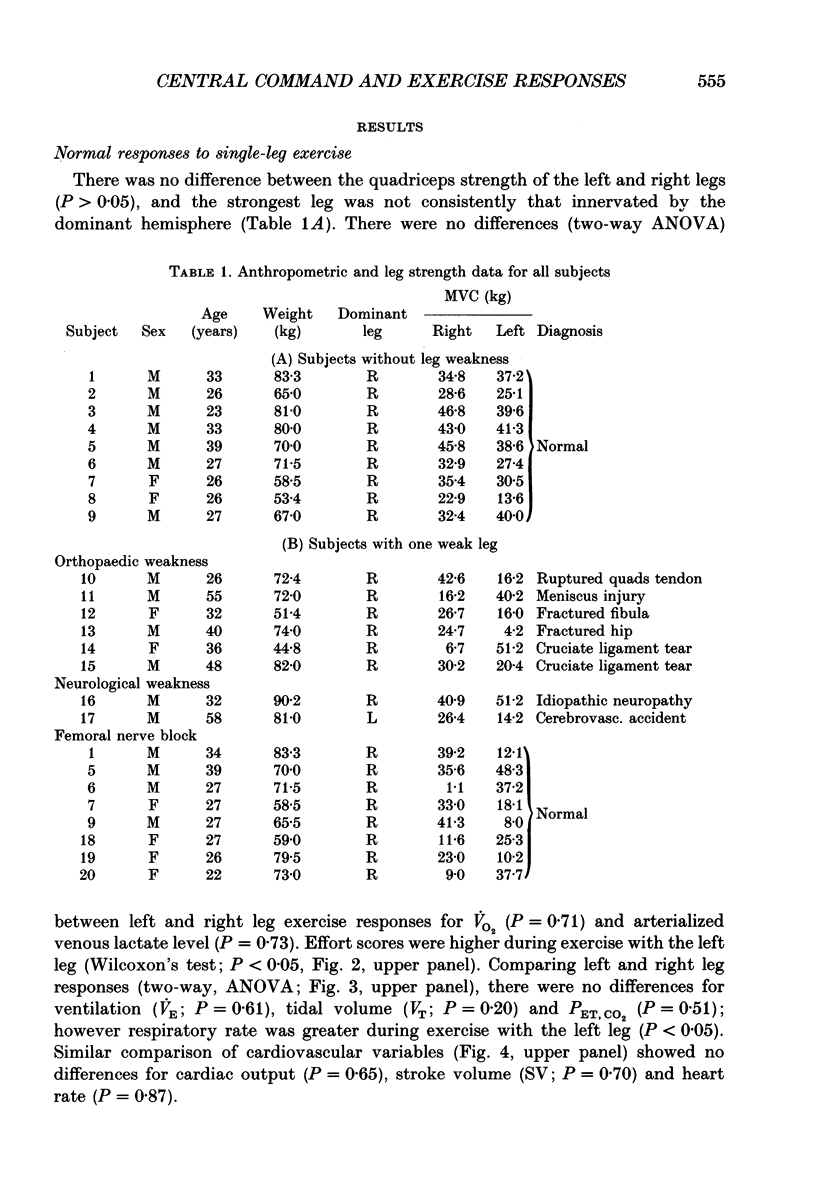
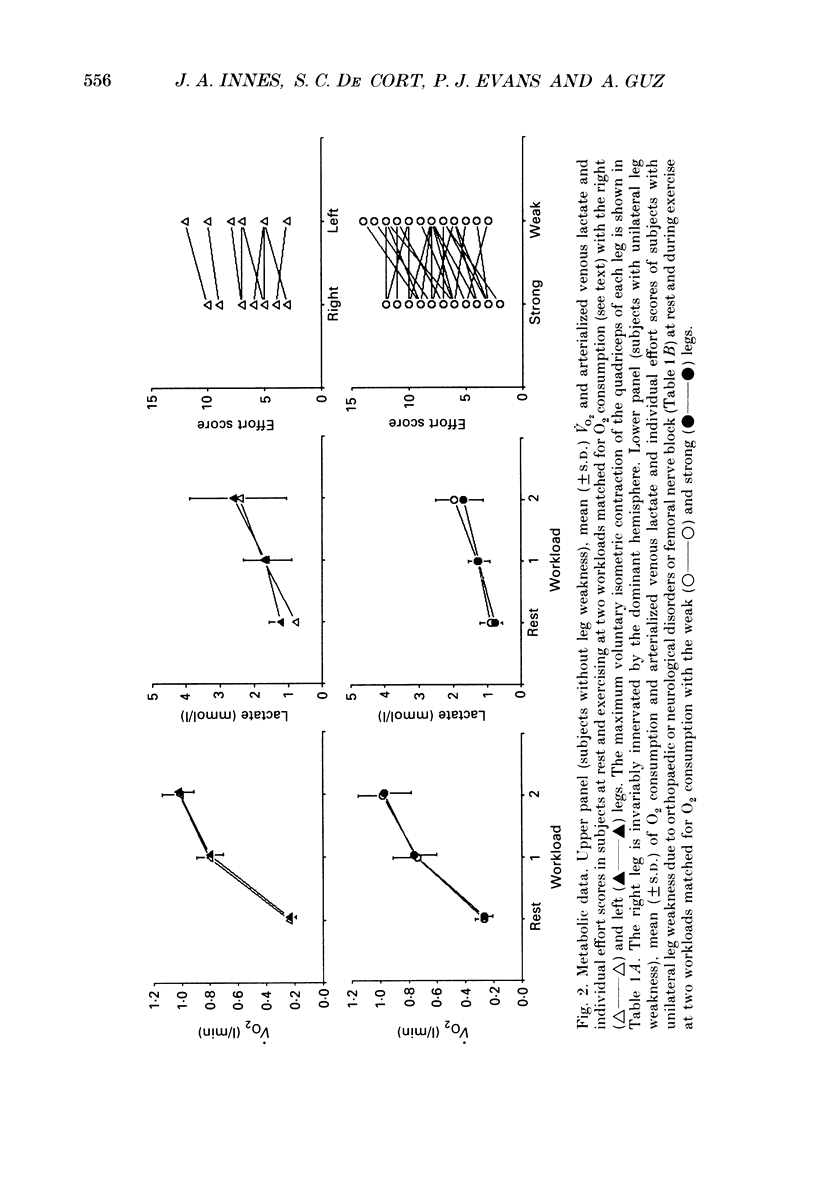
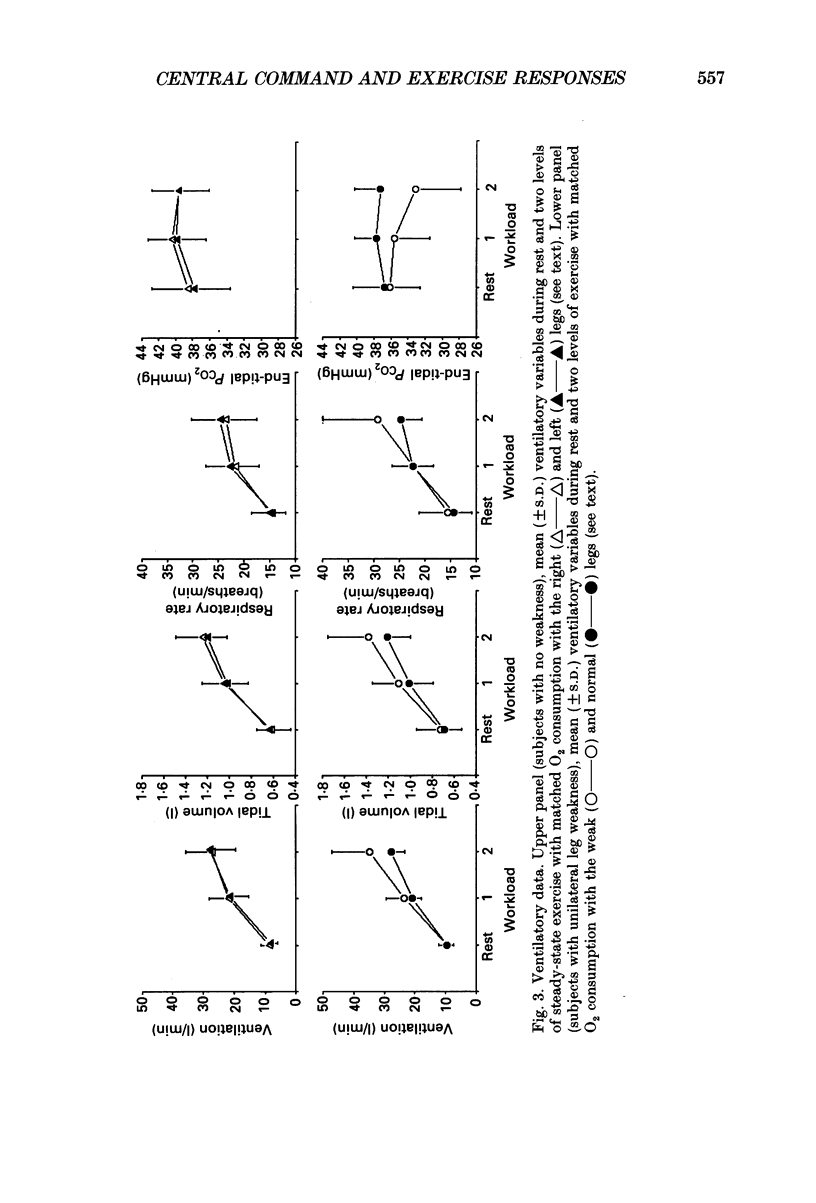





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASMUSSEN E., JOHANSEN S. H., JORGENSEN M., NIELSEN M. ON THE NERVOUS FACTORS CONTROLLING RESPIRATION AND CIRCULATION DURING EXERCISE. EXPERIMENTS WITH CURARIZATION. Acta Physiol Scand. 1965 Mar;63:343–350. doi: 10.1111/j.1748-1716.1965.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cort S. C., Innes J. A., Barstow T. J., Guz A. Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in humans. J Physiol. 1991 Sep;441:501–512. doi: 10.1113/jphysiol.1991.sp018764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Young A., Hosking G. P., Jones D. A. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med. 1977 Mar;52(3):283–290. doi: 10.1042/cs0520283. [DOI] [PubMed] [Google Scholar]
- Fernandes A., Galbo H., Kjaer M., Mitchell J. H., Secher N. H., Thomas S. N. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J Physiol. 1990 Jan;420:281–293. doi: 10.1113/jphysiol.1990.sp017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster H. V., Dempsey J. A., Thomson J., Vidruk E., DoPico G. A. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972 Jan;32(1):134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- Galbo H., Kjaer M., Secher N. H. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987 Aug;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I. Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anaesthesia in man. J Physiol. 1977 Nov;272(3):673–689. doi: 10.1113/jphysiol.1977.sp012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C. The perception of motor commands or effort during muscular paralysis. Brain. 1982 Mar;105(Pt 1):151–159. doi: 10.1093/brain/105.1.151. [DOI] [PubMed] [Google Scholar]
- Hobbs S. F., Gandevia S. C. Cardiovascular responses and the sense of effort during attempts to contract paralysed muscles: role of the spinal cord. Neurosci Lett. 1985 Jun 4;57(1):85–90. doi: 10.1016/0304-3940(85)90044-8. [DOI] [PubMed] [Google Scholar]
- Innes J. A., Mills C. J., Noble M. I., Murphy K., Pugh S., Shore A. C., Guz A. Validation of beat by beat pulsed Doppler measurements of ascending aortic blood velocity in man. Cardiovasc Res. 1987 Jan;21(1):72–80. doi: 10.1093/cvr/21.1.72. [DOI] [PubMed] [Google Scholar]
- Klausen K., Secher N. H., Clausen J. P., Hartling O., Trap-Jensen J. Central and regional circulatory adaptations to one-leg training. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):976–983. doi: 10.1152/jappl.1982.52.4.976. [DOI] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913 Oct 17;47(1-2):112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen A., Mitchell J. H., Reeves D. R., Jr, Rogers H. B., Secher N. H. Cardiovascular responses to brief static contractions in man with topical nervous blockade. J Physiol. 1989 Feb;409:333–341. doi: 10.1113/jphysiol.1989.sp017500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. H., Reeves D. R., Jr, Rogers H. B., Secher N. H. Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989 Oct;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrofsky J. S., Lind A. R. The blood pressure response during isometric exercise in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol Occup Physiol. 1980;44(3):223–230. doi: 10.1007/BF00421621. [DOI] [PubMed] [Google Scholar]
- Sargeant A. J., Davies C. T., Edwards R. H., Maunder C., Young A. Functional and structural changes after disuse of human muscle. Clin Sci Mol Med. 1977 Apr;52(4):337–342. doi: 10.1042/cs0520337. [DOI] [PubMed] [Google Scholar]


