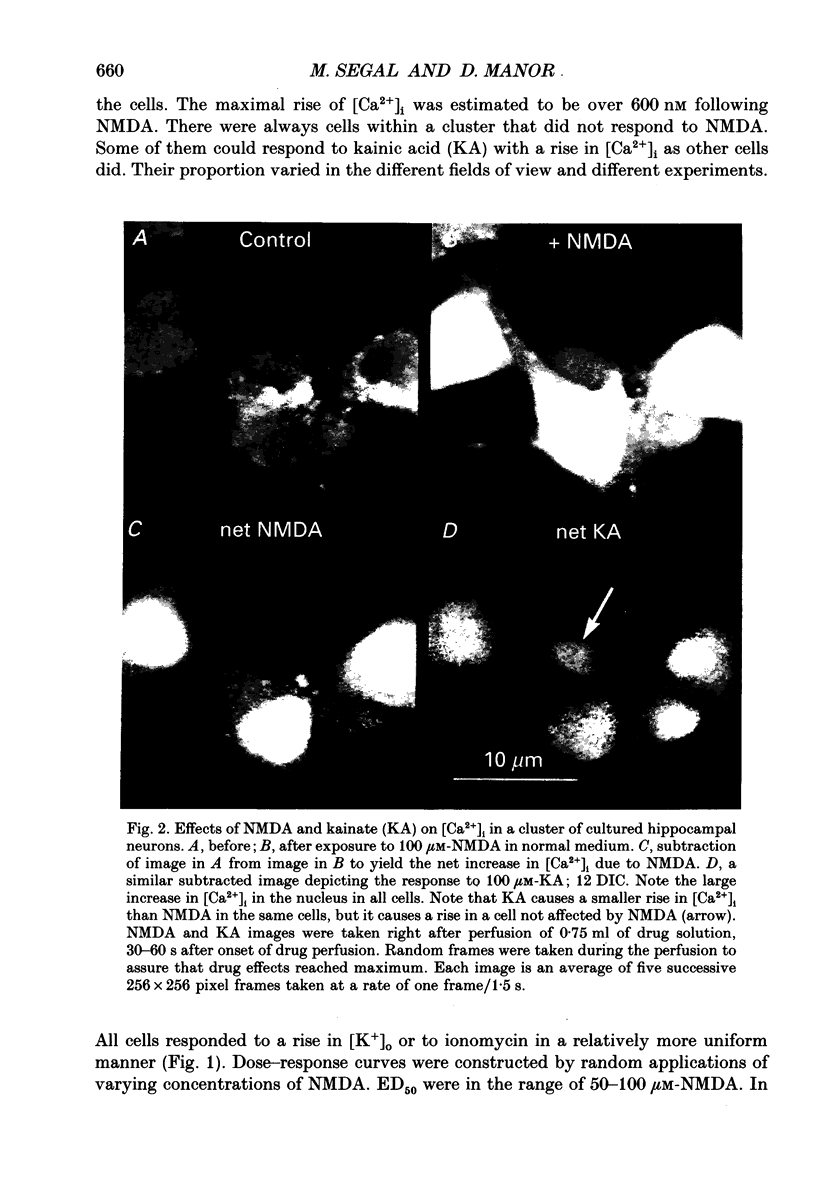
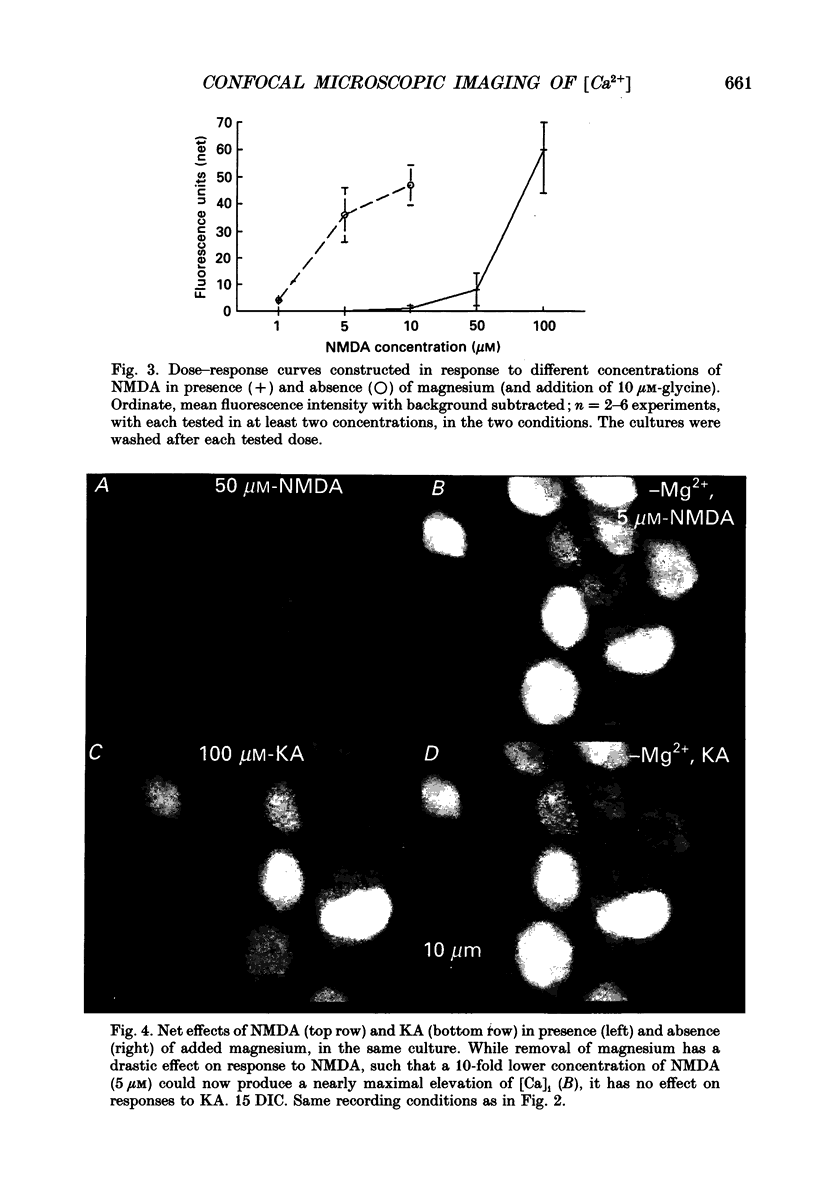
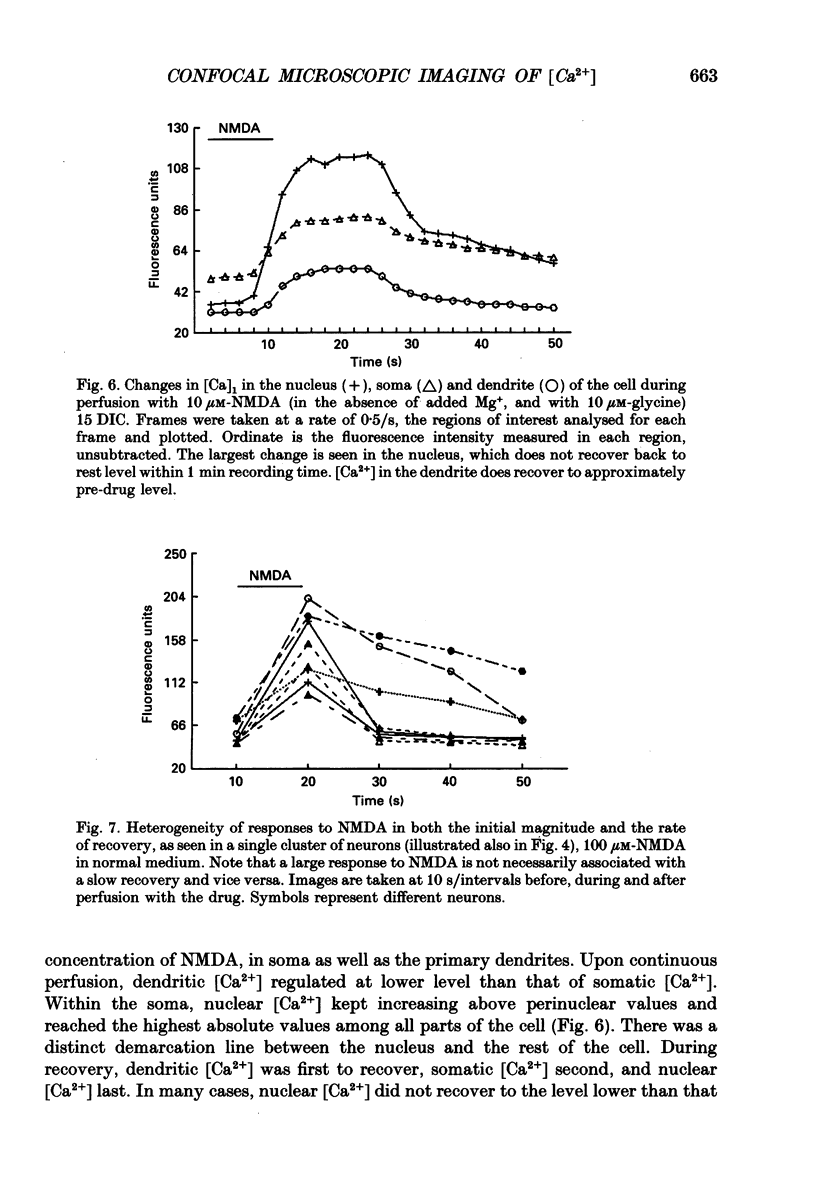
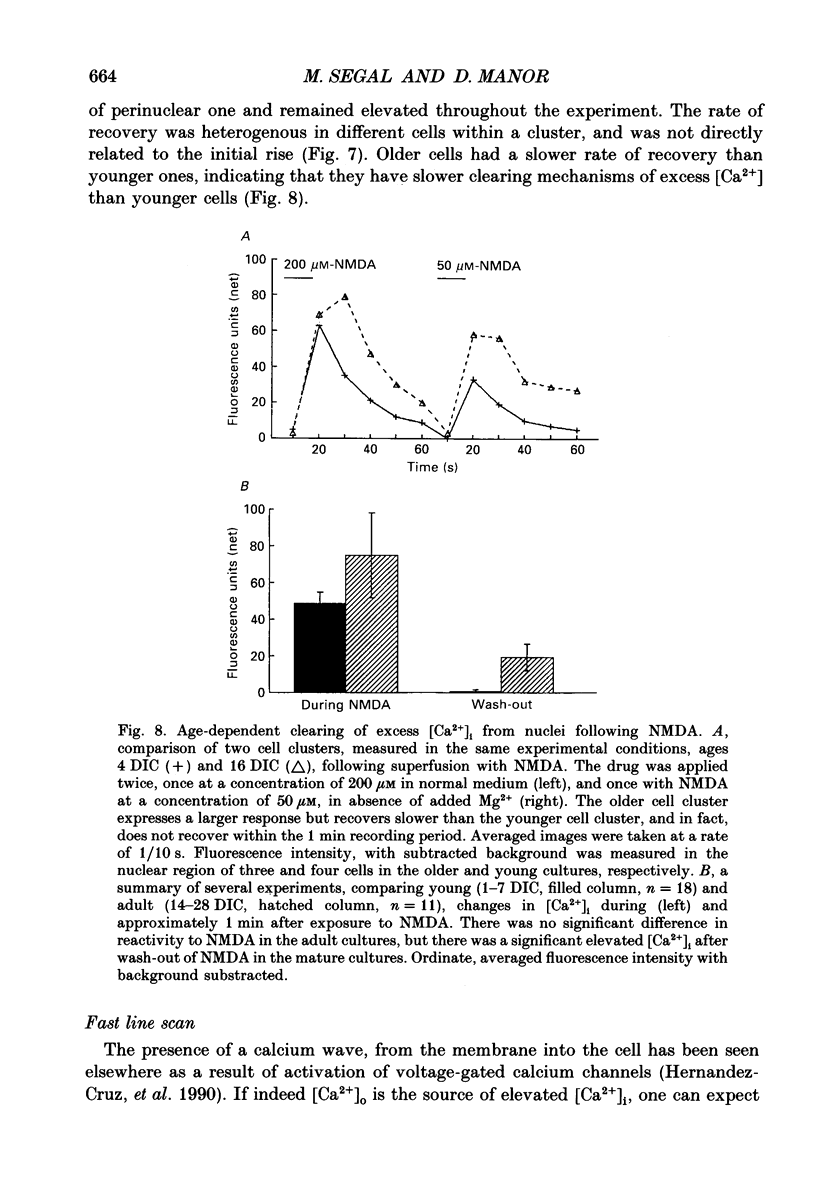
Abstract
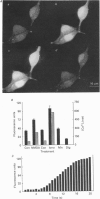
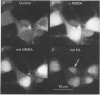
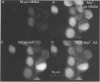
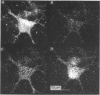
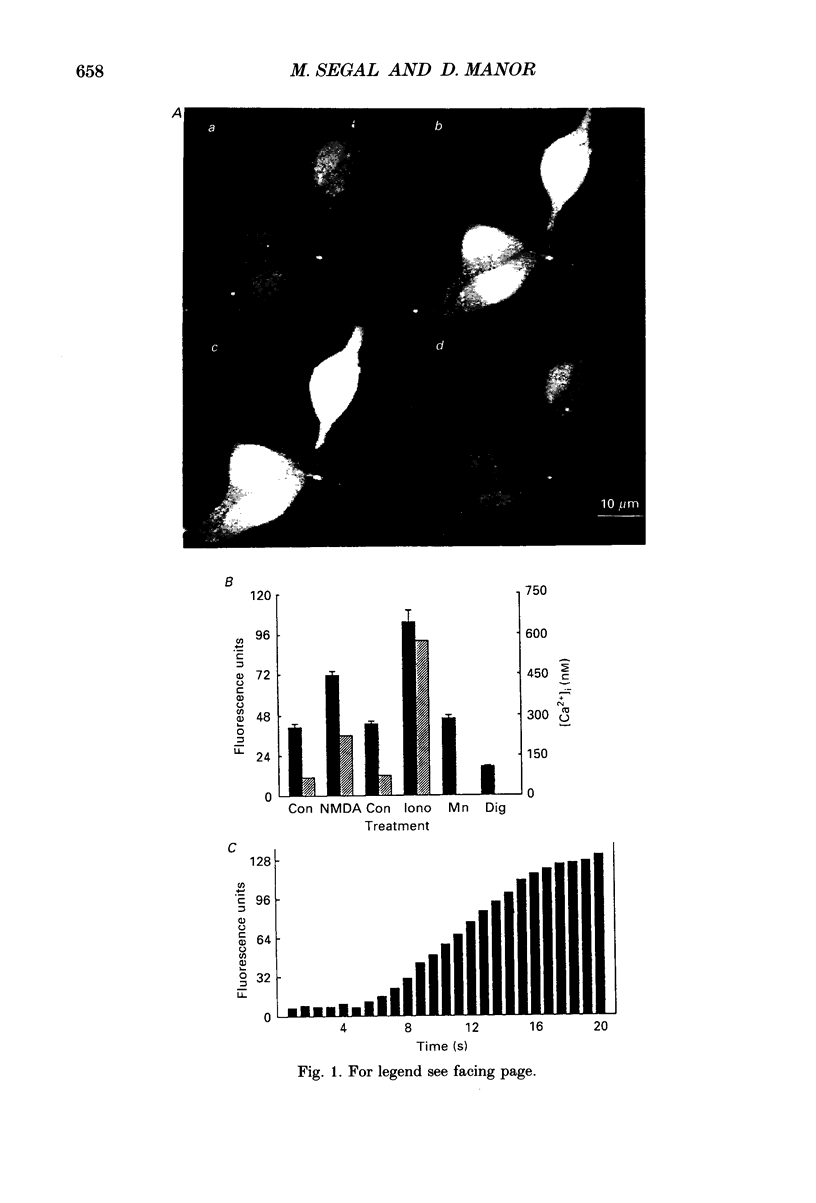
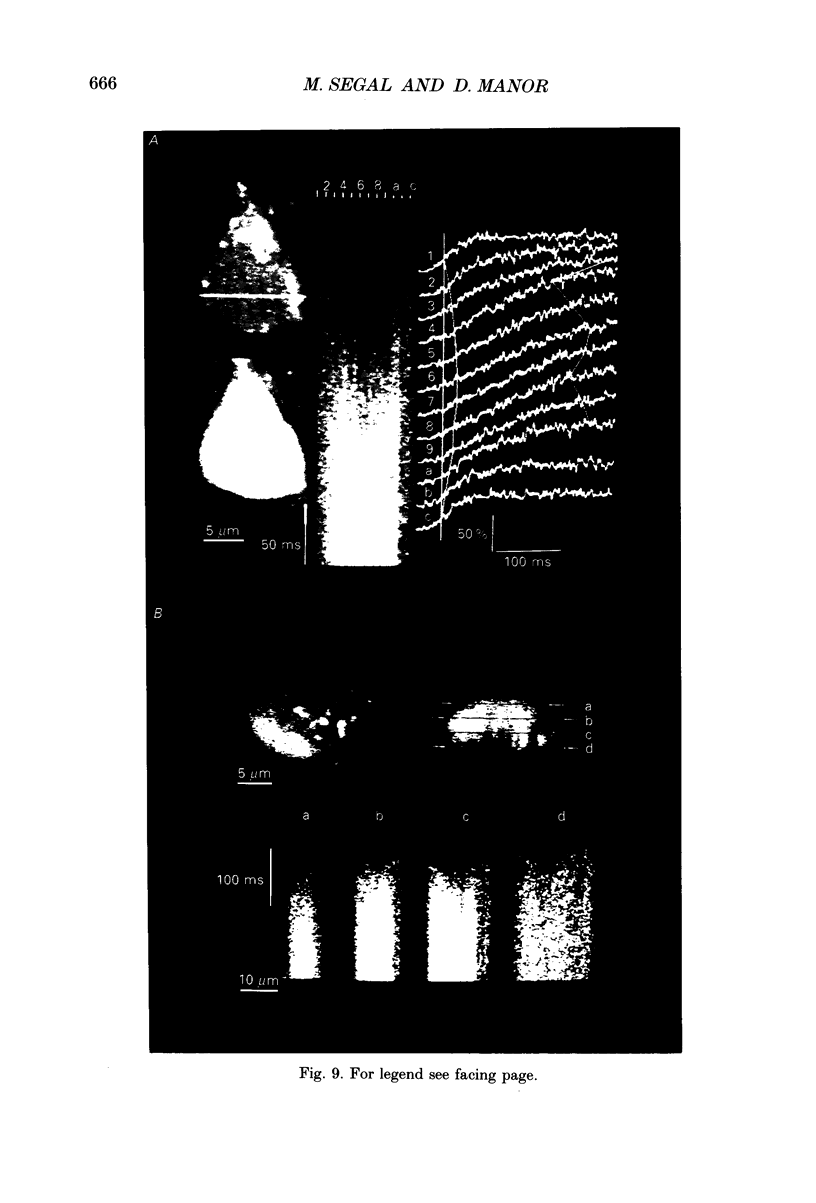
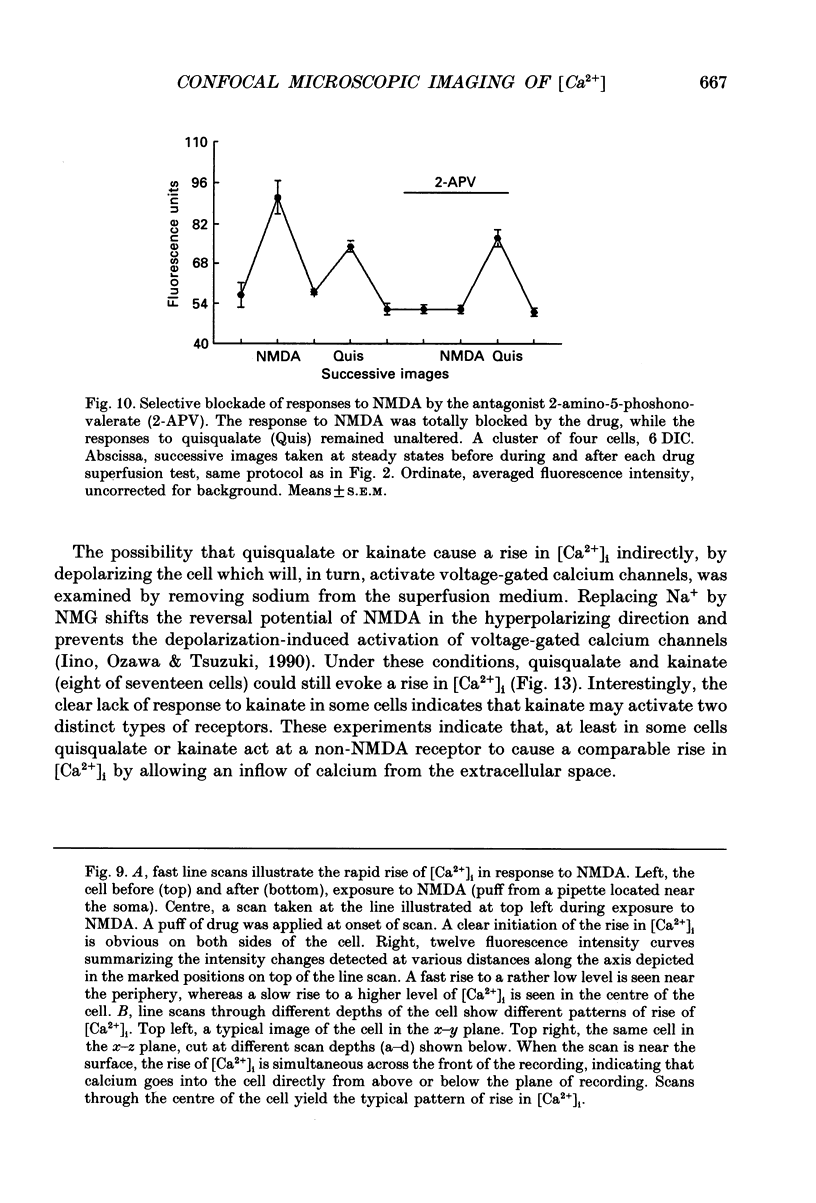
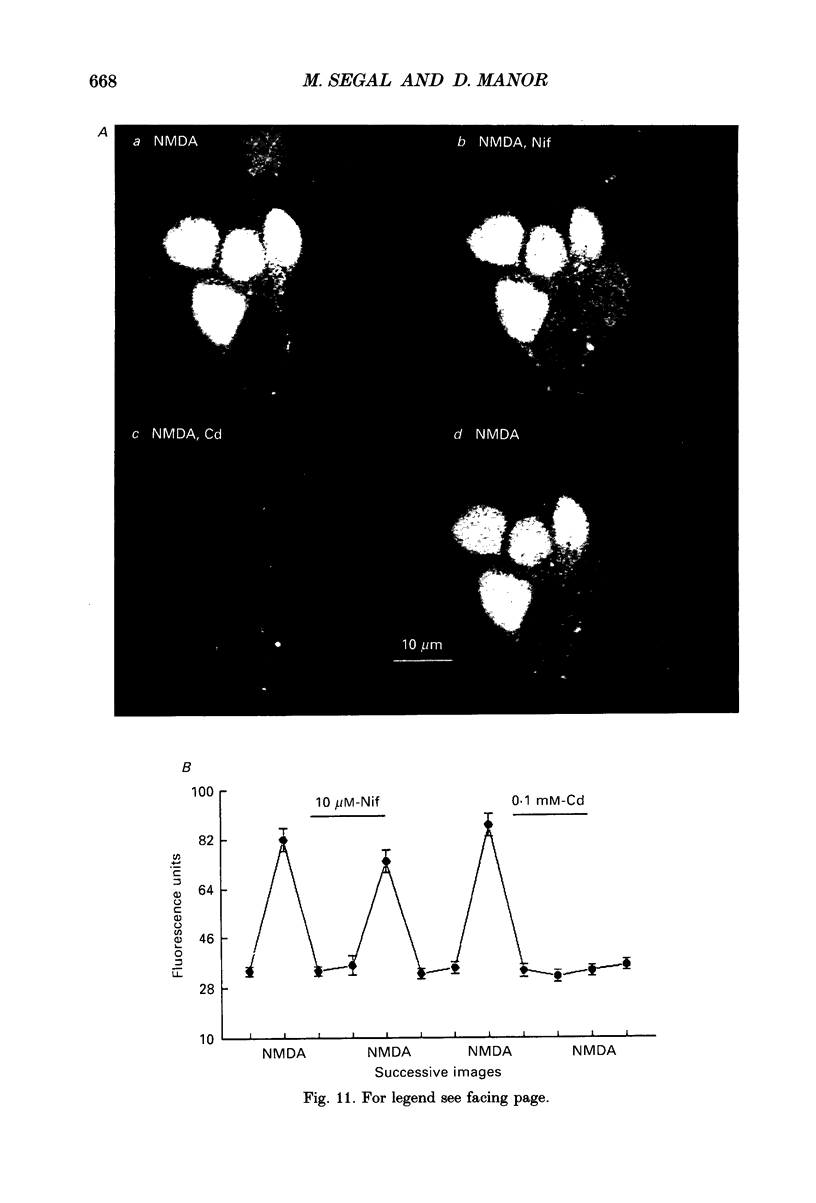
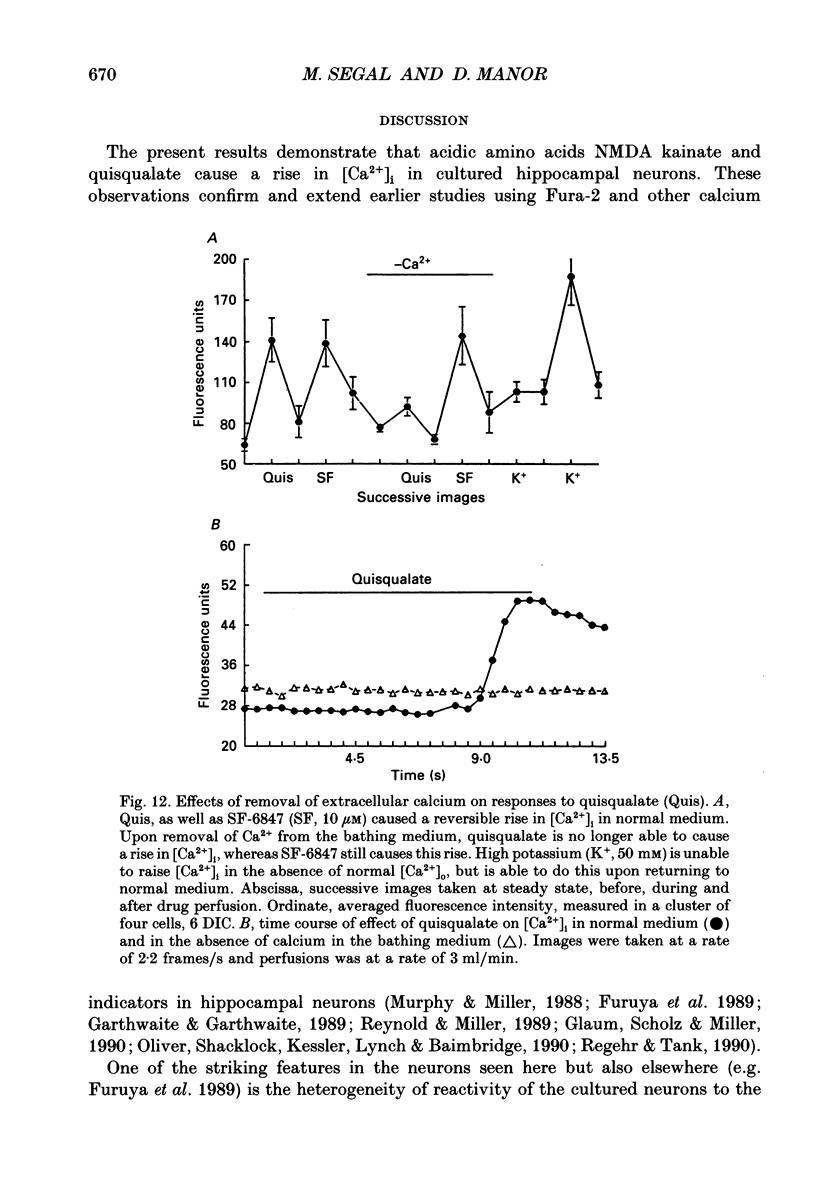
1. The confocal laser scanning microscope (CLSM) was used in conjunction with the calcium indicator dye Fluo-3 to record changes in free intracellular calcium concentration ([Ca2+]i) in cultured hippocampal neurons in response to superfusion of N-methyl-D-aspartate (NMDA). 2. NMDA caused a rapid rise in [Ca2+]i in all parts of the neuron. The rise in [Ca2+]i was dependent on activation of an NMDA receptor, was enhanced by the removal of Mg2+ and addition of glycine to the superfusion medium, and was dependent on normal [Ca2+]o. 3. The rise of [Ca2+]i was seen first near the membrane. A wave of elevated [Ca2+]i moved centripetally at a rate of 117 microns/s. 4. Dantrolene pre-incubation caused a significant reduction in the efficacy of the NMDA-induced rise in [Ca2+]i, indicating that at least part of the rise is caused by intracellular release of calcium. 5. The replacement of calcium by barium caused a reduction in the response to NMDA, but a significant response was still present in these cells, supporting the assumption that NMDA causes release of calcium from intracellular stores. 6. The removal of sodium from the superfusion medium prolonged the [Ca2+]i rise in response to NMDA indicating that the Na-Ca antiporter is instrumental in reducing [Ca2+]i. 7. These studies demonstrate the multiplicity of regulating mechanisms of [Ca2+]i following activation of NMDA receptors.
Full text
PDF






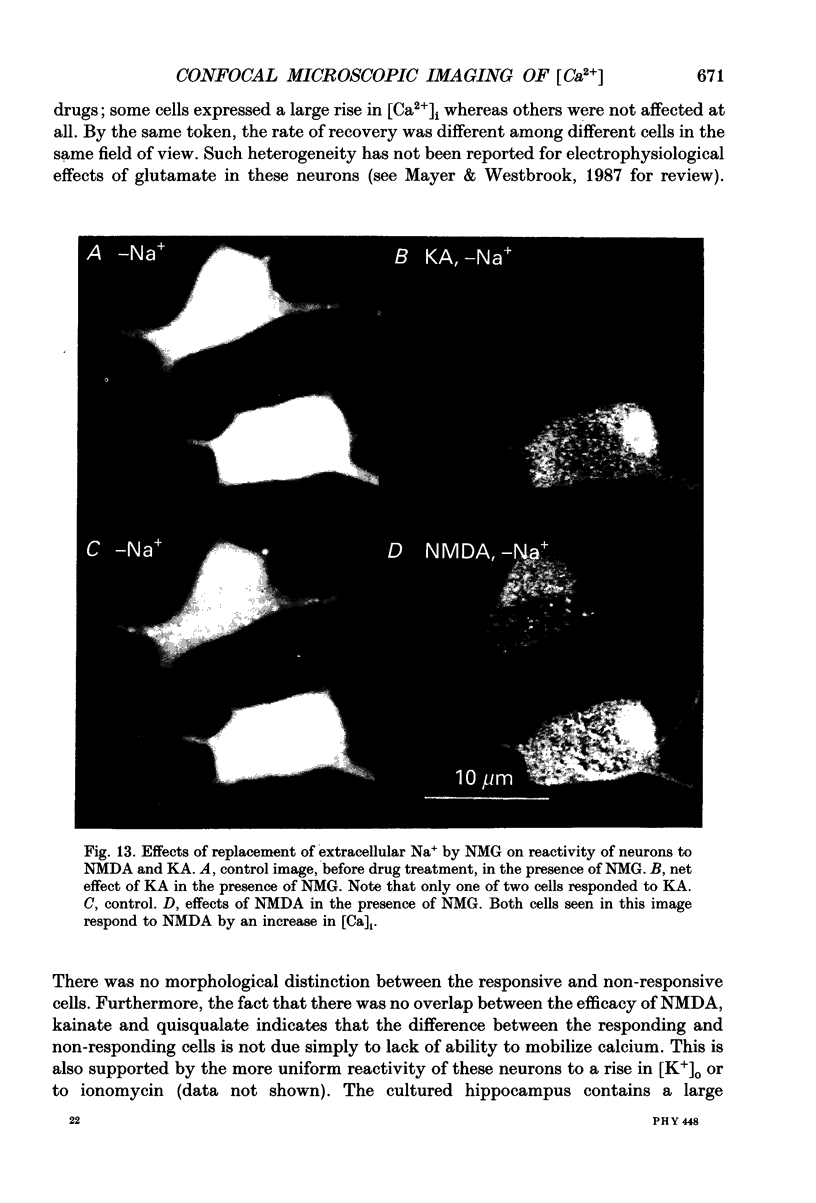
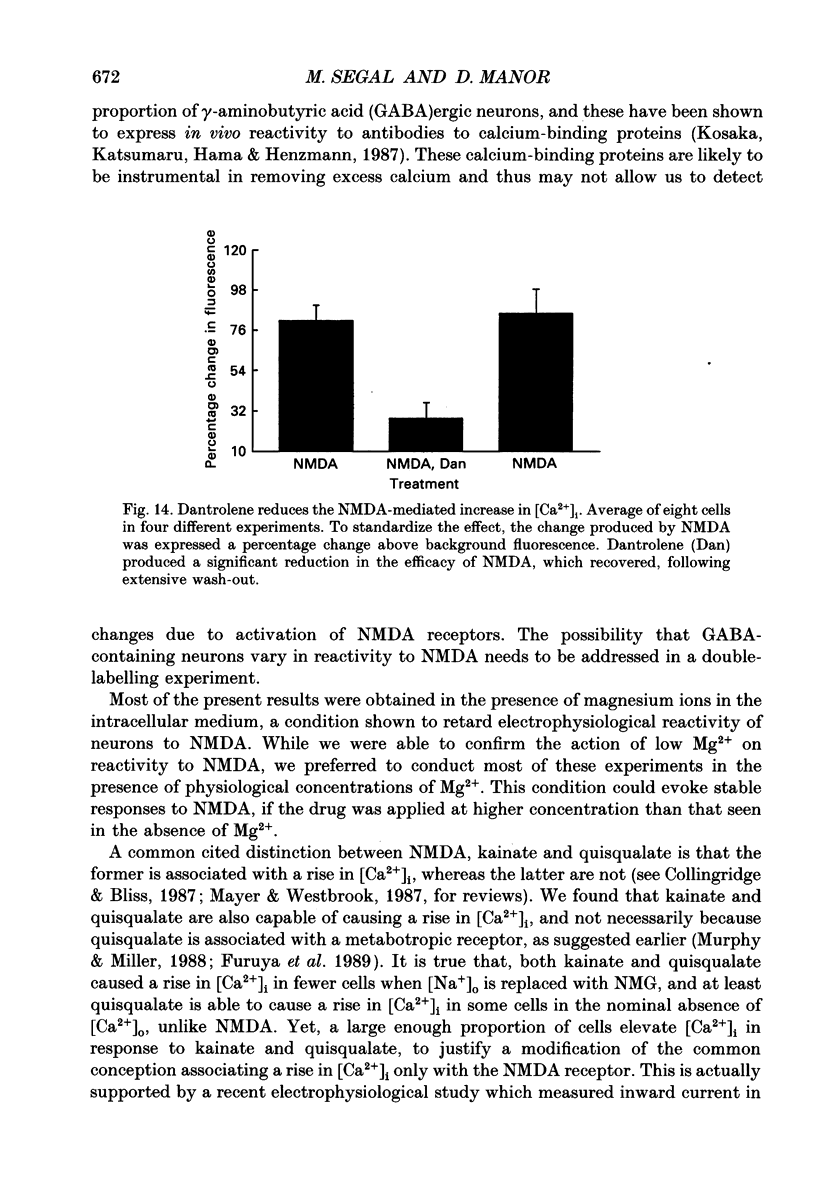
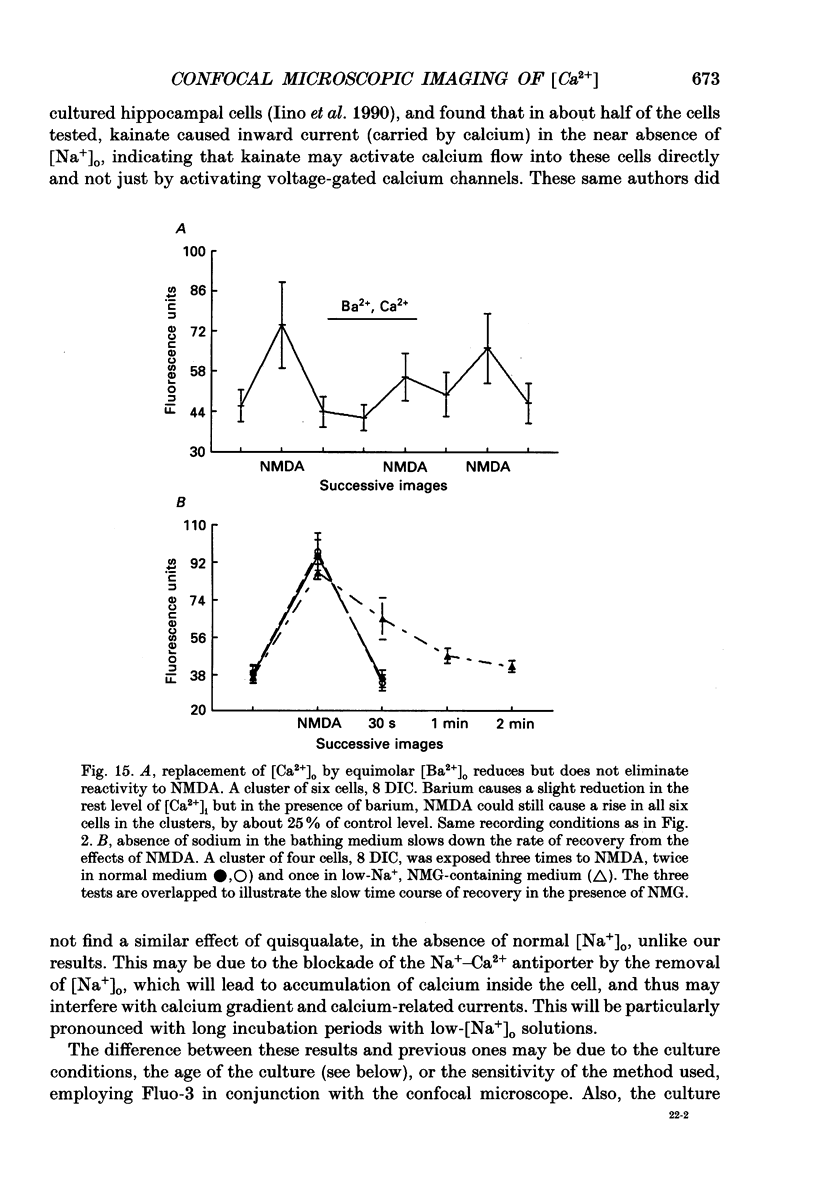



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Furuya S., Ohmori H., Shigemoto T., Sugiyama H. Intracellular calcium mobilization triggered by a glutamate receptor in rat cultured hippocampal cells. J Physiol. 1989 Jul;414:539–548. doi: 10.1113/jphysiol.1989.sp017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Differential dependence on Ca2+ of N-methyl-D-aspartate and quisqualate neurotoxicity in young rat hippocampal slices. Neurosci Lett. 1989 Feb 27;97(3):316–322. doi: 10.1016/0304-3940(89)90617-4. [DOI] [PubMed] [Google Scholar]
- Glaum S. R., Scholz W. K., Miller R. J. Acute- and long-term glutamate-mediated regulation of [Ca++]i in rat hippocampal pyramidal neurons in vitro. J Pharmacol Exp Ther. 1990 Jun;253(3):1293–1302. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Kosaka T., Katsumaru H., Hama K., Wu J. Y., Heizmann C. W. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987 Sep 1;419(1-2):119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Köller H., Siebler M., Schmalenbach C., Müller H. W. GABA and glutamate receptor development of cultured neurons from rat hippocampus, septal region, and neocortex. Synapse. 1990;5(1):59–64. doi: 10.1002/syn.890050105. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oliver M. W., Shacklock J. A., Kessler M., Lynch G., Baimbridge K. G. The glycine site modulates NMDA-mediated changes of intracellular free calcium in cultures of hippocampal neurons. Neurosci Lett. 1990 Jul 3;114(2):197–202. doi: 10.1016/0304-3940(90)90071-g. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature. 1990 Jun 28;345(6278):807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Muscarinic agonists cause calcium influx and calcium mobilization in forebrain neurons in vitro. J Neurochem. 1989 Jul;53(1):226–233. doi: 10.1111/j.1471-4159.1989.tb07318.x. [DOI] [PubMed] [Google Scholar]
- Segal M. Rat hippocampal neurons in culture: responses to electrical and chemical stimuli. J Neurophysiol. 1983 Dec;50(6):1249–1264. doi: 10.1152/jn.1983.50.6.1249. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Wahl P., Schousboe A., Honoŕe T., Drejer J. Glutamate-induced increase in intracellular Ca2+ in cerebral cortex neurons is transient in immature cells but permanent in mature cells. J Neurochem. 1989 Oct;53(4):1316–1319. doi: 10.1111/j.1471-4159.1989.tb07430.x. [DOI] [PubMed] [Google Scholar]