Abstract
Background/purpose
Dental management prior to radiotherapy is often time-critical, and there are no studies on whether manipulations such as tooth extraction influence the risk of severe radiation-induced oral mucositis (ROM) during radiotherapy. Therefore, the aim of this study was to describe the relationship between dental management and the incidence of severe ROM.
Materials and methods
A retrospective analysis was conducted on 144 head and neck cancer (HNC) patients who received dental management before radiotherapy at Peking University Cancer Hospital, from January 2016 to December 2017. Demographic characteristics, primary tumor features, cancer treatment characteristics, and severity of oral mucositis during radiotherapy were recorded. Univariate analysis and logistic regression analysis were used to explore factors associated with severe radiation-induced oral mucositis.
Results
The incidence of grade 3 mucositis was 14.5% (22/144); univariate analysis showed that the number of extracted teeth (OR = 1.313; 95%CI = 1.012–1.702; P = 0.040) and patients with primary oral cancer had a higher risk of developing grade 3 mucositis (OR = 3.848; 95% CI = 1.508–9.822; P = 0.005). No statistical correlation was found between pre-radiation therapy prophylaxis, dental restoration, endodontic treatment, and grade 3 mucositis (P > 0.05). Logistic regression analysis showed that the number of extracted teeth (OR = 1.421, 95%CI = 1.071–1.885, P = 0.015) and primary tumor location in the oral cavity (compared with other head and neck cancers) (OR = 5.165, 95%CI = 1.636–16.311, P = 0.005) were significantly associated with grade 3 mucositis.
Conclusion
In HNC patients undergoing radiotherapy, the primary site located in the oral cavity and a higher number of teeth extracted are independent risk factors for the development of severe mucositis.
Keywords: Dental management prior to radiation therapy, Head and neck cancer, Radiation-induced oral mucositis, Retrospective study, Risk factors
Introduction
Radiation therapy constitutes a pivotal aspect of the multidisciplinary treatment strategy for head and neck cancer (HNC) patients. Despite eradicating malignant tissues, radiotherapy invariably impacts the integrity of surrounding healthy tissues in the same time. This includes deleterious effects on salivary glands, the development of radiation-induced oral mucositis (ROM).1, 2, 3 Profound instances of ROM can severely impair a patient's ability to eat orally, undermining their nutritional status. Some patients may even have to suspend or discontinue radiotherapy due to an inability to tolerate ROM.4,5 Investigations into the exacerbating factors of severe ROM have pinpointed a myriad of contributing elements: poor oral hygiene, tobacco use, concurrent chemotherapy, low hemoglobin levels, advanced nodal staging, elevated body mass index (BMI) > 24 kg/m2, and a heightened neutrophil-to-lymphocyte ratio (NLR).6, 7, 8
Researchers have found that tooth extraction after radiotherapy increases the risk of osteoradionecrosis, hence it is advised to extract at-risk teeth during pre-radiotherapy dental preparation.9 The National Comprehensive Cancer Network (NCCN) guidelines for HNC recommend dental preparation before radiotherapy, including the elimination of potential sources of infection, treatment of dental caries, and periodontal disease.10
The timeframe for pre-radiotherapy dental preparation is often tight, requiring the start of radiotherapy within 7–14 days after the tooth extraction procedure.11 Currently, there are no studies on whether pre-radiotherapy dental procedures such as tooth extraction increase the risk of acute severe ROM during radiotherapy. Therefore, this study aims to describe the relationship between dental management prior to radiation therapy and the incidence rate of severe ROM.
Materials and methods
Study design
This study retrospectively analyzed the clinical medical records and dental management data of HNC patients who were treated at the Department of Stomatology, Peking University Cancer Hospital, from January 2016 to December 2017. The inclusion and exclusion criteria are as follows:
Inclusion criteria: (1) cases of HNC that have undergone radiotherapy; (2) complete clinical medical records, with documentation on the presence or absence of ROM and its severity level; (3) completion of pre-radiotherapy dental preparation at the hospital, with comprehensive dental case files and operation records.
Exclusion criteria: (1) cases with incomplete clinical medical records or lacking documentation on the severity of ROM; (2) HNC cases that did not complete pre-radiotherapy dental preparation or have incomplete dental case files and operation records.
The study adheres to the Helsinki Declaration, and the research protocol has been approved by the Biomedical Ethics Committee of Peking University School of Stomatology (Approval number: PKUSSIRB-202391129). The design of the study references the guidelines set out in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.12
Statistical content
The following information and data were collected and extracted from clinical records. Subject information including age, gender, body mass index (BMI), history of tobacco and alcohol use, presence of comorbidities such as diabetes. Tumor and treatment-related information including primary tumor sites in the head and neck region are categorized as oral or non-oral; staging (American Joint Committee on Cancer staging eighth edition); concurrent treatment details are divided into groups receiving concurrent chemoradiotherapy or targeted therapy, and those receiving radiotherapy alone; modality of radiotherapy treatment; total dose and number of radiotherapy sessions; pre-radiotherapy laboratory test results: red blood cell count, hemoglobin, absolute lymphocyte count, absolute neutrophil count, creatinine, blood glucose, and calculation of NLR.
Dental management prior to radiation therapy
Based on the NCCN Guidelines for Head and Neck Cancers (Version 2014), a treatment plan is developed, which includes eliminating potential sources of infection, treating dental caries, periodontal disease, etc.13 Data such as whether scaling was performed, the number of teeth extracted, the number of teeth restored, the number of teeth treated with root canal therapy, etc., are extracted from the records of oral diagnosis and operations, and recorded. For cases where tooth extraction was performed before radiotherapy, the number of days between the last tooth extraction and the start of radiotherapy is recorded.
Assessment of radiation-induced oral mucositis
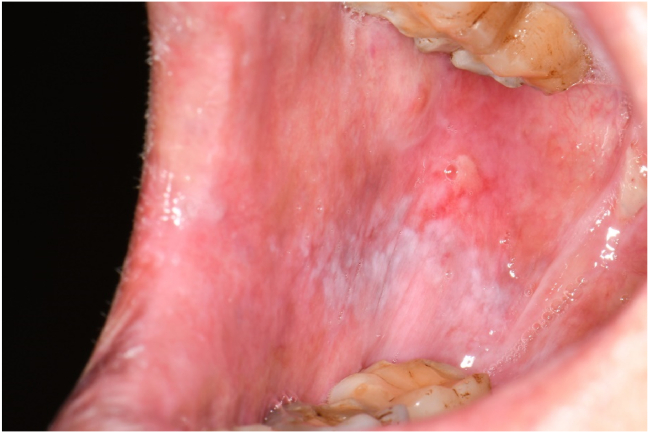
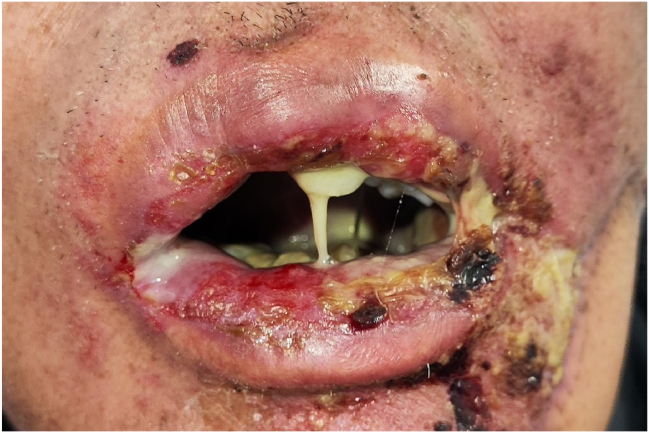
In accordance with the Common Terminology Criteria for Adverse Events (CTCAE) established by the National Cancer Institute in the United States, radiation oncologists documented the occurrence of radiation-induced oral mucositis and its severity grading within patient medical records.14 For this study, we extracted data based on the highest level of ROM severity observed during radiotherapy treatment. The ROM severity grading scale is as follows, with clinical pictures provided as examples from different patients to illustrate each grade: grade 1: asymptomatic or mild symptoms, intervention not indicated (Fig. 1); grade 2: moderate pain or ulcer that does not interfere with oral intake, modified diet indicated (Fig. 2); grade 3: severe pain, interfering with oral intake (Fig. 3); grade 4: life-threatening, urgent intervention indicated; grade 5: death.
Figure 1.
Grade 1 oral mucositis. Mild ulcer covered by yellow fibrin pseudomembrane, affecting buccal membrane after 12 times radiotherapy for nasopharyngeal carcinoma.
Figure 2.
Grade 2 oral mucositis. Large ulcer, modified diet indicated but maintaining oral intake without the necessity for analgesics, after 15 times radiotherapy for oral carcinoma.
Figure 3.
Grade 3 oral mucositis. Extensive ulcer, crusting, and exudate on the lips and oral mucosa, accompanied by limited mouth opening and oral intake impairment, requiring analgesics after 23 times radiotherapy for oral carcinoma.
Statistical analysis
SPSS26.0 statistical analysis software (IBM, Armonk, NY, USA) was used. Variables exhibiting a normal distribution were characterized by the mean ± standard deviation, and their association with ROM grades was evaluated through independent samples t-test and one-way ANOVA. Conversely, variables not adhering to a normal distribution had their distributions depicted by the median (25th percentile, 75th percentile) via the weighted average method, with non-parametric testing employed to gauge the correlation between variable distributions and ROM grades. Logistic regression models were applied to execute both univariate and multivariate analyses. The entirety of the statistical computations was carried out utilizing. Statistical significance was set at P < 0.05 unless otherwise indicated.
Results
Clinical information
This study included 144 patients with an age of 56.9 ± 14.1 years; 95 (66.0%) were male and 49 (34.0%) were female. All 144 patients experienced ROM, among which 122 patients (84.7%) had grade 1 or 2 ROM, and 22 patients (15.3%) had grade 3 ROM. No patients had grade 4 or 5 ROM.
All patients received radiotherapy using IMRT technique, with an average total dose of 66.5 ± 6.7Gy and a single dose of 2.1 ± 0.1Gy. All 144 patients underwent oral management and oral hygiene education before radiotherapy. Forty patients (27.8%) underwent tooth extraction, 98 (68.1%) had scaling and cleaning, 15 (10.4%) had dental defect bonding restoration, and 13 (9.0%) had endodontic treatment. Among the teeth extracted before radiotherapy, those with residual roots, residual crowns, severe periodontitis, and periapical inflammation accounted for 76.9% (70/91 teeth), and third molars accounted for 23.1% (21/91 teeth) (see Table 1).
Table 1.
Characteristics of the study population.
| Characteristic | Category | Radiation-induced oral mucositis |
t/χ2/H | P | Total n = 144 | |
|---|---|---|---|---|---|---|
| Grade 1&2 n = 122 | Grade 3 n = 22 | |||||
| Age | 50.1 ± 14.2 | 53.2 ± 14.5 | −0.811 | 0.419 | 56.9 ± 14.1 | |
| Sex | Male | 80 (65.6%) | 15 (68.2%) | 0.056 | 0.812 | 95 (66.0%) |
| BMI (kg/m2) | 23.1 ± 3.2 | 22.7 ± 3.1 | 0.415 | 0.679 | 23.1 ± 3.2 | |
| Diabetes mellitus | With | 9 (7.4%) | 2 (9.1%) | 0.078 | 0.781 | 11 (7.6%) |
| Hypertension | With | 19 (15.6%) | 3 (13.6%) | 0.054 | 0.816 | 22 (15.3%) |
| Smoking history | With | 34 (27.9%) | 8 (36.4%) | 0.651 | 0.420 | 42 (29.2%) |
| Alcohol history | With | 22 (18.0%) | 7 (31.8%) | 2.202 | 0.138 | 29 (20.1%) |
| Primary cancer location | Oral | 29 (23.8%) | 12 (54.5%) | 8.668 | 0.003 | 41 (28.5%) |
| Non-orala | 93 (76.2%) | 10 (45.5%) | 103 (71.5%) | |||
| Staging, n (%) | Ⅰ | 7 (5.7%) | 0 (0.0%) | 7.339 | 0.062 | 7 (4.9%) |
| Ⅱ | 15 (12.3%) | 2 (9.1%) | 17 (11.8%) | |||
| Ⅲ | 17 (13.9%) | 8 (36.4%) | 25 (17.4%) | |||
| Ⅳ | 83 (68.0%) | 12 (54.5%) | 95 (66.0%) | |||
| Surgery | With | 57 (46.7%) | 14 (63.6%) | 2.134 | 0.144 | 71 (49.3%) |
| Total radiation dose (Gy) | 67.1 ± 6.0 | 65.5 ± 5.4 | 1.047 | 0.297 | 66.5 ± 6.7 | |
| Single radiation dose (Gy) | 2.1 ± 0.1 | 2.1 ± 0.1 | 0.545 | 0.587 | 2.1 ± 0.1 | |
| Concomitant treatment | Radiotherapy only | 50 (41.0%) | 6 (27.3%) | 1.474 | 0.225 | 56 (38.9%) |
| Concurrent chemoradiotherapy | 72 (59.0%) | 16 (72.7%) | 88 (61.1%) | |||
| Number of teeth extracted | 0 (0,1) | 0 (0,1.25) | 1.240 | 0.266 | 0 (0,1) | |
| Scaling | 81 (66.4%) | 17 (77.3%) | 1.015 | 0.314 | 98 (68.1%) | |
| Red blood cell count (10˄9/L) | 4.4 ± 0.5 | 4.5 ± 0.6 | −1.312 | 0.192 | 4.4 ± 0.5 | |
| Hemoglobin (g/L) | 131.0 ± 17.5 | 134.2 ± 18.4 | −0.751 | 0.454 | 131.5 ± 17.6 | |
| NLR | 3.3 ± 2.8 | 2.7 ± 1.3 | 1.043 | 0.299 | 3.2 ± 2.6 | |
| NLR>5 | 19 (15.6%) | 0 (0%) | 4.122 | 0.047 | 19 (13.2%) | |
| Creatinine (μmol/L) | 64.4 ± 15.2 | 60.2 ± 12.8 | 1.093 | 0.277 | 63.7 ± 14.9 | |
Abbreviations: BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio.
Non-oral cancer included nasopharyngeal carcinoma, nasal cavity cancer, paranasal sinus cancer, oropharyngeal carcinoma, hypopharyngeal carcinoma, salivary gland tumor, skin cancer, external auditory canal carcinoma.
Univariate analysis of factors associated with grade 3 radiation-induced oral mucositis
Table 2 provides the results of the univariate analysis. The results indicated a significant positive correlation with the occurrence of grade 3 ROM for the following factors: primary tumor site in the oral cavity (non-oral) (OR = 3.848, 95%CI = 1.508–9.822, P = 0.005), and number of teeth extracted (OR = 1.313, 95%CI CL = 1.001–1.702, P = 0.040). Whether scaling was performed, the number of teeth restored, and the number of teeth treated endodontically showed no statistical association with the occurrence of grade 3 ROM (P > 0.05).
Table 2.
Univariate analysis of factors related to grade 3 radiation-induced oral mucositis.
| Variable | Category (reference) | OR | 95%CI | P |
|---|---|---|---|---|
| Sex | Male (female) | 0.889 | 0.338–2.349 | 0.812 |
| Age | 1.014 | 0.981–1.048 | 0.416 | |
| BMI (kg/m2) | 0.961 | 0.799–1.156 | 0.675 | |
| Smoking history | Have (none) | 1.479 | 0.569–3.842 | 0.420 |
| Alcohol history | Have (none) | 2.121 | 0.773–5.818 | 0.138 |
| Primary cancer location | Oral (none-oral) | 3.848 | 1.508–9.822 | 0.005 |
| Stage | Ⅲ,Ⅳ (Ⅰ,Ⅱ) | 2.200 | 0.479–10.110 | 0.300 |
| Surgery | With (without) | 1.996 | 0.781–5.102 | 0.144 |
| Total dose (Gy) | 0.957 | 0.880–1.039 | 0.295 | |
| Single dose (Gy) | 0.223 | 0.001–45.968 | 0.581 | |
| Induction chemotherapy | With (without) | 0.978 | 0.381–2.511 | 0.963 |
| Concomitant treatment | With (without) | 1.852 | 0.678–5.060 | 0.225 |
| Number of teeth extracted | 1.313 | 1.012–1.702 | 0.040 | |
| Scaling | With (without) | 1.721 | 0.593–4.995 | 0.314 |
| Number of teeth filled | 1.189 | 0.495–2.858 | 0.698 | |
| Number of teeth receiving pulp therapy | 0.872 | 0.218–3.486 | 0.846 | |
| Days from tooth extraction to radiation therapy begin | 0.416 | 0.136–1.274 | 0.560 | |
| Red blood cell count (10˄9/L) | 1.850 | 0.734–4.666 | 0.192 | |
| Hemoglobin (g/L) | 1.011 | 0.983–1.039 | 0.451 | |
| NLR | 0.882 | 0.695–1.119 | 0.300 | |
| Creatinine (μmol/L) | 0.980 | 0.946–1.016 | 0.275 | |
| Glucose (mmol/L) | 0.874 | 0.452–1.688 | 0.688 |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio.
Multivariate logistic regression analysis of oral preparation and grade 3 radiation-induced oral mucositis
In the multifactorial logistic regression model, with the occurrence of Grade 3 ROM as the dependent variable, independent variables included total radiation dose, primary tumor site (oral vs. non-oral), concurrent treatment (chemoradiation or targeted therapy vs. radiotherapy alone), alcohol consumption history (yes vs. no), postoperative status (postoperative vs. non-postoperative), number of extracted teeth, periodontal treatment (yes vs. no), number of bonded restorations, number of endodontically treated teeth, and red blood cell count. The binary logistic regression multifactorial analysis was conducted. The results indicated that the primary tumor site in the oral cavity (OR = 5.165, 95%CI = 1.636–16.311, P = 0.005) and the number of extracted teeth (OR = 1.421, 95%CI = 1.071–1.885, P = 0.015) were significantly positively correlated with Grade 3 ROM, with a Nagelkerke R2 value for the model at 0.182. See Table 3.
Table 3.
Multivariate logistic regression analysis for grade 3 radiation-induced oral mucositis.
| Variable | B | SE | Wald χ2 | P | EXP (B) | 95%CI of EXP (B) |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Primary cancer location | 1.642 | 0.587 | 7.833 | 0.005 | 5.165 | 1.636 | 16.311 |
| Number of teeth exacted | 0.351 | 0.144 | 5.914 | 0.015 | 1.421 | 1.071 | 1.885 |
| Constant | −2.629 | 0.457 | 33.056 | 0.000 | 0.072 | ||
Abbreviations: B, regression coefficient; SE, standard error; Wald χ2, Wald chi-square; EXP (B), exponentiated regression coefficient; CI, confidence interval.
Discussion
This study retrospectively analyzed the impact of pre-radiotherapy oral preparation on the incidence of severe ROM in 144 HNC patients undergoing radiotherapy. The primary tumor site being in the oral cavity and a higher number of teeth extractions during the preparatory phase were identified as independent risk factors for grade 3 ROM. Univariate analysis did not reveal any statistically significant correlation between other pre-radiotherapy oral preparation measures (periodontal treatment, dental restorations, endodontic treatment) and the severity of ROM.
In the present study, the incidence of grade 3 ROM was noted to be 15.3%, which is significantly lower than the rates reported in other literature, ranging from 39% to 65%.8,15, 16, 17 This may because advancements in radiotherapy technology, coupled with comprehensive oral preparation, have substantially mitigated the onset of severe radiation-induced oral mucositis. The adoption of IMRT has been proven to decrease the occurrence of severe ROM.18, 19, 20 On the other hand, though lack of evidence, scholars believe that oral health education and management are beneficial for controlling mucositis in radiotherapy patients. In this study, radiation oncologists placed great emphasis on oral management, and dental professionals were highly experienced, allowing patients to receive timely, high-quality oral care.
Besides, the method of assessing and recording ROM may lead to disparities in the judgment of ROM. The CTCAE grade does not include an actual description of the patient's oral mucosal lesions, but rather relies solely on the patient's subjective feelings of symptoms like pain for evaluation. Since patients have different levels of tolerance, those who are less sensitive to pain but have larger lesion areas may underreport their discomfort, whereas those who are more sensitive to pain might report more severe sensations even with smaller lesions. Different physicians may have varying habits in recording the severity of ROM; some patients' medical records may lack timely updated assessments of their ROM status, resulting in some patients who progress to grade 3 ROM still being recorded as grade 1 or 2 in their charts. In future ROM-related research, it is necessary to incorporate objective indicators for ROM assessment and emphasize standardized evaluation and recording to ensure the objectivity and consistency of ROM assessments.
Patients with primary HNC in the oral cavity had a higher incidence rate of grade 3 ROM than those with non-oral primary HNC. This can be attributed to the fact that patients with oral tumors have a closer radiation treatment target to the oral mucosa, which results in direct absorption of higher doses of radiation. Additionally, it has been observed clinically that some patients undergoing unilateral radiotherapy develop ROM lesions in both sides of the oral cavity, indicating that not only the direct effect of radiation irradiation but also secondary immune responses play a role in ROM occurrence.4
Risk factors associated with severe ROM included: smoking history, concurrent chemotherapy, alcohol consumption history, BMI >24.0 kg/m2, diabetes, etc.6 Studies reported by the Multinational Association of Supportive Care in Cancer (MASCC) highlighted a significant correlation between smoking and ROM.21 However, this study did not find a significant correlation between the above factors with severe ROM, possibly because the impact of tumor location and the number of teeth extracted prior to radiotherapy on severe ROM is more substantial. The aforementioned studies did not include these two factors, and only a few ROM-related studies have described the content of oral preparations. Additionally, previous research found that the severity of ROM is related to body weight, suggesting that managing nutritional status might play a positive role in alleviating the severity of ROM.17
In this study, it was found for the first time that a higher number of teeth extracted during the pre-radiation oral preparation phase is an independent risk factor for grade 3 ROM. The teeth extracted before radiotherapy often present with chronic infections and may have alveolar bone or tooth structure damage due to long-term chronic inflammation or decay, which are not expected to remain viable in the long term after radiotherapy. In this study, many of the teeth removed before radiotherapy were infected, including residual roots, residual crowns, severe periodontitis, and periapical lesion cases. Within the urgency in the treatment of head and neck cancer, and the fact that wounds from tooth extraction require at least 1–2 weeks to heal, but generally, dentists have only about 1–2 weeks to perform pre-radiation oral care.9 As a result, dentists may need to extract multiple teeth in a short period for some patients. Patients often have poor oral hygiene before starting radiation therapy, and their ability to maintain oral health independently is low. If they undergo induction chemotherapy, it leads to reduced systemic and local immunity. Extracting many teeth in a short period increases the number of oral wounds, which are difficult to heal quickly. All these factors combined lead to a severe deficiency in the patient's oral defenses during radiation therapy. Since bacterial and fungal infections are directly related to the occurrence of ROM, if the high concentration of oral bacterial and fungal infections is not effectively reduced before radiotherapy and maintained throughout the treatment, it becomes more likely to cause severe ROM, especially in patients with lowered immunity and an increased number of post-extraction wounds.4,22
The risk of developing osteoradionecrosis (ORN) of the jaw increases after tooth extraction post-radiotherapy.23 Research indicates that teeth with periodontitis that are not extracted before radiotherapy are a risk factor for ORN.24 Although there is still a lack of high-level evidence to prove that extracting at-risk teeth before radiotherapy can reduce the incidence of ORN,25, 26, 27 current international guidelines still recommend a systematic oral assessment and tooth extraction before radiotherapy.9,10
This study identified a higher number of teeth extracted before radiotherapy as an independent risk factor for severe ROM. However, this does not mean that the practice of extracting high-risk teeth before radiotherapy should be discarded. The impact of ORN on patients' quality of life is more significant compared to the severe ROM experienced during radiotherapy, and the benefits of extracting infected teeth before treatment may outweigh the risks. Therefore, for patients who have had multiple extractions before radiotherapy, adopting additional measures to prevent or reduce the incidence of ROM is a direction that requires further research emphasis. For patients with oral cancer undergoing radiotherapy, the use of enteral nutrition such as gastric tubes or gastrostomies early in radiotherapy or before starting may also be considered. This approach minimizes oral intake during radiotherapy, reducing frictional trauma from eating while ensuring that patients receive adequate and controlled nutritional intake.
No significant correlation was found in this study between pre-radiotherapy scaling and the severity of ROM in HNC patients, which may be due to the fact that some patients had maintained good oral hygiene through scaling before surgery or regular dental hygiene practices. For these patients, an additional scaling procedure before radiotherapy may not be necessary. Previous research has identified poor oral hygiene as a risk factor for severe ROM, we still recommend that HNC patients with poor oral hygiene should undergo scaling before radiotherapy.28 Therefore future studies should employ indices such as plaque index and calculus index to investigate the correlation between oral hygiene status and the severity of ROM.
This study has certain limitations, as it was conducted in a single oncology hospital, limiting the generalizability of its findings. Subsequent multicenter studies should be carried out to prospectively investigate the oral condition of patients before radiotherapy, the content of oral preparation, and the severity of ROM, in order to gain a deeper understanding of the relationship between oral health, oral preparation, and the severity of ROM.
In HNC patients undergoing radiotherapy, the primary site located in the oral cavity and a higher number of teeth extracted are independent risk factors for the development of severe mucositis. In future research, we should pay special attention to recording oral procedures before radiotherapy and tracking and follow-up of patients after radiotherapy.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
None.
Contributor Information
Yan Sun, Email: lisaysun@139.com.
Hongwei Liu, Email: hongweil2569@163.com.
References
- 1.Jensen S.B., Section S.G.H., Pedersen A.M.L., et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 2.Chow L.Q.M. Head and neck cancer. N Engl J Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 3.Sroussi H.Y., Epstein J.B., Bensadoun R.J., et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918–2931. doi: 10.1002/cam4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elad S., Yarom N., Zadik Y., Kuten-Shorrer M., Sonis S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA A Cancer J Clin. 2021;72:57–77. doi: 10.3322/caac.21704. [DOI] [PubMed] [Google Scholar]
- 5.Elad S., Cheng K.K.F., Lalla R.V., et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126:4423–4431. doi: 10.1002/cncr.33100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soutome S., Yanamoto S., Nishii M., et al. Risk factors for severe radiation-induced oral mucositis in patients with oral cancer. J Dent Sci. 2021;16:1241–1246. doi: 10.1016/j.jds.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol A.J., Ching J.C.F., Tam V.C.W., et al. Predictive factors for chemoradiation-induced oral mucositis and dysphagia in head and neck cancer: a scoping review. Cancers. 2023;15:5705. doi: 10.3390/cancers15235705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashita Y., Kitamura M., Soutome S., Ukai T., Umeda M., Saito T. Association of neutrophil-to-lymphocyte ratio with severe radiation-induced mucositis in pharyngeal or laryngeal cancer patients: a retrospective study. BMC Cancer. 2021;21:1064. doi: 10.1186/s12885-021-08793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson E., Dorna Mojdami Z., Oladega A., Hope A., Glogauer M. Clinical practice guidelines for dental management prior to radiation for head and neck cancer. Oral Oncol. 2021;123 doi: 10.1016/j.oraloncology.2021.105604. [DOI] [PubMed] [Google Scholar]
- 10.Caudell J.J., Gillison M.L., Maghami E., et al. NCCN guidelines® insights: head and neck cancers, version 1.2022. J Natl Compr Cancer Netw. 2022;20:224–234. doi: 10.6004/jnccn.2022.0016. [DOI] [PubMed] [Google Scholar]
- 11.Falek S., Regmi R., Herault J., et al. Dental management in head and neck cancers: from intensity-modulated radiotherapy with photons to proton therapy. Support Care Cancer. 2022;30:8377–8389. doi: 10.1007/s00520-022-07076-5. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfister D.G., Spencer S., Brizel D.M., et al. Head and neck cancers, version 2.2014. J Natl Compr Cancer Netw. 2014;12:1454–1487. doi: 10.6004/jnccn.2014.0142. [DOI] [PubMed] [Google Scholar]
- 14.Freites-Martinez A., Santana N., Arias-Santiago S., Viera A. Using the common terminology criteria for adverse events (CTCAE – version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Anderson C.M., Lee C.M., Saunders D.P., et al. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J Clin Orthod. 2019;37:3256–3265. doi: 10.1200/JCO.19.01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger K., Schopohl D., Bollig A., et al. Burden of oral mucositis: a systematic review and implications for future research. Oncol Res Treat. 2018;41:399–405. doi: 10.1159/000487085. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Z., Zhao X., Zhao Q., et al. The effects of early nutritional intervention on oral mucositis and nutritional status of patients with head and neck cancer treated with radiotherapy. Front Oncol. 2021;10 doi: 10.3389/fonc.2020.595632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fregnani E.R., Parahyba C.J., Morais-Faria K., et al. IMRT delivers lower radiation doses to dental structures than 3DRT in head and neck cancer patients. Radiat Oncol. 2016;11:116. doi: 10.1186/s13014-016-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzi S., Iaccarino G., Pasciuti K., et al. Analysis of salivary flow and dose–volume modeling of complication incidence in patients with head-and-neck cancer receiving intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1252–1259. doi: 10.1016/j.ijrobp.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Sanguineti G., Sormani M.P., Marur S., et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;83:235–242. doi: 10.1016/j.ijrobp.2011.06.2000. [DOI] [PubMed] [Google Scholar]
- 21.Wardill H.R., Oncology O., Sonis S.T., et al. Prediction of mucositis risk secondary to cancer therapy: a systematic review of current evidence and call to action. Support Care Cancer. 2020;28:5059–5073. doi: 10.1007/s00520-020-05579-7. [DOI] [PubMed] [Google Scholar]
- 22.Tao Z., Gao J., Qian L., et al. Factors associated with acute oral mucosal reaction induced by radiotherapy in head and neck squamous cell carcinoma. Medicine. 2017;96 doi: 10.1097/MD.0000000000008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acharya S., Pai K.M., Acharya S. Risk assessment for osteoradionecrosis of the jaws in patients with head and neck cancer. Med Pharm Rep. 2020;93:195–199. doi: 10.15386/mpr-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuurhuis J.M., Stokman M.A., Roodenburg J.L.N., et al. Efficacy of routine pre-radiation dental screening and dental follow-up in head and neck oncology patients on intermediate and late radiation effects. A retrospective evaluation. Radiother Oncol. 2011;101:403–409. doi: 10.1016/j.radonc.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Aarup-Kristensen S., Hansen C.R., Forner L., Brink C., Eriksen J.G., Johansen J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: risk factors and dose-volume correlations. Acta Oncol. 2019;58:1373–1377. doi: 10.1080/0284186X.2019.1643037. [DOI] [PubMed] [Google Scholar]
- 26.Singh A., Kitpanit S., Neal B., et al. Osteoradionecrosis of the jaw following proton radiation therapy for patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2023;149:151. doi: 10.1001/jamaoto.2022.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urquhart O., DeLong H.R., Ziegler K.M., et al. Effect of preradiation dental intervention on incidence of osteoradionecrosis in patients with head and neck cancer: a systematic review and meta-analysis. J Am Dent Assoc. 2022;153:931–942. doi: 10.1016/j.adaj.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Chan J., Filippi A., Filippi C. Clinical guidance for maintaining oral hygiene in patients undergoing chemotherapy or radiation therapy: a scoping review. Swiss Dent J. 2023;133(6):368–379. doi: 10.61872/sdj-2023-06-01. [DOI] [PubMed] [Google Scholar]