Abstract
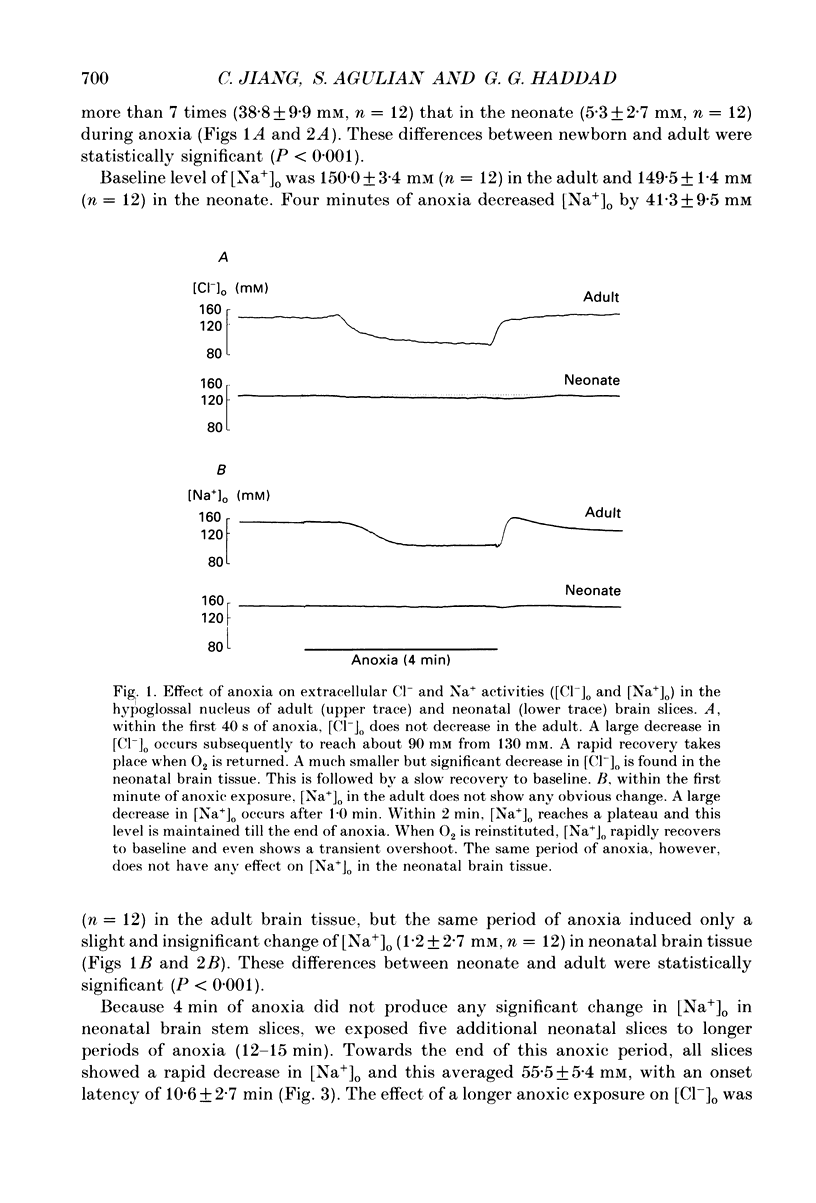
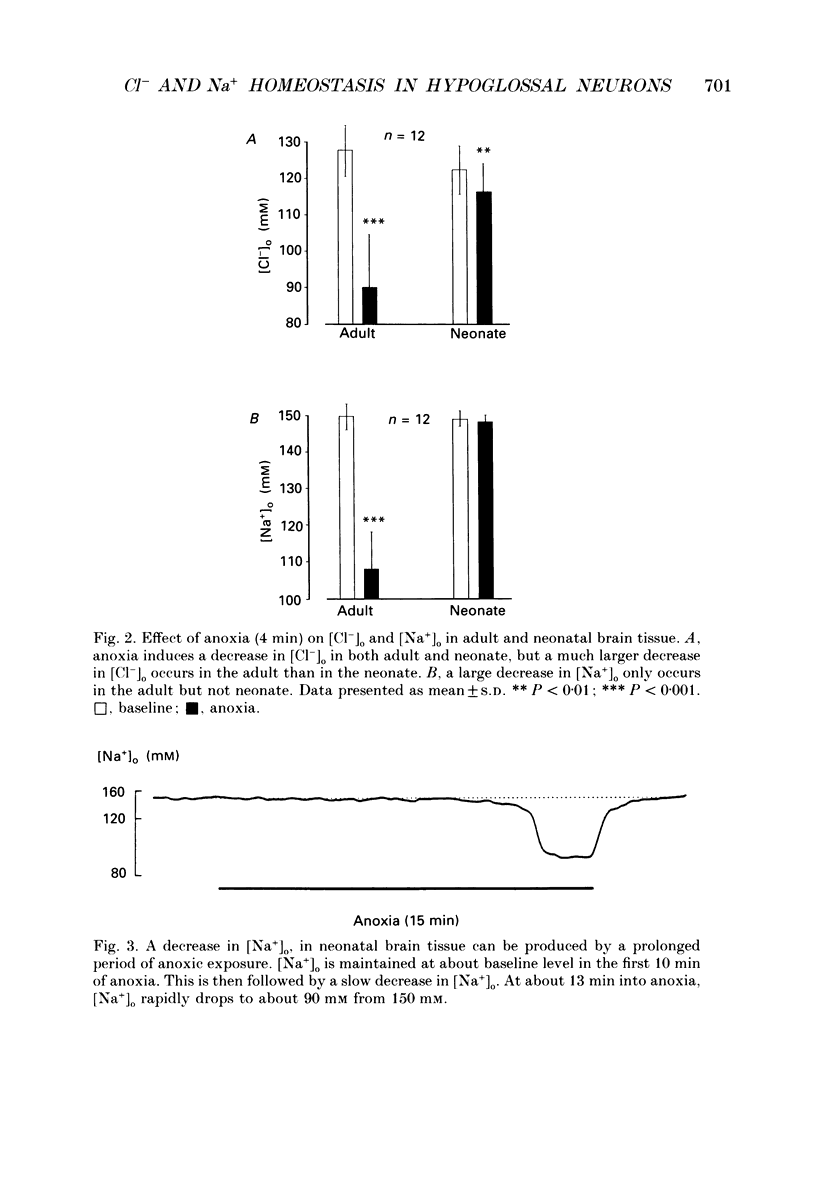
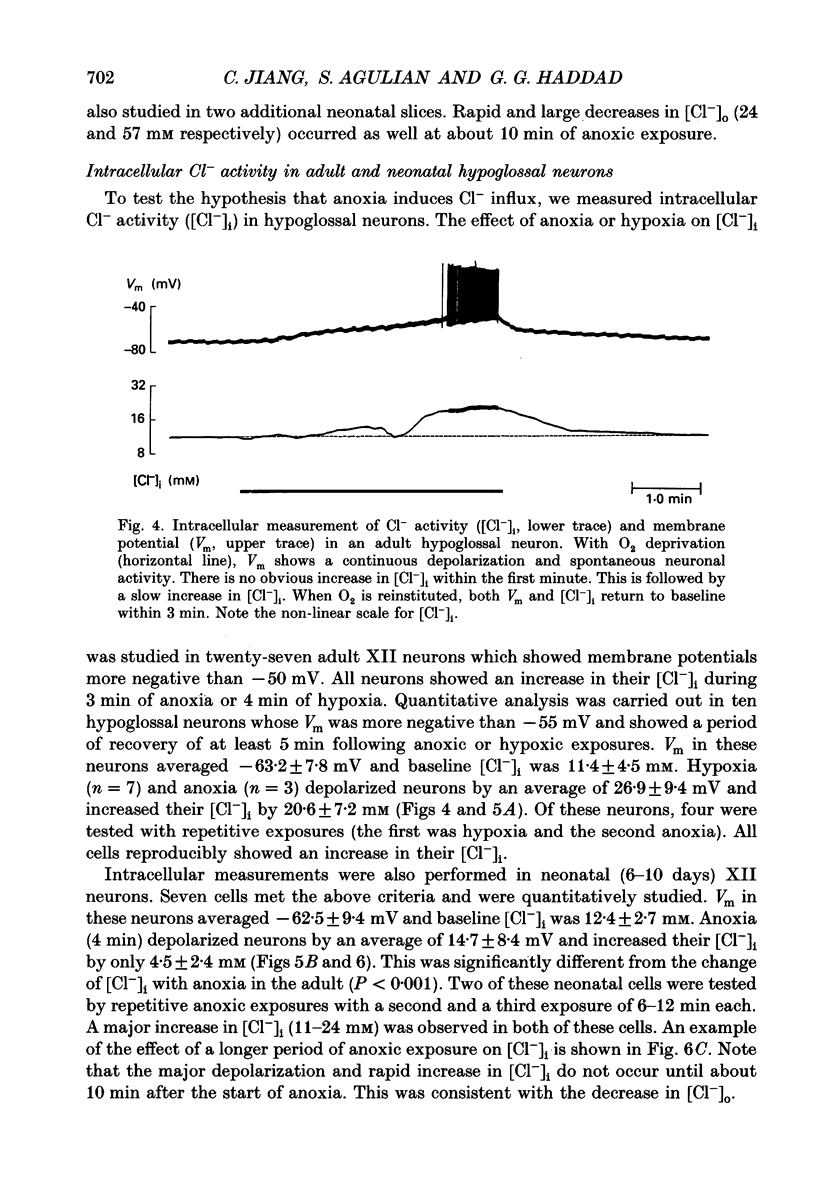
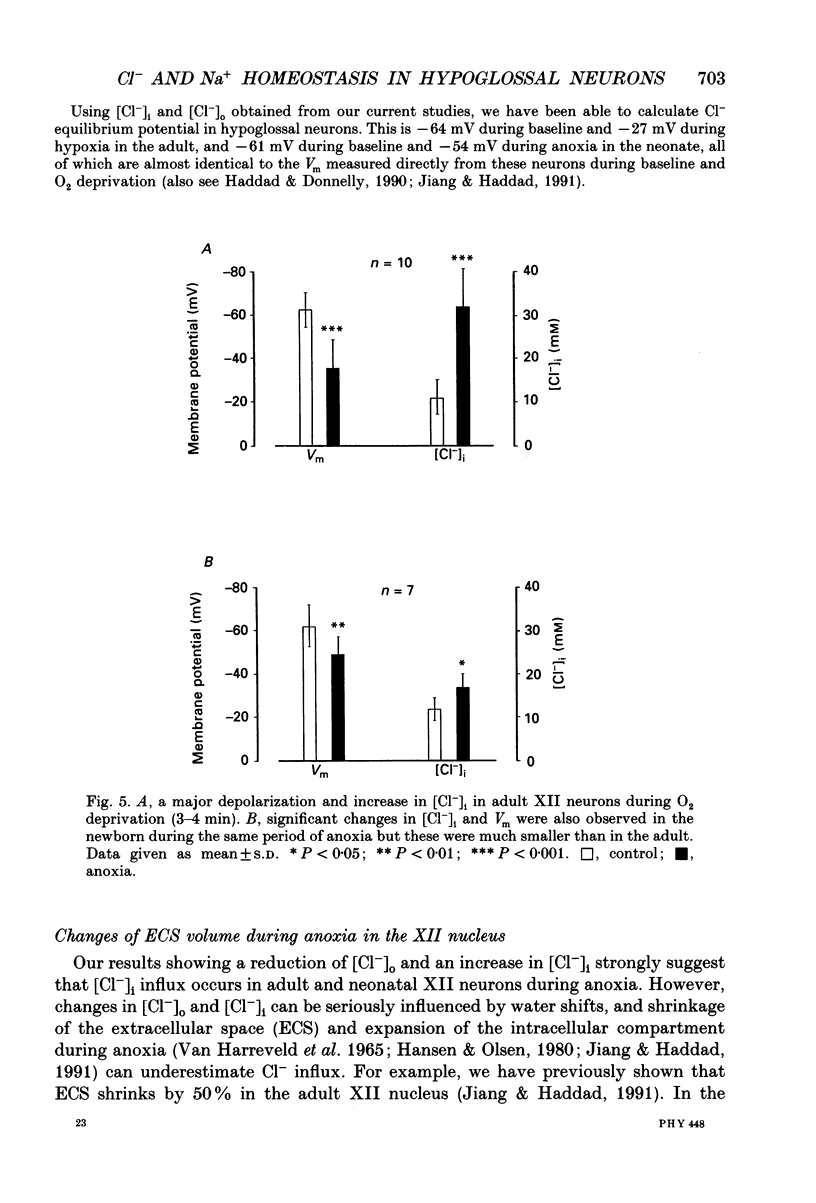
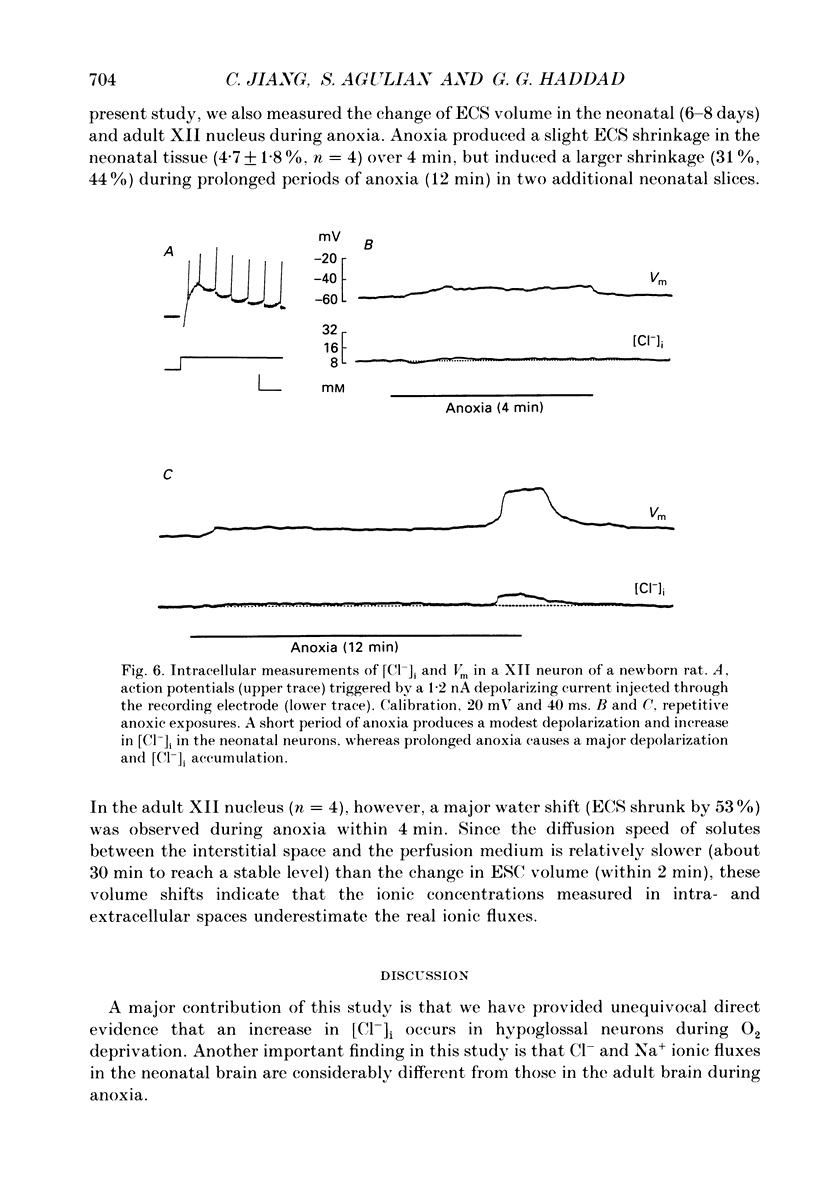
1. To understand the mechanisms which lead to acute neuronal swelling during anoxia, we studied the ionic movements of Cl- and Na+ during O2 deprivation in the hypoglossal (XII) neurons of rat brain slices using double-barrelled ion-selective microelectrodes. 2. Baseline extracellular Cl- and Na+ activities ([Cl-]o, [Na+]o) were 128.3 +/- 7.4 and 150.0 +/- 3.4 mM respectively (n = 12) in the adult. Similar baseline values were obtained from neonatal brain slices. 3. During a period of anoxia (4 min), [Na+]o decreased by about 40 mM in adult slices while [Na+]o did not show any significant change in the neonate (n = 12). Although anoxia induced a significant decrease of [Cl-]o in both adult and neonate, [Cl-]o dropped 7 times more in the adult than in the neonate (n = 12). 4. Intracellular Cl- activity ([Cl-]i) was studied in twenty-seven adult hypoglossal cells. All showed an increase in [Cl-]i) was studied in twenty-seven adult hypoglossal cells. All showed an increase in [Cl-]i with O2 deprivation. Detailed analysis carried out on ten hypoglossal neurons showed a baseline [Cl-]i of 11.4 +/- 4.5 mM and an increase in [Cl-]i by 20.6 +/- 7.2 mM during O2 limitation. 5. Baseline [Cl-]i in neonatal XII neurons was similar to that of the adult. Anoxia, however, produced an increase in [Cl-]i by only 4.5 +/- 2.4 mM (n = 7). This increase in [Cl-]i was significantly less than that in the adult (P less than 0.001). Prolonged anoxia (6-12 min) in the neonate led to a more substantial increase in [Cl-]i, an observation consistent with the decrease in [Cl-]o after prolonged O2 deprivation. 7. We conclude that during anoxia: (1) intracellular [Cl-] increases in the adult and this most likely occurs because of entry of extracellular Cl- into the cytosol and (2) there is a major maturational difference in mechanisms regulating Cl- and Na+ homeostasis between newborn and adult brain tissue. We speculate that these mechanisms may account, at least partially, for the relative tolerance to anoxia in the newly born.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bührle C. P., Sonnhof U. Intracellular ion activities and equilibrium potentials in motoneurones and glia cells of the frog spinal cord. Pflugers Arch. 1983 Feb;396(2):144–153. doi: 10.1007/BF00615519. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990 Aug;10(8):2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985 Aug 5;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Dietzel I., Heinemann U., Hofmeier G., Lux H. D. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40(4):432–439. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- Goldberg W. J., Kadingo R. M., Barrett J. N. Effects of ischemia-like conditions on cultured neurons: protection by low Na+, low Ca2+ solutions. J Neurosci. 1986 Nov;6(11):3144–3151. doi: 10.1523/JNEUROSCI.06-11-03144.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F., Getting P. A. Biophysical properties of hypoglossal neurons in vitro: intracellular studies in adult and neonatal rats. J Appl Physiol (1985) 1990 Oct;69(4):1509–1517. doi: 10.1152/jappl.1990.69.4.1509. [DOI] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol. 1990 Oct;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Getting P. A. Repetitive firing properties of neurons in the ventral region of nucleus tractus solitarius. In vitro studies in adult and neonatal rat. J Neurophysiol. 1989 Dec;62(6):1213–1224. doi: 10.1152/jn.1989.62.6.1213. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Olsen C. E. Brain extracellular space during spreading depression and ischemia. Acta Physiol Scand. 1980 Apr;108(4):355–365. doi: 10.1111/j.1748-1716.1980.tb06544.x. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand. 1981 Dec;113(4):437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Hartley D. M., Choi D. W. Delayed rescue of N-methyl-D-aspartate receptor-mediated neuronal injury in cortical culture. J Pharmacol Exp Ther. 1989 Aug;250(2):752–758. [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991 Jul;66(1):103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Jiang C., Xia Y., Haddad G. G. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol. 1992 Mar;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Mares P., Kríz N., Brozek G., Bures J. Anoxic changes of extracellular potassium concentration in the cerebral cortex of young rats. Exp Neurol. 1976 Oct;53(1):12–20. doi: 10.1016/0014-4886(76)90277-6. [DOI] [PubMed] [Google Scholar]
- Meyer F. B. Calcium, neuronal hyperexcitability and ischemic injury. Brain Res Brain Res Rev. 1989 Jul-Sep;14(3):227–243. doi: 10.1016/0165-0173(89)90002-7. [DOI] [PubMed] [Google Scholar]
- Morris M. E., Leblond J., Agopyan N., Krnjević K. Temperature dependence of extracellular ionic changes evoked by anoxia in hippocampal slices. J Neurophysiol. 1991 Feb;65(2):157–167. doi: 10.1152/jn.1991.65.2.157. [DOI] [PubMed] [Google Scholar]
- Nakaya H., Hattori Y., Tohse N., Shida S., Kanno M. Beta-adrenoceptor-mediated depolarization of the resting membrane in guinea-pig papillary muscles: changes in intracellular Na+, K+ and Cl- activities. Pflugers Arch. 1990 Oct;417(2):185–193. doi: 10.1007/BF00370698. [DOI] [PubMed] [Google Scholar]
- Phillips J. M., Nicholson C. Anion permeability in spreading depression investigated with ion-sensitive microelectrodes. Brain Res. 1979 Sep 21;173(3):567–571. doi: 10.1016/0006-8993(79)90254-3. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Yamate C. L., Connors B. W. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci. 1985 Feb;5(2):532–535. doi: 10.1523/JNEUROSCI.05-02-00532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. M. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985 Jun;5(6):1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B. K., Bengtsson F., Grampp W., Theander S. Calcium, excitotoxins, and neuronal death in the brain. Ann N Y Acad Sci. 1989;568:234–251. doi: 10.1111/j.1749-6632.1989.tb12513.x. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]


