Abstract
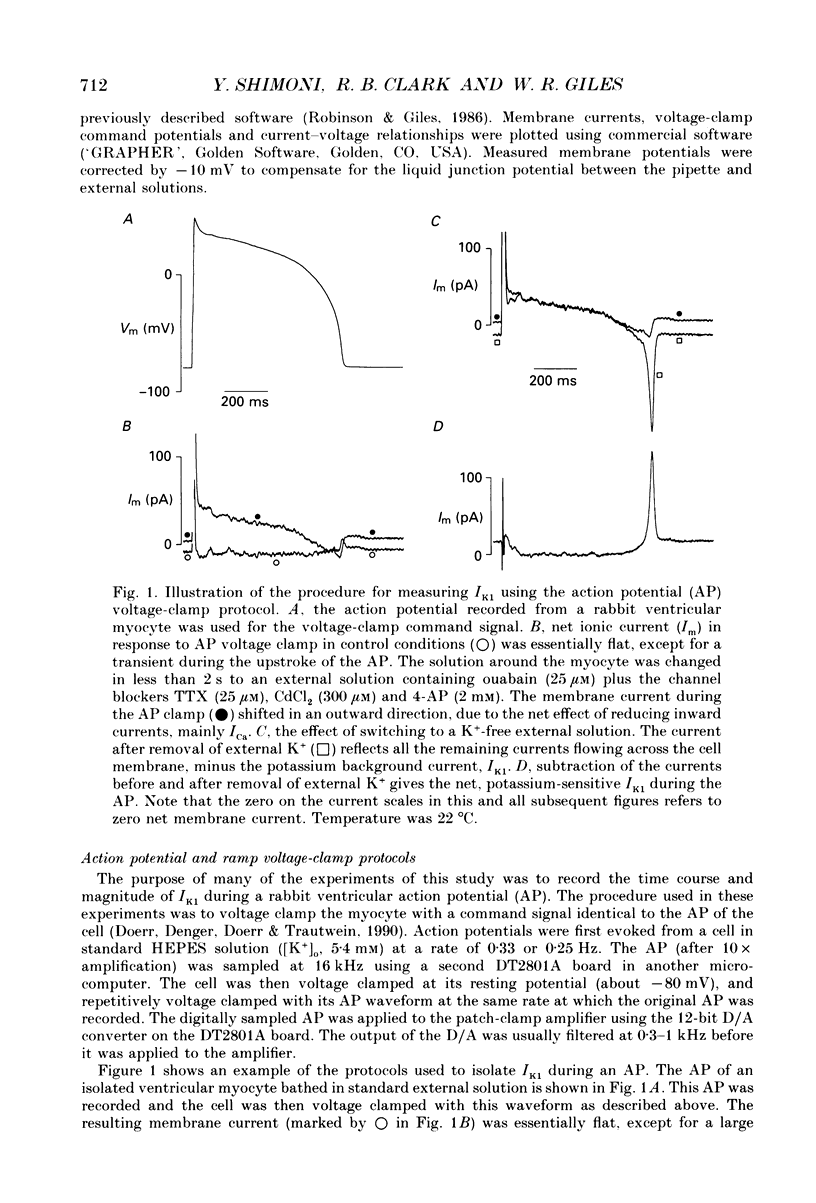
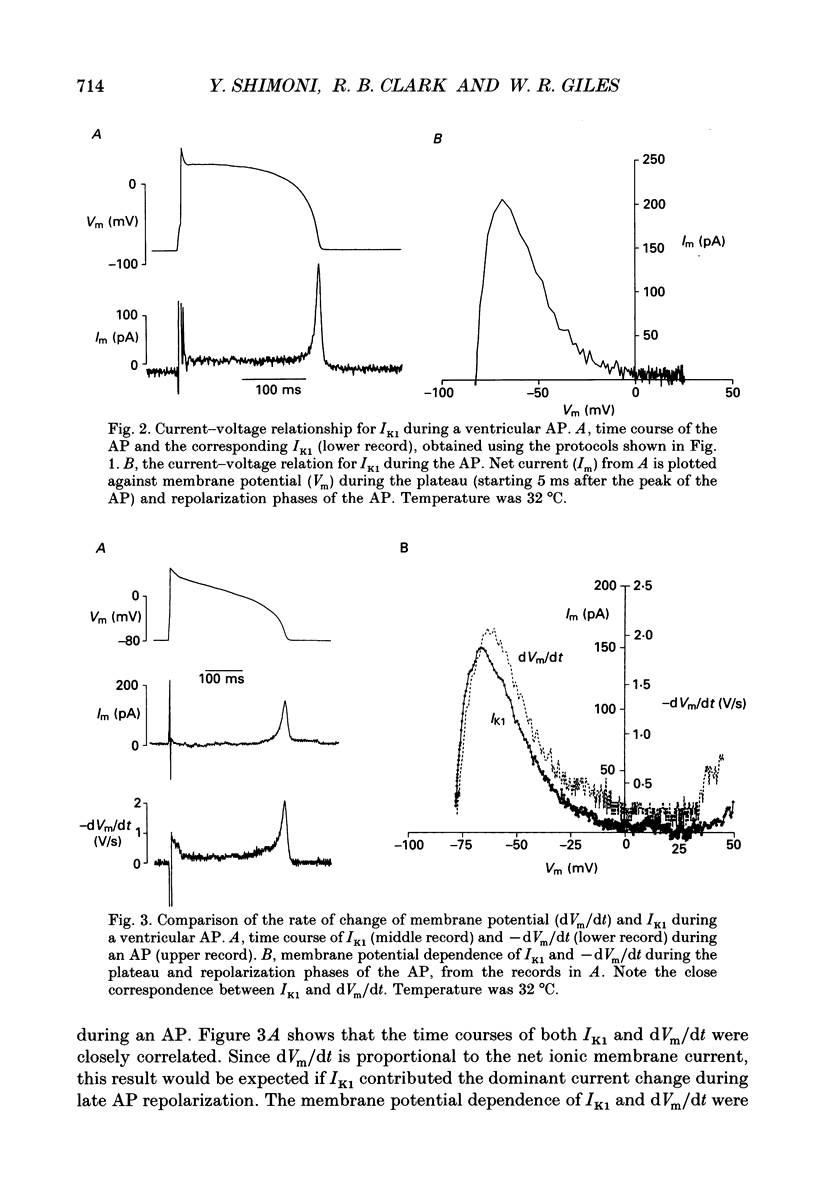
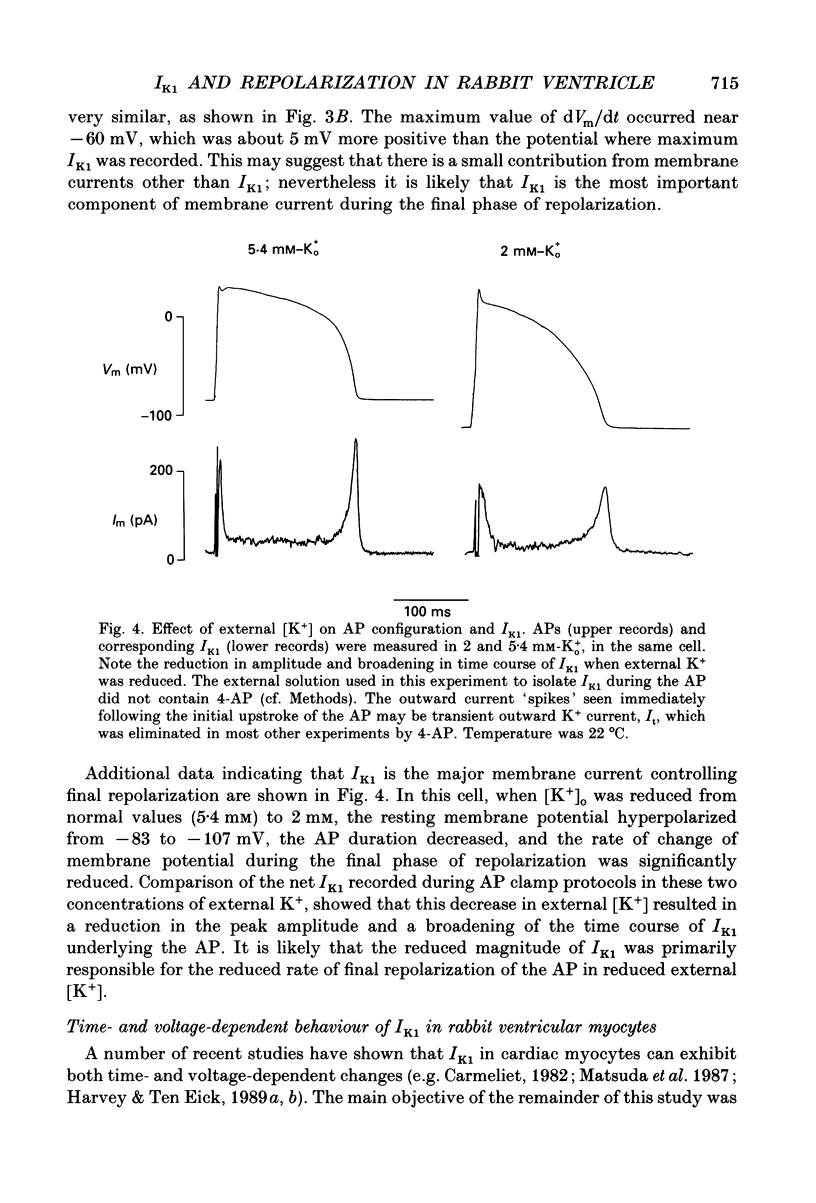
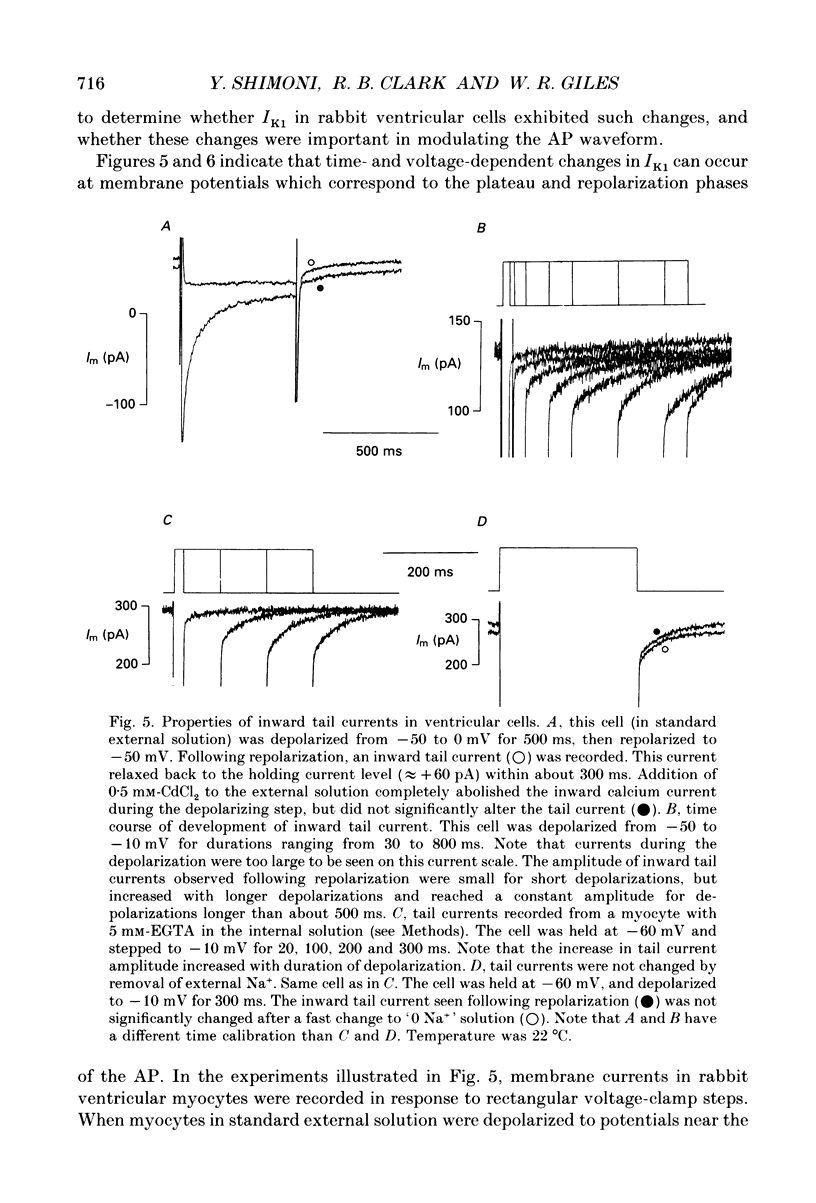
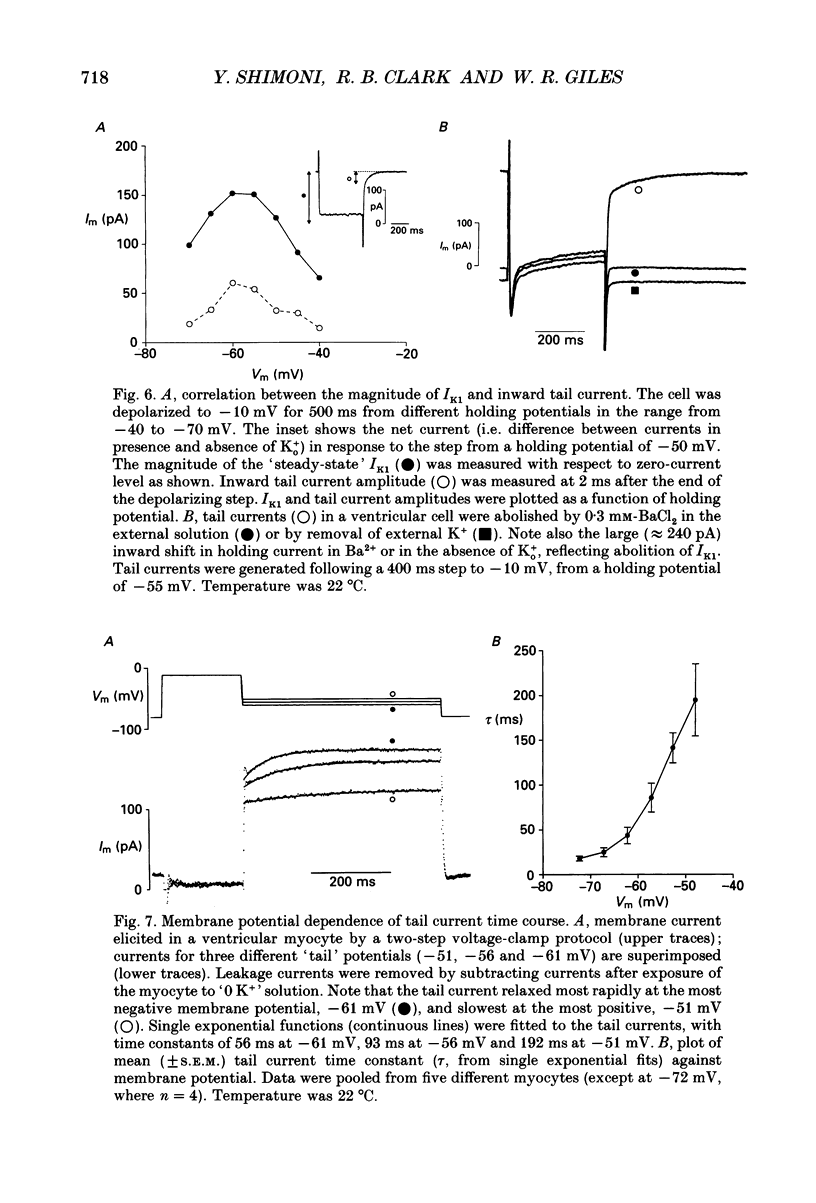
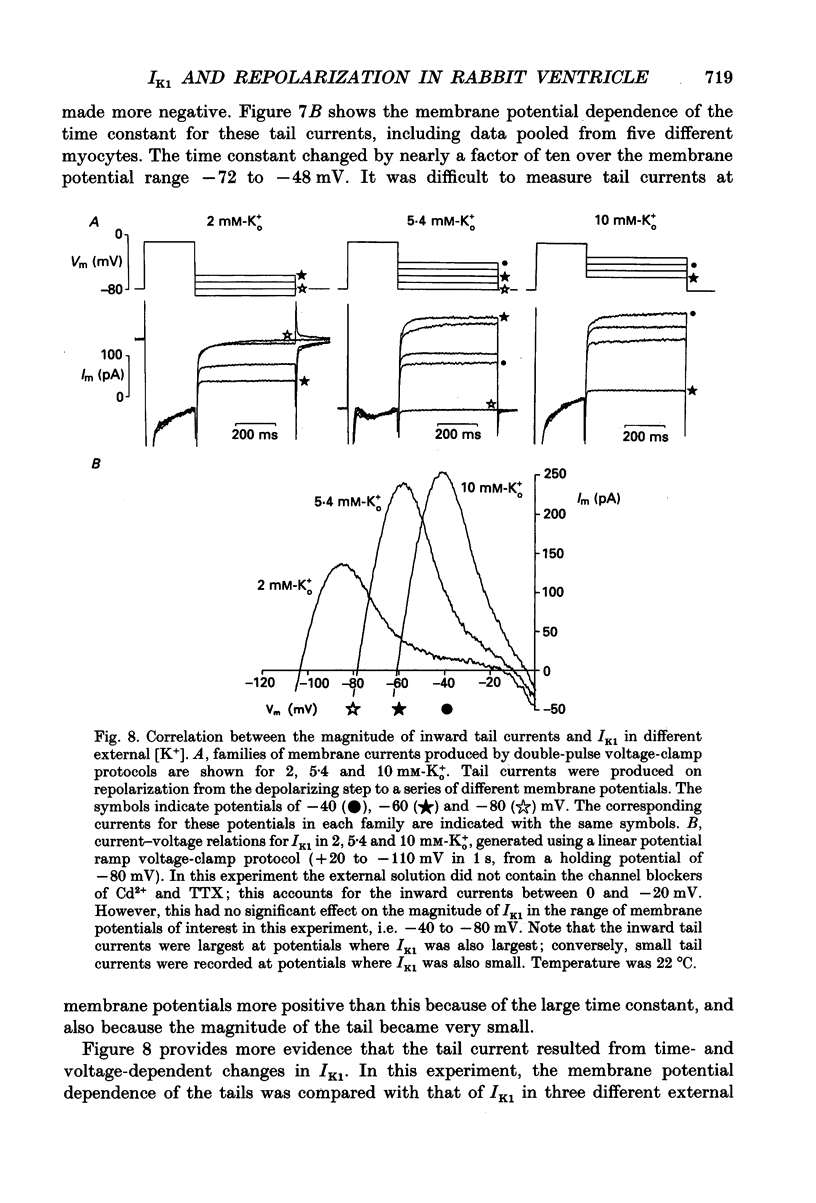
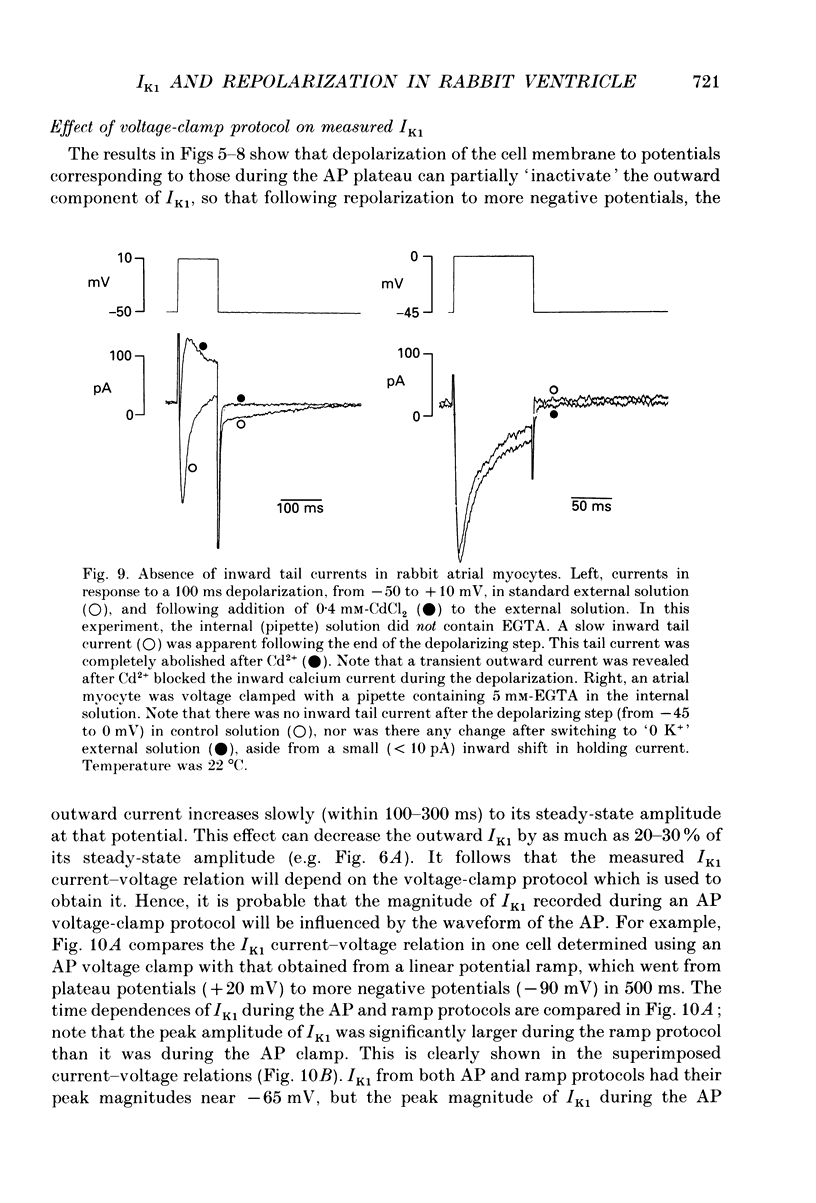
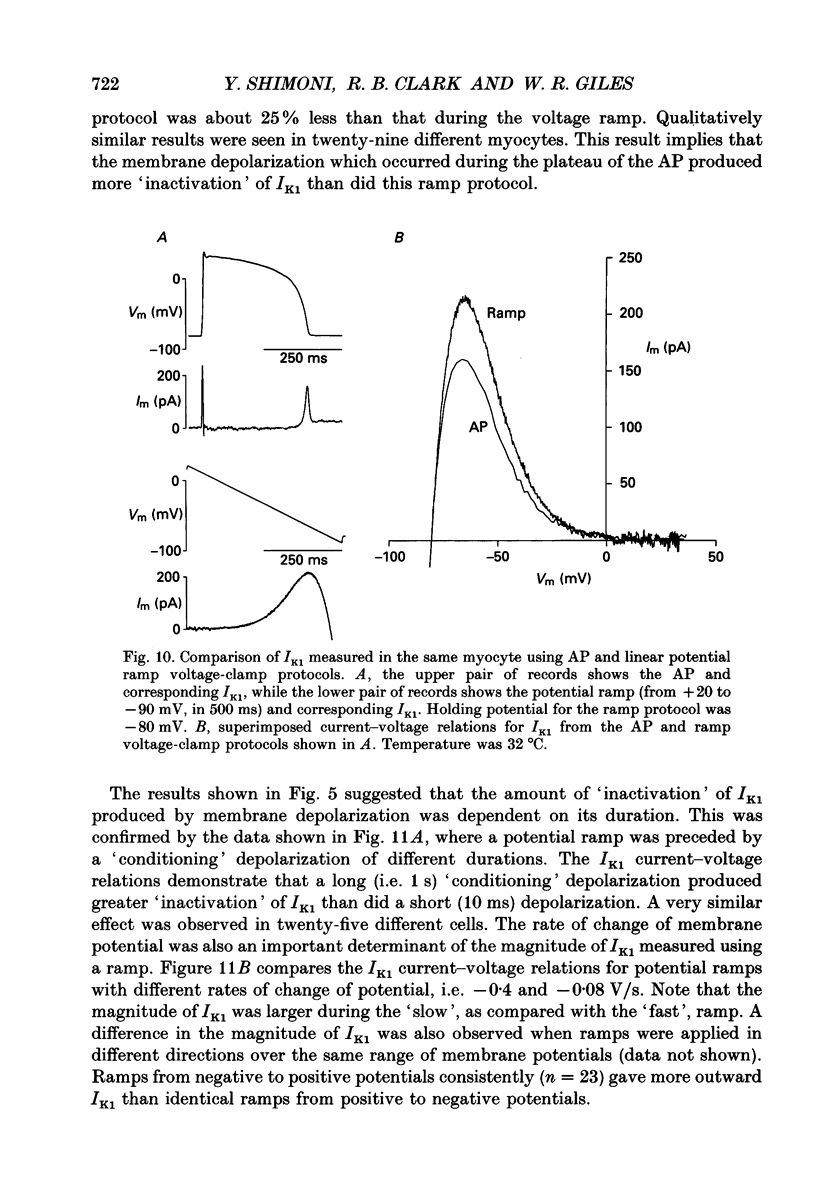
1. Whole-cell voltage-clamp measurements were made of the time- and voltage-dependent properties of the inwardly rectifying background potassium current IK1, in single myocytes from rabbit ventricle. The main goal of these experiments was to define the role of IK1 in the plateau and repolarization phases of the action potential (AP). 2. Action potentials from single ventricular myocytes were used as the command signals for voltage-clamp measurements. In these 'action potential voltage-clamp' experiments, IK1 was isolated from other membrane currents by taking the difference between control currents and currents in K(+)-free bathing solution. The results show that IK1 is small during the plateau, but then rapidly increases during repolarization and declines in early diastole. 3. Evidence of an important functional role for IK1 in AP repolarization was obtained by comparing the magnitude of IK1 and the rate of change of membrane potential (dVm/dt) in the same cell during the AP. The time courses of IK1 and dVm/dt during the AP were closely correlated, indicating that IK1 was the principal current responsible for final repolarization. 4. Rectangular voltage-clamp steps were used to study time- and voltage-dependent changes in IK1 at membrane potentials corresponding to the repolarization phase of the AP. 'Slow' relaxations or tail currents, lasting 100-300 ms, were consistently recorded when the cell was repolarized to potentials in the range -30 to -70 mV, following depolarizations between +10 and -10 mV. 5. The close correlation between the magnitude of the steady-state IK1 (in an external K+ concentration of 5.4 mM), which was outward for membrane potentials in the range -30 to -70 mV, and the magnitude of the tail currents, suggests that they resulted from a slow increase, or reactivation, of IK1. 6. The component of the slow tails due to reactivation of IK1 can be separated from a previously described component due to Na(+)-Ca2+ exchange since the IK1 component: (i) does not depend on the presence of the calcium current, ICa; (ii) can be recorded when internal EGTA (5 mM) suppresses large changes in [Ca2+]i; (iii) does not depend on the Na+ electrochemical gradient; (iv) is abolished in K(+)-free external solution; and (v) is not present in rabbit atrial myocytes, in which IK1 is very small. 7. The time- and voltage-dependent properties of IK1 revealed by these tail current experiments suggest that the measured magnitude of IK1 will be dependent on the voltage-clamp protocol.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumonwo J. M., Delmar M., Jalife J. Electrophysiology of single heart cells from the rabbit tricuspid valve. J Physiol. 1990 Jun;425:145–167. doi: 10.1113/jphysiol.1990.sp018097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Induction and removal of inward-going rectification in sheep cardiac Purkinje fibres. J Physiol. 1982 Jun;327:285–308. doi: 10.1113/jphysiol.1982.sp014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Visentin S. Barium-induced blockade of the inward rectifier in calf Purkinje fibres. Pflugers Arch. 1984 Dec;402(4):446–453. doi: 10.1007/BF00583946. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Doerr T., Denger R., Doerr A., Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch. 1990 May;416(3):230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Earm Y. E., Ho W. K., So I. S. Inward current generated by Na-Ca exchange during the action potential in single atrial cells of the rabbit. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):61–81. doi: 10.1098/rspb.1990.0027. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Shimoni Y. Slow inward tail currents in rabbit cardiac cells. J Physiol. 1989 Oct;417:447–463. doi: 10.1113/jphysiol.1989.sp017812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol. 1988 Apr;91(4):593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. On the role of sodium ions in the regulation of the inward-rectifying potassium conductance in cat ventricular myocytes. J Gen Physiol. 1989 Aug;94(2):329–348. doi: 10.1085/jgp.94.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Voltage-dependent block of cardiac inward-rectifying potassium current by monovalent cations. J Gen Physiol. 1989 Aug;94(2):349–361. doi: 10.1085/jgp.94.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. The interaction of potassium with the activation of anomalous rectification in frog muscle membrane. J Physiol. 1981 Aug;317:497–508. doi: 10.1113/jphysiol.1981.sp013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K., Mitsuiye T., Noma A., Takano M. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol. 1989 Dec;419:297–320. doi: 10.1113/jphysiol.1989.sp017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R. Properties of inwardly rectifying K+ channels in ventricular myocytes. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):21–26. doi: 10.1007/BF00231000. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Magnesium gating of the inwardly rectifying K+ channel. Annu Rev Physiol. 1991;53:289–298. doi: 10.1146/annurev.ph.53.030191.001445. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca modulates outward current through IK1 channels. J Membr Biol. 1990 Jun;116(1):41–45. doi: 10.1007/BF01871670. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DiFrancesco D. Intracellular Ca modulates K-inward rectification in cardiac myocytes. Pflugers Arch. 1989 Jan;413(3):322–324. doi: 10.1007/BF00583549. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Pennefather P. The mechanism of rectification of iK1 in canine Purkinje myocytes. J Gen Physiol. 1990 Aug;96(2):299–318. doi: 10.1085/jgp.96.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K., Giles W. A data acquisition, display and plotting program for the IBM PC. Comput Methods Programs Biomed. 1986 Dec;23(3):319–327. doi: 10.1016/0169-2607(86)90067-2. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Inward rectification in skeletal muscle: a blocking particle model. Pflugers Arch. 1978 Dec 28;378(2):173–176. doi: 10.1007/BF00584452. [DOI] [PubMed] [Google Scholar]
- Toyotomi S., Momose Y. Temperature-controlled perfusion apparatus for microscope using transparent conducting film heater. Am J Physiol. 1989 Jan;256(1 Pt 1):C214–C217. doi: 10.1152/ajpcell.1989.256.1.C214. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]


