Abstract
Plant growth-promoting bacteria (PGPB) are considered an effective eco-friendly biostimulator. However, relatively few studies have examined how PGPB affect the native bacterial community of major crops. Thus, this study investigates the impact of a PGPB consortium, comprising Pseudomonas sp. G31 and Azotobacter sp. PBC2 (P1A), on the soil bacterial community of wheat under field conditions. As a result of PGPB application, we observed a significant increase in seed yield, as well as in nitrate content (1st and 3rd time points) and available phosphorus (2nd time point) in the rhizosphere compared to control. For the metataxonomic study, Next-Generation Sequencing was performed using the Illumina NovaSeq 6000 system. The consortium used did not have a significant impact on the diversity of native soil bacteria and slightly affected the taxonomic composition of bacteria with no significant changes in bacterial dominants at the phylum and genus level. Nevertheless, 3 weeks after application, P1A increased the relative abundance of Nitrospira which could have influenced the increase in nitrates in the rhizosphere, and also decreased Bdellovibrio. The results indicate that the P1A consortium, due to its ability to promote plant growth without detrimental alternations in the bacterial community of the soil, may be a potential candidate for commercialization.
Keywords: Biostimulator, Native bacteria, Sustainable agriculture
Subject terms: Applied microbiology, Environmental microbiology, Microbial ecology, Agroecology, Microbial ecology
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops (along with rice and maize), used for food, animal feed, and industrial raw materials, with global production exceeding 780 million tons1,2. The largest wheat producers in the European Union are Germany, the United Kingdom, France, Romania, and Poland. For instance, in 2023, wheat was grown on 2.4 million hectares in Poland, providing production of 13.0 million tons1,2.
The ever-increasing human population contributes to the continuous intensification of agriculture, resulting in, among other things, an increase in the need for inorganic fertilizers that are harmful to the agricultural environment3–6. The long-term use of inorganic fertilizers has been proven to cause soil acidification, a decline in soil quality, and a decrease in soil microbial diversity7–9. Therefore, in order to balance the intensification of agriculture with the need to feed growing populations, a number of new solutions should be implemented, including increasing stress tolerance, improving nutrient use efficiency, or introducing effective biofertilizers, the wider use of which will help to reduce the use of mineral fertilizers. One solution to reducing mineral fertilization may be plant growth-promoting bacteria (PGPB) whose importance in agriculture is growing every year. Plant growth-promoting bacteria (PGPB) enhance plant growth through both direct and indirect mechanisms. Directly, they secrete phytohormones such as indole-3-acetic acid (IAA), which stimulates root development, and gibberellins, which promote cell elongation and seed germination. PGPB also improve nutrient availability by fixing atmospheric nitrogen into plant-usable forms and by solubilizing phosphorus and potassium, releasing these essential nutrients from insoluble soil compounds. Indirectly, PGPB protect plants by suppressing pathogens through the secretion of antibiotic substances, including cyclic lipopeptides, and hydrolytic enzymes, such as chitinase and cellulase, which degrade the cell walls of harmful fungi and other pathogens. Additionally, they support plant resilience by triggering Induced Systemic Resistance (ISR), priming the plant’s immune system to better withstand biotic and abiotic stresses. PGPB also play a critical role in the detoxification of heavy metals in agricultural soils through mechanisms such as bioaccumulation, bioprecipitation, and biotransformation. These attributes make PGPB indispensable in sustainable agriculture, as they not only enhance crop yields and reduce reliance on chemical fertilizers but also improve plant resistance to diseases and contribute to bioremediation efforts10–15. Bacteria that stimulate plant growth include, for instance, bacteria of the following genera: Azotobacter, Azospirillum, Bacillus, Paenibacillus, Pseudomonas, and Serratia16,17.
So far, numerous studies have been conducted on wheat and other cereal crops, demonstrating the growth-stimulating effect of beneficial bacteria18–21. However, despite the large number of reports and the ever-increasing number of new biopreparations with PGPB, there are still relatively few studies showing how PGPB affects the native microbiota of inoculated soils and plants. As proven, in this case, the rhizosphere microbiota is particularly important and plays a key role in maintaining the plant health and growth and the development of the plant22,23. Hence, major changes in biodiversity and the bacterial community structure of the native rhizosphere microbiota can have a negative impact on plant health and proper plant development24,25. For instance, shifts in the abundance of dominant bacterial taxa can significantly influence soil biochemical processes, potentially leading to adverse effects on soil functioning26,27. One particularly critical factor is the decline in taxa belonging to the phyla Proteobacteria and Actinobacteriota. These phyla often constitute the core of bacterial communities and include numerous species involved in carbon and nitrogen cycling28,29. Specifically, they encompass microorganisms capable of decomposing biopolymers such as cellulose and chitin, as well as nitrifying and ammonifying bacteria28,30,31. In addition, a decline in bacteria from the phylum Acidobacteriota represents another significant adverse change. These bacteria are predominantly oligotrophic microorganisms, and their reduced abundance may disrupt soil homeostasis29,32. Moreover, the vast majority of studies on the effects of PGPB on the native microbiota are carried out only once during the growing season, and only a few reports show results at different stages of plant development33,34. Such an approach should be implemented on a regular basis due to the seasonal variability in the composition of rhizodeposits, including root exudates, which affects both the native microbiota and the inoculants used.
Therefore, our study aims to evaluate the impact of the PGPB consortium consisting of Pseudomonas sp. G31 and Azotobacter sp. PBC2 on the native bacterial community of the wheat rhizosphere and bulk soil.
Results
Identification of Pseudomonas sp. G31 and Azotobacter sp. PBC2 and their plant growth promoting traits
Bacterial strains were identified by sequencing 16S rRNA gene; using BLAST, sequences (contigs) of strain G31 were assigned to the genus Pseudomonas and of strain PBC2 to the genus Azotobacter. After BLAST, the 16S rRNA gene sequences of studied bacteria strains have been deposited in GenBank under accession numbers: PP499654 (Pseudomonas sp. G31) and PP500530 (Azotobacter sp. PBC2) (Table 1).
Table 1.
Bacterial isolates with the closest related strains from the NCBI database.
| Species | Strain name | Query cover (%) | Percent identity (%) | Accession length | Accession number |
|---|---|---|---|---|---|
| Pseudomonas sp. G31 | |||||
| Pseudomonas helmanticensis | OHA11 | 100 | 99.88 | 1472 | NR_126220.1 |
| Pseudomonas rustica | MBT-4 | 100 | 99.75 | 1537 | NR_181703.1 |
| Pseudomonas germanica | FIT28 | 100 | 99.63 | 1532 | NR_181838.1 |
| Pseudomonas atagonensis | PS14 | 100 | 99.63 | 1460 | NR_180743.1 |
| Pseudomonas crudilactis | UCMA 17988 | 100 | 99.63 | 1525 | NR_179985.1 |
| Azotobacter sp. PBC2 | |||||
| Azotobacter chroococcum | IAM 12666 | 97 | 96.94 | 1430 | NR_041035.1 |
| Azotobacter chroococcum | NBRC 102613 | 98 | 96.90 | 1461 | NR_114167.1 |
| Azotobacter chroococcum | LMG 8756 | 98 | 96.83 | 1501 | NR_116305.1 |
| Azotobacter beijerinckii | ICMP 8673 | 93 | 95.86 | 1384 | NR_042071.1 |
| Azotobacter bryophylli | L461 16S | 96 | 95.16 | 1392 | NR_179675.1 |
Importantly, the bacterial strains did not exhibit antagonistic interactions with each other. The isolates were capable of producing IAA on LB medium supplemented with tryptophan, Pseudomonas sp. G31 and Azotobacter sp. PBC2. Strain Azotobacter sp. PBC2 is capable of nitrogen fixation. Additionally, Pseudomonas sp. G31 demonstrated phosphorus solubilizing properties. However, neither strain showed the ability to solubilize zinc or exhibit cellulolytic activity (CMCase), as shown in Table 2.
Table 2.
PGP traits of studied bacterial strains
| Traits | Pseudomonas sp. G31 | Azotobacter sp. PBC2 |
|---|---|---|
| IAA production | 95.4 µg mL−1 | 43.6 µg mL−1 |
| Nitrogen fixation | − | + |
| P solubilization | + | − |
| Zn solubilization | − | − |
| CMCase | − | − |
Biometric measurements of plants
After harvesting the plants, several biometric parameters were determined. There were no differences in the inoculated samples in terms of root length or length of the aboveground part. In terms of shoot weight (per m2) and seed yield (per m2), higher values were observed in inoculated plants than in control plants by 12.69% and 27.14%, respectively. However, statistically significant values were recorded only for seed yield, where it was 415.75g for the control and 528.57 for P1A (Table 3).
Table 3.
Biometric traits of inoculated and control plants.
| Treatment | Root length (cm) | Root mass (g) | Shoot length (cm) | Shoot yield (g) | Seed yield (g) |
|---|---|---|---|---|---|
| C | 18.67a | 101.2a | 156.57a | 1573.67a | 415.75a |
| P1A | 18.36a | 113.97a | 156.98a | 1773.3a | 528.57b |
C, control; P1A, consortium application (Pseudomonas sp. G31 and Azotobacter sp. PBC2)
Means in columns with the same letter do not differ significantly at p < 0.05 in Tukey’s HSD test.
Physico-chemical analysis of the soil
Soil properties rarely differed significantly. The observed pH values were: 6.76–7.2 in bulk soil and 6.77–6.9 in rhizosphere soil (Table 4). Significant difference in pH values was observed only at the first time point, when a significantly higher value was recorded in the control compared to the inoculated soil. In terms of nitrate nitrogen (N-NO3), higher values were observed in inoculated soils. However, significantly higher values were recorded only in the rhizosphere soil at first and third time points, when values in inoculated samples were higher than in control. In most cases, the differences in AP content were in favor of the inoculated soils. However, significantly higher values were recorded only at the second time point, when the AP content in the inoculated soil (48.03 mg kg−1) was higher than in the control soil (40.23 mg kg−1). In contrast, for TN and TOC, there were no significant differences between samples.
Table 4.
Physico-chemical properties of bulk soil and rhizosphere
| Time point | Treatment | pH | N-NO3 mg kg−1 | AP mg kg−1 | TN mg kg−1 | TOC mg kg−1 |
|---|---|---|---|---|---|---|
| Bulk soil | ||||||
| I | C.WB | 7.2b | 4.3a | 19.73a | > 0.1a | 1.31a |
| P1.WB | 6.99a | 11.2a | 22.04a | > 0.1a | 1.16a | |
| II | C.WB.2 | 6.76a | 6.73a | 17.90a | > 0.1a | 1.11a |
| P1A.WB.2 | 6.87a | 5.93a | 16.75a | > 0.1a | 1.15a | |
| III | CW.B.3 | 7.02a | 3.3a | 9.23a | > 0.1a | 1.15a |
| P1A.WB.3 | 6.89a | 5.2a | 12.55a | > 0.1a | 1.35a | |
| Rhizosphere | ||||||
| I | C.WR | 6.9a | 1.13a | 40.23a | > 0.1a | 1.0a |
| P1A.WR | 6.77a | 3.7b | 42.8a | > 0.1a | 1.03a | |
| II | C.WR.2 | 6.8a | 1.47a | 40.23a | > 0.1a | 1.05a |
| P1A.WR.2 | 6.9a | 2.67a | 48.03b | > 0.1a | 1.11a | |
| III | C.WR.3 | 6.9a | 2.03a | 48a | > 0.1a | 1.05a |
| P1A.WR.3 | 6.83a | 3.43b | 49.67a | > 0.1a | 1.05a | |
C, control; P1A, consortium application Pseudomonas sp. G31 and Azotobacter sp. PBC2
AP, available phosphorus; TN, total nitrogen; TC, total carbon
Means in columns with the same letter do not differ significantly at p < 0.05 in Tukey’s HSD test.
Soil bacterial communities
Alfa-diversity
At the first study point time, i.e. three weeks after consortium application, there was a minimally lower biological diversity (Shannon—10.39) and species richness (Chao1—3136.6 and observed features—3066) in P1A rhizosphere compared to the control rhizosphere; however, the differences were not statistically significant and, in addition, the Simpson index (P1A—0.997; C—0.998) showed almost no difference (Table 5). However, in terms of bulk soil, the slightly higher values of Shannon (10.46) and Chao1 (3263.0) indices were noted in inoculated soil compared to control (Shannon—10.38; Chao1—2918); the differences were also not significant. The soil taken in the middle of the growing season (plant stage: 39 BBCH) and at harvest also showed no significant differences in Chao1 and diversity indices between the studied treatments. However, a noticeable decrease in Chao1 and values of other studied features was observed at the second and third time point compared to the first time point (Table 5).
Table 5.
Alfa-diversity of bacterial community: C.WR, control rhizosphere soil; P1A.WR, rhizosphere inoculated with P1A; C.WB, control bulk soil; P1A.WB, bulk soil inoculated with P1A.
| Time point | Treatment | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| I | C.WR | 3436.5a | 10.58a | 0.998a |
| P1A.WR | 3136.6a | 10.39a | 0.997a | |
| C.WB | 2918.6a | 10.38a | 0.998a | |
| P1.WB | 3263.0a | 10.46a | 0.998a | |
| II | C.WR.2 | 2058.7a | 10.02a | 0.997a |
| P1A.WR.2 | 2282.4a | 10.036a | 0.997a | |
| C.WB.2 | 2234.4a | 10.001a | 0.997a | |
| P1A.WB.2 | 2313.0a | 9.836a | 0.993a | |
| III | C.WR.3 | 2303.4a | 10.017a | 0.997a |
| P1A.WR.3 | 2299.6a | 9.875a | 0.990a | |
| CW.B.3 | 2623.9a | 10.411a | 0.998a | |
| P1A.WB.3 | 2506.2a | 10.437a | 0.999a |
Means in columns with the same letter do not differ significantly at p < 0.05 in Tukey’s HSD test.
Beta-diversity
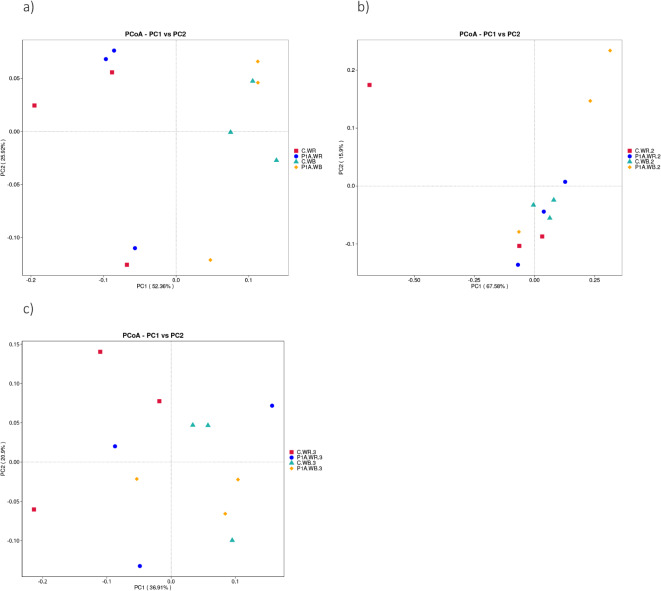
At the first time point PCoA analysis using the weighted unifrac algorithm showed a moderate clustering of bacterial communities from the non-rhizosphere soil, with one sample inoculated with P1A being clearly distinct from the others along PC2 axis, which explains 25.92% of the variance (Fig 1a). While a clustering of two P1A.WR samples was observed in the rhizosphere soil, the other sample from the same treatment was clearly isolated. At the second time point, three samples from the non-rhizosphere control soil formed a cluster, and two rhizosphere control samples were also located next to each other (Fig 1b). However, samples from the inoculated soil were relatively more heterogeneous, as shown on the PCoA plot by smaller or larger distance between samples. At the third time point, similarly as before, the non-rhizospheric control samples formed a cluster and the rest of the samples in individual treatments was relatively variable (Fig 1c). Interestingly, at each time point, one sample from the rhizosphere control differed significantly from the other two from the treatment.
Fig. 1.
PCoA of weighted UniFrac distances of the bacterial community of rhizospheric soil in different treatments: C.WR, control rhizosphere soil; P1A.WR, rhizosphere inoculated with P1A; C.WB, control bulk soil; P1A.WB, bulk soil inoculated with P1A; a, first time point; b, second time point; c, time point.
Based on the PCoA analysis, greater variation was observed in the inoculated and rhizosphere soil samples compared to the control samples from bulk soil.
Structure of bacterial communities
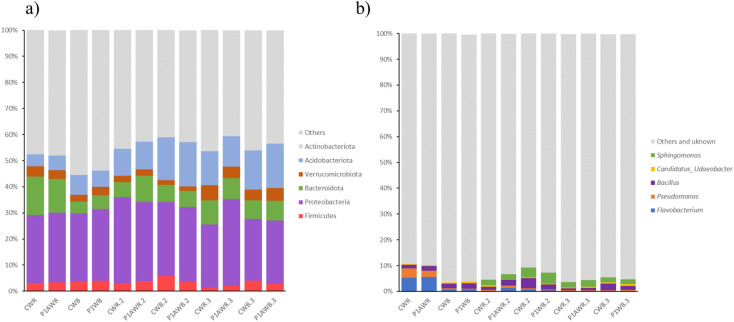
Analysis of the bacterial community response to the consortium application was conducted by sequencing the V3–V4 regions of the genes encoding 16S rRNA, resulting in 3,100,501 raw sequences (1st time point—1,510,310; 2nd time point—806,708; 3rd time point—783,483). After the removal of chimeras and further bioinformatics processing, including annotation of sequences according to the SILVA database, we identified 6 dominant bacterial phyla in the soil samples. In most samples, among the dominant phyla, Proteobacteria (23.39–33.17%), Actinobacteriota 12.78–23.46%), Acidobacteriota (4.6–17%), Bacteroidota (4.57–14.72%), Firmicutes (1.23–5.88%), and Verrucomicrobiota (1.67–5.78%) were present. These dominant phyla accounted for more than 50% of the bacterial community (Fig. 2). Three weeks (first time point) after the consortium application, a slight increase in the relative abundance of phylum Proteobacteria was observed in both rhizosphere and non-rhizosphere soil, although this increase was not statistically significant (Fig. 2, Supplement; Table 1). For the other dominant phyla, no statistically significant differences were found between the inoculated soil and control samples. However, significant differences were detected between the control samples, with significantly more sequences belonging to Bacteroidota (up to 10%) in the CWR than the CWB. This pattern persisted at later dates but without statistically significant differences. Interestingly, Acidobacteriota were consistently more abundant in bulk soil samples compared to the rhizosphere, with the differences being significant only at the third time point (Fig. 2a, Supplement—Table 1).
Fig. 2.
Relative abundance of the dominant bacterial phyla and genera in different treatments: C.WR, control rhizosphere soil; P1A.WR, rhizosphere inoculated with P1A; C.WB, control bulk soil; P1A.WB, bulk soil inoculated with P1A; 1—first time point, 2—second time point, 3—third time point.
In terms of the bacterial community at the genus level, the dominant genera in the soils studied were as follows: at the 1st time point—Flavobacterium (0.60–5.65%), Pseudomonas (0.3–3.62%), and Bacillus (1.45–2.09%); at the 2nd time point—Bacillus (1.09–3.91%), Pseudomonas (0.42–0.93%), Sphingomonas (1.9–4.28%), and Flavobacterium (0.21–1.33%); and at the 3rd time point—Sphingomonas (1.83–2.58%), Bacillus (0.76–2.57%), and Pseudomonas (0.37–0.64%) (Fig. 2b). Sequences classified as ‘others’ accounted for between 88.51 and 96.14% of the amplicons. As with the phyla, differences in relative abundance of dominant bacterial genera were statistically insignificant (Fig. 3, Supplement—Table 2).
Fig. 3.
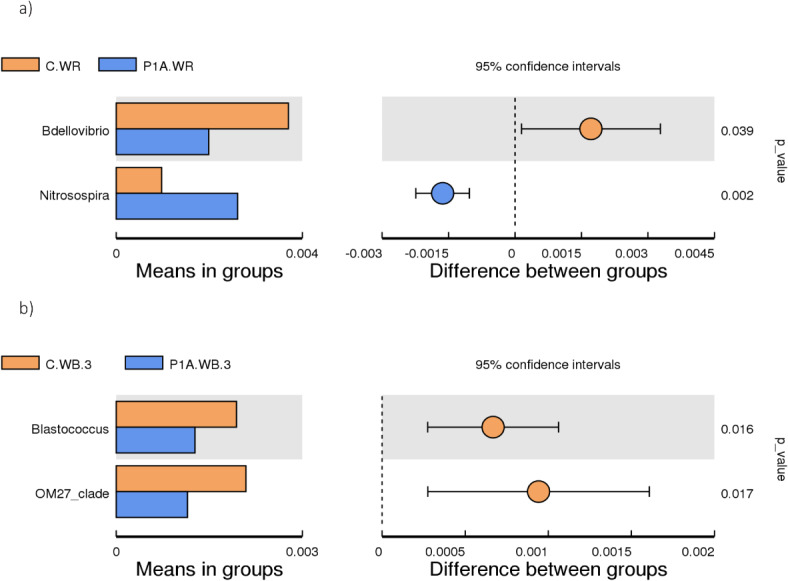
T-test analysis of the rhizosphere bacterial community between the following treatments: C.WR, control rhizosphere soil; P1A.WR, rhizosphere inoculated with P1A; C.WB, control bulk soil; P1A.WB, bulk soil inoculated with P1A; a, first time point; b, third time point (only first time point and third time point; there were no significant differences at the second time point).
The LEfS analysis, intended to detect bioindicators, also did not show statistically significant differences between the studied samples. Nevertheless, for non-dominant bacteria, the t-test detected a significant increase in Nitrospira and a decrease in Bdellovibrio in the inoculated soil compared to the control (Fig. 3a). At the second time point, no significant differences were detected by the t-test, while at the third time point, it revealed a significantly higher abundance of the genera Blastococcus and OM27_clade in the control compared to the inoculated non-rhizosphere samples. These results were also confirmed by the MetaStat (Supplement—Fig. 1).
Discussion
Plant growth-promoting bacteria (PGPB) can be a robust alternative or supplement to mineral fertilizers. In recent years, there has been an increasing amount of research on this group of bacteria under field conditions. To date, several reports have documented the use of PGPB in wheat cultivation; however, these studies differ in terms of the bacterial strain used, soil type, climatic conditions, and wheat variety33,35,36. Therefore, the more studies on different PGPB in wheat cultivation, the more data there will be to establish patterns. This increasing body of research will help in identifying the most effective bacterial strains and conditions for enhancing wheat growth and yield.
IAA-producing, phosphate-solubilising and atmospheric nitrogen-fixing bacterial strains were selected for the study. The experiment with the application of the consortium of Pseudomonas sp. G31 and Azotobacter sp. PBC2 under field conditions was conducted on Luvisol in a temperate climate. The bacteria introduced into the soil had an effect on plant length, root mass, and seed yield. Previously, similar effects after PGPB application were also reported in wheat (field conditions) following the application of plant growth-promoting bacteria34,37,38. For instance, Kumar et al.38, after using Bacillus megaterium, Arthrobacter chlorophenolicus and a consortium of B. megaterium, A. chlorophenolicus and Enterobacter sp., reported significantly higher values in wheat grain yield than in inoculated wheat in a field experiment (Bhadohi district, Uttar Pradesh, India). The strains studied by the authors also increased plant height, for example, significantly higher values were recorded for B. megaterium and the consortium containing all strains, while for A. chlorophenolicus similarly to our study recorded no statistical differences. Other authors, Chen et al.34 studied several consortia stimulating plant growth in wheat cultivation (Jining City, China). The research revealed that two consortia, one with B. subtilis and Paenibacillus polymyxa and the other with B. subtilis and B. licheniformis increased i.a. plant height and grain yield, however significant increase they noted only for the first consortium.
The P1A effects can be explained by the ability of the strains to produce IAA, fix atmospheric nitrogen or solubilise phosphorus, and especially the latter two capabilities, as reflected in the increase in N-NO3 and AP in the inoculated rhizosphere soil. Previously, the effect of PGPB on soil properties from wheat cultivation was reported by the aforementioned34. Regardless of sampling time, two formulations with plant growth-promoting bacteria increased AN compared to the control (irrespective of sampling time). However, they did not considerably increase available phosphorus34. More recently, in other crop cultivation, an increase in both available nitrogen and phosphorus in the maize rhizosphere was detected following the application of Lysobacter antibioticus 13-6; the ability to solubilise phosphorus was also previously detected in this strain39. Interestingly, similar patterns were also obtained by Shi et al.40, who, using Bacillus velezensis YH-18 in a Prunus davidiana experiment, observed a significant increase in available nitrogen and phosphorus in the rhizosphere compared to the control.
The native bacterial community is a major contributor to soil biochemical processes, thus the structure and diversity of native bacteria is an important determinant of soil health. Therefore, as mentioned above, one of the most crucial issues associated with the application of PGPB to soil is maintaining or increasing bacterial diversity and not disturbing the structure of the native bacterial community. However, prompted by the sequencing techniques development, it has only been some time since the effects of PGPB on the native microbiota of inoculated plants and soil began to be studied. To the best of our knowledge, the assessment of the impact of the consortium consisting of two species of genera Pseudomonas and Azotobacter on the bacterial community in winter wheat cultivation under field conditions with three different plant vegetative stages is the first such study to date.
Alpha diversity values between treatments (in both soil) changed slightly over time, but without significant changes. At the first sampling point, a very slight decrease in Chao1 index was observed in the inoculated rhizosphere bacterial community, and an inverse relationship in the non-rhizosphere soil, but subsequent sampling times the index values were already more similar to each other. However, various patterns can be found in the literature. The significant decrease in bacterial biodiversity of cucumber rhizosphere was observed 35 days after the application of B. amyloliquefaciens FH-1 in cucumber cultivation, whereas the inoculant had no effect on the diversity of bacteria in the bulk soil41. Moreover, similar to our results, the highest values of the richness and biodiversity indices regardless of treatment were observed for the first point time and then progressively decreased. The decline in biodiversity during the growing season may be associated with alterations in the composition of nutrients and rhizodeposits including root exudates and has been previously observed by other authors42.
Beta-diversity visualized using PCoA analysis showed different patterns in various time points; a slightly greater differentiation between the bacterial community of the inoculated soils in comparison with non-inoculated soils (in each time point) was observed—this result may indicate that the inoculant changed the bacterial community in each plot slightly differently. These multidirectional effects of PGPB can be explained by the variability of the soil within the experimental field. Previously, divergences between samples from the same treatment were also observed41,43–45. Furthermore, at the first time point, samples from the two soil types differed much more markedly than the same samples from the second time point, which were more similar to each other. These discrepancies may be due to the different factors modulating the microbiota, for example rhizodeposits which change over time and depends on vegetative stages, but it could also have been due to abiotic factors.
According to the previously studies, the phylum dominants in the untreated wheat rhizosphere occur in the following ranges of percentages: Proteobacteria 32–55%, Actinobacteria 7–35%, Acidobacteria 3–15%, Firmicutes 1–3%, Chloroflexi 2–5%, Bacteroidetes 2–11%, Verrucomicrobiota 2–4%33–36. Overall, the results obtained in this study are in the line with the above.
In our study, after application the PGPB, the relative abundance of bacterial dominants at individual time points was generally not significantly different. The results obtained are in line with some previous reports. Khanghahi et al. (2022) reported no significant differences in the relative abundance of the wheat rhizosphere bacterial community (pot experiment) at the phylum level (e.g. in Proteobacteria, Acidobacteria, Actinobacteria or Firmicutes populations) and genus level (Pseudomonas or Sphingomonas) after application of PGPB (consortium: Acinetobacter spp. and Comamonas spp.). On the other hand, depending on the formulation used, Chen et al.34 noted different patterns in the bacterial community of wheat rhizosphere (at the maturity stage, field conditions). After the introduction of unspecified PGPR, the authors observed the lack of shift in bacterial community at phylum level, and a significant increase in the genus Actinoallomurus compared to the control. But in the case of another inoculant called a microbial agent (containing bacteria of the genus Bacillus), they found an increase in the relative abundance of Actinobacteria, Acidobacteria, Chloroflexi and decrease in the abundance of the Proteobacteria and Bacteroidetes in rhizobacterial community of wheat (maturity stage) compared to the control34. Also minor alterations in the bacterial community after PGPR application (bacterial strains from the following genera Azotobacter, Bacillus, Pseudomonas and Rahnella) in wheat cultivation were reported by Graziano et al.36, who, for example, observed a slight decrease in the Proteobacteria and Acidobacteria populations, a decrease in the phylum Actinobacteria in the inoculated wheat rhizosphere compared to the control (in a greenhouse experiment).
Nevertheless, our analyses showed significant changes in non-dominant taxa at the genus level, namely an increase in the relative abundance of Nitrospira and a decrease in Bdellovibrio after 3 weeks of PGPB application. While the decrease in the number of Bdellovibrio is difficult to assess in terms of its benefits, the increase in the population of Nitrospira, the bacteria responsible for nitrification, which probably led to an increase in plant-available nitrate46, appears to be a positive change. Particularly in view of the fact that the content of ammonium, which is not leached like nitrate (it binds to the soil sorption complex)47, increased after the application of PGPB, so the inoculation did not lead to a loss of nitrogen in the soil.
Our findings, along with previous studies, suggest that the direction of change induced in the bacterial community by PGPB applications varies and depends on multiple factors.
Conclusion
In summary, the bacterial consortium studied moderately promoted plant growth while slightly altering the chemical properties of the soil, particularly the rhizosphere soil. Additionally, the investigated bacterium did not negatively affect the diversity and species richness of the native soil bacterial community, nor did it disturb the abundance of the main dominant soil bacteria. However, the application of PGPB resulted in a significant increase in the relative abundance of Nitrospira during the first sampling period, a positive outcome given the functional role of this genus. Conversely, a decrease in the abundance of Bdellovibrio was observed in the same period. Additionally, a reduction in the abundance of two other non-dominant genera in the inoculated bulk soil during the third sampling period is more challenging to interpret.
In the future, studying the impact of the P1A consortium on soil microbiome functions using RNA-Seq technology could offer valuable insights. This approach would enable the analysis of gene expression profiles and microbial interactions within the soil, providing a deeper understanding of how the consortium influences microbial communities. Nonetheless, the current findings are promising and support the potential commercial application of the P1A consortium.
Materials and methods
PGPB isolation, selection and taxonomic identification
In the study, approximately 300 bacterial strains were isolated from various soils including bulk soil from permanent grassland and grass rhizosphere. Firstly, 10 grams of roots or bulk soil were suspended in 100 mL of a sterile NaCl solution (0.85%) and shaken (200 rpm). Then, the suspension was diluted to the appropriate concentration (usually 10−5 or 10−6), 1 mL was inoculated into a Petri dish and poured over with nutrient agar. Subsequently, colonies were selected and passaged until pure strains were obtained. The selected isolates were then assessed for mutual antagonism using the cross-streak plate method.
Identification of bacterial strains was carried out based on 16S rRNA gene sequencing. The PCR template was genomic DNA isolated using the Genomic Mini (A&A Biotechnology) from a tested bacterial strain (16 h of cultivation). To amplify the 16S rRNA genes, universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) were used, as described by Kim et al.48. Amplification was performed in the following conditions: initial denaturation at 95 °C for 3 min, denaturation at 95 °C for 30 s (30 cycles), annealing at 55 °C for 2 min, extension at 72 °C for 2 min, and then incubation at 72 °C for 10 min for DNA amplification. The purified PCR products were sequenced by the Sanger technique (NEXBIO, Lublin, Poland). The obtained sequences (forward and reverse reads) were assembled to contig (BioEdit ver. 7.2) and compared with the sequences from GenBank, EMBL (European Molecular Biology Laboratory), using BLAST (the Basic Local Alignment Search Tool). The 16S rRNA gene sequences of studied bacteria strains have been deposited in GenBank under accession numbers: Pseudomonas sp. G31 – PP499654 and Azotobacter sp. PBC2 – PP500530.
Assay of plant growth promoting traits of Pseudomonas sp. G31 and Azotobacter sp. PBC2
IAA production
IAA production was assayed using a slightly modified method previously described by Bric et al.49. The bacterial strains for quantitative assay of IAA were cultured in Luria-Bertani (LB) broth supplemented with 1000 μg L−1 tryptophan for 72 h at 30±2 °C. The cultures were then centrifuged at 12000 rpm for 8 min (at 3 °C). The obtained supernatant (1 mL) was mixed with 2 mL of Salkowski reagent (49 mL, 35% perchloric acid, 1 mL 0.5 M FeCl3 solution). Absorbances were measured at 530 nm using a spectrophotometer (Epoch 2 Plate Reader, BioTek) after 60 min in the dark. The IAA production by the bacterial strains studied was quantified using an IAA standard curve ranging from 10 to 100 μg ml⁻1.
Nitrogen fixation
Nitrogen-fixing assays of the bacterial strains were performed using nitrogen-free Burk’s medium (MgSO4 0.2 g, K2HPO4 0.8 g, KH2PO4 0.2 g, CaSO4 0.13 g, FeCl3 (0.001 g), NaMoO4 0.0003 g and sucrose 20 g) with agar (18 g L−1). Plates were incubated at 30 °C for 7 days, and bacteria that grew on the medium were considered diazotrophs50.
P and Zn solubilization
Isolates were spot inoculated onto Pikovaskya’s agar (per liter: yeast extract 0.5 g, dextrose 10.0 g, Ca3(PO4)2 5.0 g, (NH4)2 SO4 0.5, KCl 0.2 g, MgSO4 0.1 g, MnSO4 0.0001 g, FeSO4 0.0001 g, Agar 15.0 g) supplemented with 2% tricalcium phosphate (TCP) and incubated at 30±2 °C for 94 h. After incubation, the appearance of clear zones around the colonies was considered positive phosphate solubilizers. The solubilisation capacity of zinc compounds was determined by spot inoculation of isolates in Tris-minimal medium (per liter: Tris-HCl 6.06 g, NaCl 4.68 g, KCl 1.49 g, NH4Cl 1.07 g, Na2SO4 0.43 g; MgCl2·2H2O 0.2 g; CaCl2·2H2O, 30 mg, pH 7.0), with 2% agar and 0.1% insoluble zinc in the form of zinc sulphate (ZnSO4)51. Cultures were incubated for 14 days, and after this time the presence of clear zones around the colonies, which were indicative of zinc solubilisation, was checked.
Carboxymethylcellulase (CMCase) activity
The capacity for cellulolytic production (CMCase production) was assayed by spot inoculating the culture medium according to Park (per liter: (NH4)2 SO4 0.5 g; KH2PO4 1.0 g; KCl 0.5 g; MgSO40.2 g; CaCl2 0.1 g) with a 1% solution of carboxymethylcellulose (CMC). Cultures were incubated for 120 h at 28 ± 2° and then checked for the presence of clear zones.
Experimental site, design and soil sampling
The research was conducted in the winter wheat field belonging to Awista Pierwsza Company in Kobierzycko, (Sieradz county, Łódź voivodeship—51° 37ʹ 59.2ʺ N 18° 36ʹ 50.9ʺ E) in 2023. The experiment was established on mineral soil classified as Luvisol (World Reference Base (WRB) for Soil Resources). At the beginning of the growing season, the average pH value in the soil was 6.5. The average temperature and precipitation for the months in which the experiment lasted was: April—2.20 mm; 8.1 °C, May—0.70 mm; 13.3 °C, June—0.48 mm; 18.2 °C, July—70 mm; 20 °C.
Winter wheat was sown at a density of 550 germinating grains per 1 m2 in the second decade of September 2022. In autumn, ammonium nitrate was applied (180 kg ha−1), however, P and K fertilization was not applied (on the site were previously grown potatoes).
The experiment was set up in April 2023 in Randomized Complete Block Design—RCBD in triplicate The size of a single plot was 2 m × 5 m (10 m2). The buffer zone between plots was 1 m wide. The study included two treatments: control (C) and consortium application Pseudomonas sp. G31 and Azotobacter sp. PBC2 (P1A). The bacterial strains intended for application were cultured on LB (lysogeny broth) for 24 h at 30 °C. Subsequently, using a densitometer, inocula with a McFarland value of 0.5 (approximately 1.5 × 108 CFU mL⁻1) were prepared and mixed in a 1:1 ratio. The bacterial application procedure was performed on April 18, 2023 (plant stage: 23 BBCH). The inoculum was applied using a hand sprayer, held approximately 50 cm from the soil; 600 mL per plot. For soil physico-chemical analysis and 16S rRNA sequencing, roots (rhizosphere soil) and bulk soil were sampled three times during the growing season, 3 weeks after the consortium application (early May; wheat development phase: 33 BBCH), in the middle of the growing season (middle of June; 39 BBCH) and before harvest (late July; 92 BBCH). For testing, roots were taken from 5 plants per plot and soil was collected with a soil sampler from 5 points in the plot from a depth of 0 to 20 cm (samples were collected into sterile bags). Samples were taken in triplicate (3 replicates from each variant). To obtain the rhizosphere, the loosely bound soil was shaken off and the tightly bound soil was extracted with a sterile brush and sieved through a sterile sieve52. After collection, samples were frozen at − 20 °C degrees for chemical analyses and at − 80 °C for molecular analyses. Plant samples were taken once a year; before harvest, plants were taken from 1 m2 and the following measurements were taken: root length (cm), root mass (g), shoot length (cm), shoot yield (g), seed yield (g).
Soil physico-chemical analysis
Soil samples for pH, total carbon (TC), total nitrogen (TN), and available phosphorus (AP) determinations were air-dried and passed through a 2 mm sieve53–56. At the same time, N-NO3 was measured in fresh soil mass. Parameters were determined by the following methods: pH in 1M KCl by PN-EN ISO 10390:2022-09, TC by PN-R-04024:1997, total TN by ISO 13878:1998, the content of mineral forms of nitrogen was performed using the Continuous Flow Analysis (CFA) with detection spectrophotometric, and AP by PN-R-04023:1996.
16S rRNA sequencing and statistical and bioinformatic analyses
Soil DNA was extracted by using Magnetic Soil and Stool DNA Kit (TianGen, China). PCR amplification of targeted regions was performed using specific primers connecting with barcodes; region V3–V4 -314F (5′-CCTACGGNGGCWGCAG-3′) and 785R (5′ GACTACHVGGTATCTAATCC-3′). PCR reactions were carried out with 15 μL of Phusion® High - Fidelity PCR Master Mix; 0.2 μM of forward and reverse primers, and about 10 ng template DNA. Thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s and 72 °C for 5 min. Sequencing was performed on a sequencer Illumina NovaSeq 6000 System (Novogene, Germany). Raw data was filtered and denoised using DADA2 method57,58. Each de-duplicated sequence generated after noise reduction using DADA2 was called ASVs (Amplicon Sequence Variants), or feature sequence (corresponding to the OTU representative sequence). Taxonomic assignment was performed using a classifier trained Naive Bayes59,60 and annotation database was Silva 138.1. According to the taxonomic annotation results, the top taxa of phylum and genus level were selected to form the distribution histogram of the relative abundance of taxa.
Alpha diversity and beta diversity
In order to analyze the diversity, richness in the sample, alpha diversity was calculated from four indices in QIIME2, including Observed_features, Chao1, Shannon, and Simpson. In order to evaluate the complexity of the community composition and compare the differences between samples (groups), beta diversity was calculated based on weighted unifrac distances in QIIME2. PCoA analysis was displayed by the ade4 package and ggplot2 package in R software (Version 4.0.3).
Statistical analysis
LEfSe, t-test, and MetaStat were performed to determine significant differences in the bacterial community at the phylum and genus level using vegan package and ggplot2 package within R.
Tukey’s HSD (honest significant difference test; level of p = 0.05) were processed statistically using Statistica 6.0.
Supplementary Information
Acknowledgments
English language revision and translation assistance provided by Katarzyna Rafalska.
Author contributions
J.D. contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.D., I.K., Z.J., B.W. The first draft of the manuscript was written by J.D. and B.W. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research was funded by the European Agricultural Fund under the Rural Development Programme for 2014-2020. Grant number: DDD.6509.00267.2022.15.
Data availability
Sequencing data has been deposited in Sequence Read Archive (SRA) data (NCBI) under the following accession number: PRJNA1092096
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86820-3.
References
- 1.Food and Agriculture Organization (FAO). Crop Prospects and Food Situation—Triannual Global Report No. 1, March 2024. Rome (2024).
- 2.Food and Agriculture Organization (FAO). World Food and Agriculture—Statistical Yearbook 2023. Rome. 10.4060/cc8166en (2023).
- 3.Smagacz, J. & Martyniuk, S. Soil properties and crop yields as influenced by the frequency of straw incorporation in a rape-wheat-triticale rotation. J. Water Land Dev.56, 1–6 (2023). [Google Scholar]
- 4.Sosnowski, J., Wróbel, B. & Truba, M. Effect of Tytanit on selected morphological, physiological and chemical characteristics of Lolium multiflorum dry matter. J. Water Land Dev.56, 7–13 (2023). [Google Scholar]
- 5.Wierzchowski, P. S., Dobrzyński, J. & Mazur, K. Chemical properties and bacterial community reaction to acidified cattle slurry fertilization in soil from maize cultivation. Agronomy11, 601 (2021). [Google Scholar]
- 6.Zörb, C., Ludewig, U. & Hawkesford, M. J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci.23, 1029–1037. 10.1016/j.tplants.2018.08.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L. et al. Organic amendment mitigates the negative impacts of mineral fertilization on bacterial communities in Shajiang black soil. Appl. Soil Ecol.150, 103457. 10.1016/j.apsoil.2020.103457 (2020). [Google Scholar]
- 8.Dobrzyński, J. et al. The reaction of cellulolytic and potentially cellulolytic spore-forming bacteria to various types of crop management and farmyard manure fertilization in bulk soil. Agronomy11, 772. 10.3390/agronomy11040772 (2021). [Google Scholar]
- 9.Zielewicz, W. et al. Effect of forage plant mixture and biostimulants application on the yield, changes of botanical composition, and microbiological soil activity. Agronomy11, 1786. 10.3390/agronomy11091786 (2021). [Google Scholar]
- 10.Dobrzyński, J. et al. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol.14, 1069053. 10.3389/fmicb.2023.1069053 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrarezi, J. A. et al. Effects of inoculation with plant growth-promoting rhizobacteria from the Brazilian Amazon on the bacterial community associated with maize in field. Appl. Soil Ecol.170, 104297. 10.1016/j.apsoil.2021.104297 (2022). [Google Scholar]
- 12.Joshi, S. et al. Rhizospheric bacteria: The key to sustainable heavy metal detoxification strategies. Front. Microbiol.14, 1229828. 10.3389/fmicb.2023.1229828 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkova, I., Wróbel, B. & Dobrzyński, J. Serratia spp. as plant growth-promoting bacteria alleviating salinity, drought, and nutrient imbalance stresses. Front. Microbiol.15, 1342331. 10.3389/fmicb.2024.1342331 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra, P., Mishra, J. & Arora, N. K. Plant growth promoting bacteria for combating salinity stress in plants—Recent developments and prospects: A review. Microbiol. Res.252, 126861. 10.1016/j.micres.2021.126861 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Dobrzyński, J. & Naziębło, A. Paenibacillus as a Biocontrol Agent for Fungal Phytopathogens: Is P. polymyxa the Only One Worth Attention?. Microb. Ecol.87(1), 134 (2024). [DOI] [PMC free article] [PubMed]
- 16.Abdelaal, K. et al. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology10, 520. 10.3390/biology10060520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrzyński, J., Wróbel, B. & Górska, E. B. Taxonomy, ecology, and cellulolytic properties of the genus Bacillus and related genera. Agriculture13, 1979. 10.3390/agriculture13101979 (2023). [Google Scholar]
- 18.Çığ, F., Sönmez, F., Nadeem, M. A. & El Sabagh, A. Effect of biochar and PGPR on the growth and nutrients content of Einkorn wheat (Triticum monococcum L.) and post-harvest soil properties. Agronomy11, 2418. 10.3390/agronomy11122418 (2021). [Google Scholar]
- 19.Paul, G. K. et al. Volatile compounds of Bacillus pseudomycoides induce growth and drought tolerance in wheat (Triticum aestivum L.). Sci. Rep.12, 19137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana, A. et al. Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann. Microbiol.61, 893–900. 10.1007/s13213-011-0211-z (2011). [Google Scholar]
- 21.Sedri, M. H. et al. Comparative analysis of plant growth-promoting rhizobacteria (PGPR) and chemical fertilizers on quantitative and qualitative characteristics of rainfed wheat. Agronomy12, 1524. 10.3390/agronomy12071524 (2022). [Google Scholar]
- 22.de Vries, F. T. et al. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science368, 270. 10.1126/science.aaz5192 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Mendes, R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science332, 1097–1100. 10.1126/science.1203980 (2011). [DOI] [PubMed] [Google Scholar]
- 24.de Faria, M. R. et al. The rhizosphere microbiome: Functions, dynamics, and role in plant protection. Trop. Plant Pathol.46, 13–25. 10.1007/s40858-020-00390-5 (2021). [Google Scholar]
- 25.Xiong, W. et al. Rhizosphere protists are key determinants of plant health. Microbiome8, 1–9. 10.1186/s40168-020-00799-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrzyński, J., Jakubowska, Z. & Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol.13, 1069053. 10.3389/fmicb.2022.1069053 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manfredini, A. et al. Current methods, common practices, and perspectives in tracking and monitoring bioinoculants in soil. Front. Microbiol.12, 698491. 10.3389/fmicb.2021.698491 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boubekri, K. et al. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res.261, 127059. 10.1016/j.micres.2022.127059 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Ho, A., Di Lonardo, D. P. & Bodelier, P. L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol.93, fix006. 10.1093/femsec/fix006 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Korsa, G. et al. Microbial cellulase production and its potential application for textile industries. Ann. Microbiol.73, 13. 10.1186/s13213-023-01715-w (2023). [Google Scholar]
- 31.Li, Y., Chapman, S. J., Nicol, G. W. & Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem.116, 290–301. 10.1016/j.soilbio.2017.10.023 (2018). [Google Scholar]
- 32.Kalam, S. et al. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol.11, 580024. 10.3389/fmicb.2020.580024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assainar, S. K. et al. Response of wheat to a multiple species microbial inoculant compared to fertilizer application. Front. Plant Sci.9, 1601. 10.3389/fpls.2018.01601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, Y. et al. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut.28, 23036–23047. 10.1007/s11356-020-12203-y (2021). [DOI] [PubMed] [Google Scholar]
- 35.Chen, S. et al. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome7, 136. 10.1186/s40168-019-0750-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graziano, S. et al. A metagenomic and gene expression analysis in wheat (Triticum durum) and maize (Zea mays) biofertilized with PGPM and biochar. Int. J. Mol.23, 10376. 10.3390/ijms231810376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dal Cortivo, C. et al. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ.247, 396–408. 10.1016/j.agee.2017.07.006 (2017). [Google Scholar]
- 38.Kumar, A., Maurya, B. R. & Raghuwanshi, R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol.3, 121–128. 10.1016/j.bcab.2014.06.005 (2014). [Google Scholar]
- 39.Dai, Z. et al. Seed coat treatment by plant-growth-promoting rhizobacteria Lysobacter antibioticus 13–6 enhances maize yield and changes rhizosphere bacterial communities. Biol. Fertil. Soils59, 317–331 (2023). [Google Scholar]
- 40.Shi, H. et al. Effects of two Bacillus velezensis microbial inoculants on the growth and rhizosphere soil environment of Prunus davidiana. Int. J. Mol. Sci.23, 13639. 10.3390/ijms232113639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, J. et al. Bacillus amyloliquefaciens FH-1 significantly affects cucumber seedlings and the rhizosphere bacterial community but not soil. Sci. Rep.11, 12055. 10.1038/s41598-021-91399-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao, Y. et al. Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: A greenhouse study. J. Soil Sediment19, 3597–3607. 10.1007/s11368-019-02306-0 (2019). [Google Scholar]
- 43.Liu, Y. et al. Unraveling mechanisms and impact of microbial recruitment on oilseed rape (Brassica napus L.) and the rhizosphere mediated by plant growth-promoting rhizobacteria. Microorganisms9, 161. 10.3390/microorganisms9010161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, Y. et al. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol.12, 693535. 10.3389/fmicb.2021.693535 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaghoubi Khanghahi, M., Crecchio, C. & Verbruggen, E. Shifts in the Rhizosphere and Endosphere Colonizing Bacterial Communities Under Drought and Salinity Stress as Affected by a Biofertilizer Consortium. Microb. Ecol.84, 483–495 (2022). [DOI] [PubMed]
- 46.Daims, H. et al. Complete nitrification by Nitrospira bacteria. Nature528, 504–509. 10.1038/nature16461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieder, R., Benbi, D. K. & Scherer, H. W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils.47, 1–14. 10.1007/s00374-010-0506-4 (2011). [Google Scholar]
- 48.Kim, S. J. et al. Polyphasic assignment of an aromatic-degrading Pseudomonas sp., strain DJ77, in the genus Sphingomonas as Sphingomonas chungbukensis sp. nov. Int. J. Syst. Evol. Microbiol.50, 1641–1647. 10.1099/00207713-50-5-1641 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Bric, J. M., Bostock, R. M. & Silverstone, S. E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol.57, 535–538 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakraborty, P. & Tribedi, P. Functional diversity performs a key role in the isolation of nitrogen-fixing and phosphate-solubilizing bacteria from soil. Folia Microbiol.64, 461–470 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Sharma, S. K. et al. Characterization of zinc-solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol.22, 352–359. 10.4014/jmb.1106.05063 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Mendes, L. W. et al. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J.12, 212–224. 10.1038/ismej.2017.158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ISO 13878. Soil quality—Determination of total nitrogen content by dry combustion (“elemental analysis”). International Organization for Standardization (1998).
- 54.Polish Committee for Standardization (PN). Agrochemical Soil Analyses—Determination of Assimilated Phosphorus Contents (PN-R-04023:1996).
- 55.Polish Committee for Standardization (PN). Analysis of the Chemical-Agricultural Soil—Determination of the Content of Available Phosphorus, Potassium, Magnesium and Manganese in Organic Soils (PN-R-04024:1997).
- 56.Polish Committee for Standardization (PN). Soil, Treated Bio-Waste and Sewage Sludge—Determination of pH (PN-EN ISO 10390:2022-09).
- 57.Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods13, 581–583. 10.1038/nmeth.3869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li, M. et al. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res.32, 755–767. 10.21147/j.issn.1000-9604.2020.06.09 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome6, 90. 10.1186/s40168-018-0470-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol.37, 852–857. 10.1038/s41587-019-0209-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data has been deposited in Sequence Read Archive (SRA) data (NCBI) under the following accession number: PRJNA1092096