Abstract
Acute B-lymphoblastic leukemia (B-ALL) is a highly heterogeneous hematologic malignancy, characterized by significant molecular differences among patients as the disease progresses. While the PI3K-Akt signaling pathway and metabolic reprogramming are known to play crucial roles in B-ALL, the interactions between lipid metabolism, immune pathways, and drug resistance remain unclear. In this study, we performed multi-omics analysis on different patient cohorts (newly diagnosed, relapsed, standard-risk, and poor-risk) to investigate the molecular characteristics associated with metabolism, signaling pathways, and immune regulation in B-ALL. Our findings indicate that the PI3K-Akt signaling pathway is significantly enriched across all groups, highlighting its critical role in B-ALL pathogenesis and progression. Furthermore, metabolomic analysis revealed that lipid metabolism, ferroptosis, and glutathione metabolism are closely linked to disease progression. Notably, in relapsed patients, dysregulated lipid metabolism and the activation of antioxidant mechanisms may contribute to treatment resistance. Immune-related pathways, such as the complement system and coagulation cascade, were also significantly enriched in patients with B-ALL. This suggests that these pathways, alongside the PI3K-Akt pathway, play a role in forming the tumor microenvironment, thereby promoting disease progression and relapse. Based on these findings, this study provides novel potential therapeutic targets for the personalized treatment of B-ALL and lays the foundation for further development of PI3K-Akt pathway inhibitors and immunometabolism-targeted therapies.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87684-3.
Keywords: B-ALL, Multi-omics analysis, PI3K-Akt signaling pathway, Immune pathways, Lipid metabolism, Treatment strategy
Subject terms: Leukaemia, Acute lymphocytic leukaemia, Computational biology and bioinformatics
Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is a malignant clonal disorder originating from precursor B cells, characterized by distinct clinical features that vary depending on genetic rearrangements, chromosomal abnormalities, and gene mutations. B-ALL is the most common type of acute leukemia in children, accounting for approximately 80% of cases, while it represents around 20% of adult leukemia cases1. Over recent decades, significant advancements in pediatric B-ALL treatment have been made, with current chemotherapy regimens achieving cure rates exceeding 90% in many subtypes. In contrast, similar outcomes have not been replicated in adult B-ALL. Current treatment options for adult B-ALL include chemotherapy, targeted therapies, monoclonal antibodies, hematopoietic stem cell transplantation, and cellular immunotherapy. However, long-term survival rates remain suboptimal2, with approximately 40–50% of patients experiencing relapse or refractory disease and a 5-year survival rate of around 50%3. Therefore, there is an urgent need to explore new therapeutic breakthroughs for adult B-ALL.
B-ALL is a highly heterogeneous hematologic malignancy with significant biological differences across patient groups. These differences not only reflect distinct molecular mechanisms but also directly influence disease progression and treatment response. Leukemic cells in newly diagnosed patients typically exhibit relatively simple molecular characteristics, with specific gene mutations and signaling pathway abnormalities predominantly associated with early tumorigenesis. However, as the disease progresses, particularly in relapsed patients, tumor cells often acquire more complex genetic alterations, leading to increased treatment resistance and closely associated metabolic and immune-related changes4,5.
In the risk stratification of ALL, patients are classified into standard-risk (SR) and poor-risk (PR) categories, exhibiting notable biological differences. PR patients often present with more complex gene mutations. Accurate risk stratification at the initial diagnosis is vital for tailoring individualized therapies aimed at minimizing toxicity for standard-risk patients while intensifying treatment for poor-risk patients to achieve the best possible outcome with minimal side effects6.
Recent studies on the pathogenesis of B-ALL suggest that disease onset is associated with aberrant signaling pathways7 and immune-metabolic dysregulation8,9. Metabolic reprogramming is a key mechanism by which tumor cells reset their energy metabolism network to support growth, proliferation, and metastasis under the metabolic stress of the tumor microenvironment and immune surveillance10. The tumor microenvironment refers to the non-cancerous cells and components within a tumor, including the molecules they produce and release. Continuous interactions between tumor cells and the the tumor microenvironment are crucial in determining tumor initiation, progression, metastasis, and therapeutic response11. Despite significant advances in the molecular understanding of ALL, challenges remain in developing precise therapies targeting these molecular abnormalities, underscoring the need for further research to elucidate the heterogeneity across different patient populations. This study aims to investigate the molecular characteristics of B-ALL in various patient groups (newly diagnosed, relapsed, SR, and PR) through multi-omics analysis. By comparing metabolic, signaling, and immune regulatory differences among these groups, we hope to identify new therapeutic targets and strategies for the personalized treatment of B-ALL.
Results
Exosomal proteomics analysis in healthy controls and newly diagnosed patients (5 vs. 5)
Proteomic sequencing analysis was performed on five healthy controls and five newly diagnosed patients with B-ALL. PCA revealed a significant separation between healthy controls and newly diagnosed patients (Fig. 1A). Sample correlation analysis based on reliable proteins demonstrated clear groupings but also identified reproducibility issues between samples (Supplementary Fig. 1A). Differential protein expression between the two groups was assessed using FC and p value criteria. Proteins with P < 0.05 and FC > 2 were considered upregulated, while those with P < 0.05 and FC < 0.5 were considered downregulated. A total of 195 proteins were upregulated, and 147 were downregulated in newly diagnosed patients compared to healthy controls. Differential expression proteins (DEPs) were visualized using a volcano plot (Fig. 1B) and a heatmap (Supplementary Fig. 1B). GO enrichment analysis was performed on DEPs across three categories: biological processes (BP), cellular components (CC), and molecular functions (MF). The top 30 enriched GO terms were presented as bar graphs (Fig. 1C), and the top 15 as bubble plots (Supplementary Fig. 1C). Significant enrichment of terms such as antigen binding, cadherin binding, extracellular exosome, extracellular space, blood coagulation, and immunoglobulin-mediated immune response indicated possible inflammation in patients. KEGG pathway analysis of DEPs identified immune-related pathways such as complement and coagulation cascades, neutrophil extracellular trap formation, and B cell receptor signaling pathway as significantly enriched (Fig. 1D). Notably, the PI3K-Akt signaling pathway was also prominently enriched. STRING analysis was conducted to investigate protein–protein interactions, identifying the top 25 proteins based on connectivity, including GAPDH, ACTB, ALB, and FN1, and visualized in a network diagram (Fig. 1E).
Fig. 1.
Proteomic analysis of healthy donors and newly diagnosed patients (5 vs. 5). (A) PCA analysis of proteomics in healthy donor and newly diagnosed patients. (B) Volcano plot of differential proteins between healthy donors and newly diagnosed patients. (C) Bar chart of GO enrichment analysis of differential proteins in healthy donors and newly diagnosed patients (top 10 for BP, MF, CC based on significance). (D) Bubble plot of KEGG enrichment analysis of differential proteins in healthy donors and newly diagnosed patients (top 20 based on significance ranking). KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories. (E) PPI network diagram of differential proteins in healthy donors and newly diagnosed patients (top 25 ranked by degree connectivity).
Metabolomics analysis in healthy controls and newly diagnosed patients (17 vs. 20)
PCA of metabolomic data from healthy controls and newly diagnosed patients demonstrated a clear separation between the two groups (Fig. 2A). Orthogonal partial least squares discriminant analysis (OPLS-DA) was performed to maximize differentiation between the groups (Fig. 2B), with variable importance in projection (VIP) scores used to identify statistically significant metabolites (VIP > 1). A total of 41 metabolites were upregulated, while 17 were downregulated in newly diagnosed patients compared to healthy controls (Fig. 2C). KEGG pathway enrichment analysis identified glutathione metabolism and ferroptosis as significantly enriched pathways (Fig. 2D), suggesting a potential involvement of oxidative stress and apoptosis mechanisms in disease progression.
Fig. 2.
Metabolomic analysis of healthy donors and newly diagnosed patients (17 vs. 20). (A) PCA analysis of metabolomics in healthy donors and newly diagnosed patients. (B) OPLS-DA analysis of metabolomics in healthy donors and newly diagnosed patients. (C) Volcano plot of differential metabolites between healthy donors and newly diagnosed patients. (D) Bubble plot of KEGG enrichment analysis of differential metabolites in healthy donors and newly diagnosed patients (top 20 based on significance ranking). KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories.
Transcriptomic analysis in standard-risk and poor-risk patients (15 vs. 9)
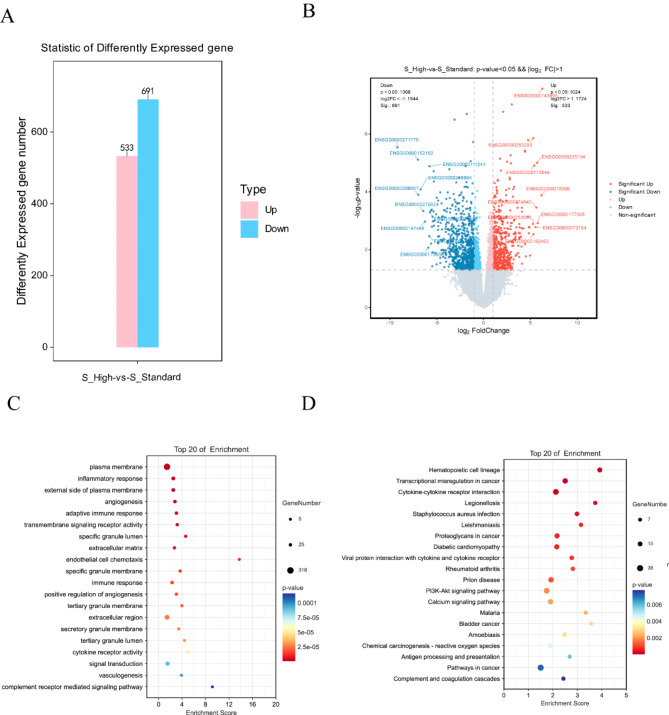
To explore differences between SR and PR patients, gene expression was analyzed using FC and P value criteria. The analysis identified 533 upregulated genes and 691 downregulated genes in PR patients compared to SR patients (Fig. 3A). According to the NCCN guidelines (version 1.2023) for acute lymphoblastic leukemia, the classification of SR and PR patients was based on cytogenetic and molecular prognostic risk stratification. DEGs were visualized in a volcano plot (Fig. 3B). GO/KEGG enrichment analysis of DEGs highlighted immune-related and metabolic pathways, with significant enrichment of the PI3K-Akt signaling pathway (Fig. 3C,D).
Fig. 3.
Transcriptomic analysis of standard-risk and poor-risk patients (15 vs. 9). (A) Bar chart of differential gene statistics in standard-risk and poor-risk patients. (B) Volcano plot of differential genes between standard-risk and poor-risk patients. (C) Bubble plot of GO enrichment analysis of differential genes in standard-risk and poor-risk patients (top 20 based on significance ranking). (D) Bubble plot of KEGG enrichment analysis of differential genes in standard-risk and poor-risk patients (top 20 based on significance ranking). KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories.
Metabolomic analysis in standard-risk and poor-risk patients (13 vs. 7)
Metabolomic analysis of SR and PR patients was conducted, and OPLS-DA was performed to distinguish between the two groups. The VIP scores for each metabolite were obtained from the OPLS-DA model, with the score plot presented in Fig. 4A. Metabolites were selected as differential metabolites (DEMs) using a threshold P value < 0.05 and VIP > 1.0. A total of 8 metabolites were upregulated in the PR group compared to the SR group, including PC 18:1_18:1, Oleamide, DL-Carnitine, Hexadecanamide, Inositol, PC 34:1, PC 38:4, PC 18:2_18:2, and Levulinic acid. Conversely, 2 metabolites were downregulated in the PR group, specifically DL-Carnitine and N-Acetylglycine. Many of these metabolites are lipids or related to lipid metabolism. Box plots of the differential metabolites are shown in Supplementary Fig. 2. The volcano plot and hierarchical clustering of the differential metabolites are shown in Fig. 4B and C, respectively. KEGG enrichment analysis was performed on the DEMs, and a bubble plot of the top 20 significantly enriched pathways is presented in Fig. 4D. Notably, pathways such as galactose metabolism, inositol phosphate metabolism, ascorbate and aldarate metabolism, phosphatidylinositol signaling system, and ABC transporters were significantly enriched.
Fig. 4.
Metabolomic analysis of standard-risk and poor-risk patients (13 vs. 7). (A) OPLS-DA analysis of metabolomics in standard-risk and poor-risk patients. (B) Volcano plot of differential metabolites between standard-risk and poor-risk patients. (C) Heatmap of differential metabolites in standard-risk and high-poor patients. (D) Bubble plot of KEGG enrichment analysis of differential metabolites in standard-risk and poor-risk patients. KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories.
Integration of transcriptomics and metabolomics in standard-risk and poor-risk patients
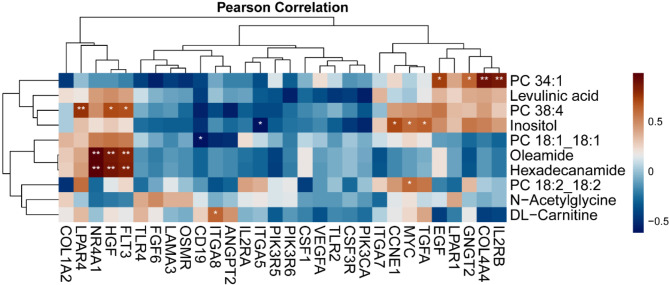
Based on our findings, we hypothesize that the PI3K-Akt signaling pathway may play a crucial role in disease progression and may be associated with lipid metabolism. Correlation analysis between key genes and differential metabolites identified significant positive correlations between IL2RB, COL4A4, GNGT2, EGF genes, and lipid metabolites (Fig. 5), suggesting a link between the PI3K-Akt signaling pathway and lipid metabolism.
Fig. 5.
Correlation analysis heatmap of transcriptomics and metabolomics in standard-risk and poor-risk patients.
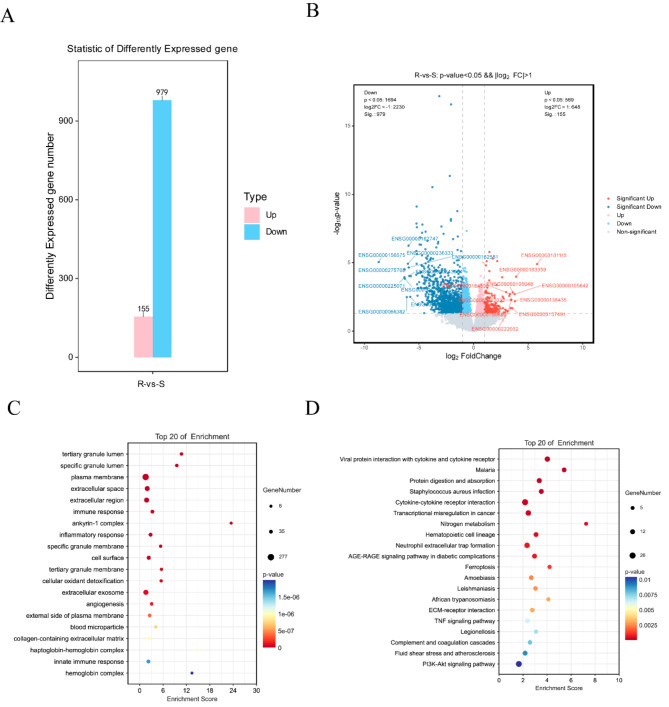
Transcriptomic analysis in newly diagnosed and relapsed patients (24 vs. 8)
Trascriptomic analysis comparing newly diagnosed and relapsed patients identified 155 upregulated and 979 downregulated genes in relapsed patients (Fig. 6A). Based on differential analysis results, differentially expressed genes (DEGs) were identified and visualized in a volcano plot (Fig. 6B). Enrichment analysis identified the PI3K-Akt signaling pathway, ferroptosis, and TNF signaling pathway as significantly enriched (Fig. 6C,D).
Fig. 6.
Transcriptomic analysis of newly diagnosed and relapsed patients (24 vs. 8). (A) Bar chart of differential gene statistics in newly diagnosed and relapsed patients. (B) Volcano plot of differential genes between newly diagnosed and relapsed patients. (C) Bubble plot of GO enrichment analysis of differential genes in newly diagnosed and relapsed patients (top 20 based on significance ranking). (D) Bubble plot of KEGG enrichment analysis of differential genes in newly diagnosed and relapsed patients (top 20 based on significance ranking). KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories.
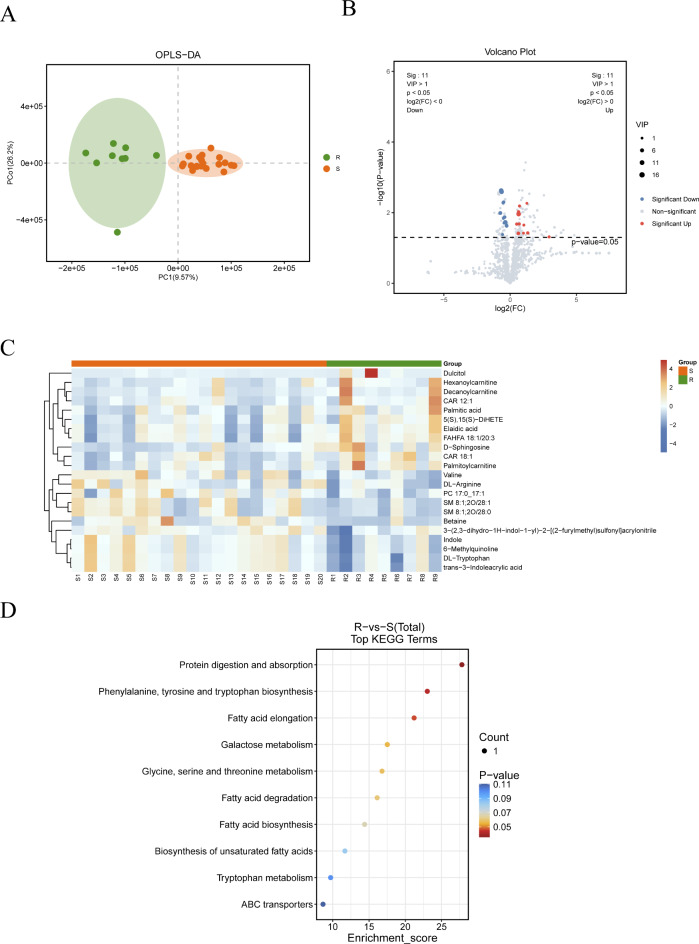
Metabolomic analysis in newly diagnosed and relapsed patients (20 vs. 9)
OPLS-DA analysis of metabolomic data from newly diagnosed and relapsed patients revealed significant separation (Fig. 7A). Differentially expressed metabolites (DEMs) were identified based on the criteria of P value < 0.05 and VIP > 1.0. Among these, 11 metabolites were upregulated in relapsed patients compared to newly diagnosed patients, including Elaidic acid, Palmitic acid, Decanoylcarnitine, 5(S),15(S)-DiHETE, FAHFA 18:1/20:3, CAR 18:1, Dulcitol, Palmitoylcarnitine, CAR 12:1, Hexanoylcarnitine, and D-Sphingosine. Additionally, 11 metabolites were downregulated in relapsed patients, including DL-Tryptophan, trans-3-Indoleacrylic acid, 3-(2,3-dihydro-1H-indol-1-yl)-2-[(2-furylmethyl)sulfonyl]acrylonitrile, Betaine, PC 17:0_17:1, Valine, Indole, 6-Methylquinoline, DL-Arginine, SM 8:1;2O/28:1, and SM 8:1;2O/28:0. The differential analysis volcano plot and clustering results are presented in Fig. 7B and C. KEGG pathway analysis indicated that lipid metabolism-related pathways, such as fatty acid elongation, degradation, and biosynthesis, were enriched in relapsed patients (Fig. 7D).
Fig. 7.
Metabolomic analysis of newly diagnosed and relapsed patients (20 vs. 9). (A) OPLS-DA analysis of metabolomics in newly diagnosed and relapsed patients. (B) Volcano plot of differential metabolites between newly diagnosed and relapsed patients. (C) Heatmap of differential metabolites in newly diagnosed and relapsed patients. (D) Bubble plot of KEGG enrichment analysis of differential metabolites in newly diagnosed and relapsed patients. KEGG pathways were identified and visualized using the KEGG database (Kanehisa Laboratories, www.kegg.jp/kegg/kegg1.html). Permission to use KEGG pathways was obtained from Kanehisa Laboratories.
Discussion
This study employed multi-omics analysis to explore the molecular characteristics of B-ALL across different patient cohorts (newly diagnosed, relapsed, SR, and PR), revealing significant differences related to signaling pathways, metabolism, and immune regulation. Our findings provide novel insights into the molecular mechanisms of B-ALL and suggest potential targets for personalized therapeutic strategies.
One key finding was the significant enrichment of the PI3K-Akt signaling pathway across all patient cohorts, indicating its critical role in the pathogenesis and progression of B-ALL. The PI3K-Akt pathway is essential for the malignant transformation of B cells12. Previous research has linked its activation in pediatric B-ALL with poor prognosis and drug resistance13. Specifically, PI3K-Akt activation has been observed in glucocorticoid-resistant cells14, and inhibiting this pathway can restore drug sensitivity in tumor cells15. Our results align with these findings, supporting the association between PI3K-Akt activation, drug resistance, disease relapse, and poor prognosis. Previous studies have shown that cancer cells use acetyl-CoA as a substrate for fatty acid synthesis, generated from cytosolic citrate via ACLY, promoting glycolysis. The PI3K/Akt pathway can activate ACLY and enhance the Warburg effect16. Moreover, PI3K/Akt signaling enhances lipid synthesis, which is necessary for cancer cell growth and metastasis17. Another study similarly demonstrated that sustained activation of the IGF1R-PI3K-Akt axis, in conjunction with GLUT1 upregulation, synergistically enhances energy metabolism and glycolytic activity in BCP-ALL. Neutralization of IGFBP7 using a monoclonal antibody or pharmacological inhibition of the PI3K-Akt pathway effectively abrogated this effect, restoring GLUT1 to physiological levels on the cell surface. These findings underscore the critical relationship between the PI3K-Akt pathway and metabolic regulation in BCP-ALL cells18. Additionally, Minematsu et al. identified differences in lipid composition between tumor stem cells and pluripotent stem cells, noting that prostaglandin E2 (PGE2) may promote tumor stem cell generation via the PI3K/Akt pathway19. These findings suggest that the PI3K/Akt pathway interacts with lipid metabolism and inflammatory responses, contributing to cancer development and progression. mTOR, a downstream molecule of the PI3K/AKT pathway, plays a pivotal regulatory role in the outcomes of antigen recognition. By sensing the immune microenvironment, mTOR influences the differentiation and maturation of T cells, B cells, and antigen-presenting cells20. In tumor cells, inhibition of the PI3K/Akt pathway induces apoptosis, leading to anti-tumor effects21. Based on our results, PI3K/Akt may play a role in lipid metabolism, apoptosis, and inflammatory responses, contributing to the development and progression of B-ALL.
Metabolomic analysis of normal and newly diagnosed patients revealed significant enrichment of pathways such as glutathione metabolism and ferroptosis. Glutathione is known to counteract oxidative stress in cells22, and downregulating the glutathione pathway can inhibit tumor cell growth23. Therefore, we hypothesize that glutathione metabolism in B-ALL may help leukemia cells resist oxidative damage by modulating oxidative stress, enabling them to evade immune-mediated apoptosis. Ferroptosis, a form of cell death dependent on lipid peroxidation24, was also enriched, suggesting a link between disrupted lipid metabolism and oxidative stress. In relapsed patients, hyperactivation of the ferroptosis pathway may indicate that leukemia cells evade apoptosis by inhibiting lipid peroxidation and enhancing antioxidant capabilities, thereby increasing treatment resistance. In contrast to drug-resistant B-ALL, other tumors enhance resistance by suppressing ferroptosis, while inducing ferroptosis promotes the death of cancer cells25,26. In summary, metabolic pathway alterations reflect adaptive changes in leukemia cells during disease progression, and these pathways may serve as novel therapeutic targets, particularly in relapsed ALL.
Our study also revealed significant differences in the immune microenvironment of patients with ALL. Proteomic analysis highlighted enriched immune-related pathways, such as complement and coagulation cascades and neutrophil extracellular trap (NET) formation, which are closely associated with PI3K-Akt signaling activation. The complement system and coagulation cascades are involved not only in immune responses27 but also in regulating the tumor microenvironment28. The surveillance function of the complement system plays a critical role in cancer development29, and recent studies have identified complement-related genes as predictors of leukemia prognosis30. Our omics analysis suggests that immune-related pathways, including the complement system and coagulation cascades, are enriched in B-ALL. We speculate that these pathways may promote tumor cell survival and proliferation by inducing chronic inflammation. Additionally, the activation of immune pathways is often accompanied by hyperactivation of the PI3K-Akt pathway21. This pathway plays a key role in regulating cell proliferation, survival, and metabolism, and its interaction with the immune system may enhance tumor cell drug resistance. Furthermore, the activation of immune pathways may contribute to the formation of an inflammatory microenvironment, providing a survival advantage for tumor cells and promoting disease progression. Overall, the synergy between immune-related pathways and the PI3K-Akt signaling pathway not only drives leukemia progression but may also support leukemia cell survival and recurrence by shaping a favorable tumor microenvironment.
In conclusion, PI3K-Akt pathway activation, along with its strong association with lipid metabolism, apoptosis, and inflammatory responses, was observed in newly diagnosed, PR, and relapsed patients with B-ALL. Therefore, future research could consider incorporating relevant biomarkers into risk stratification to better distinguish PR patients and administer more intensive treatment to improve patient outcomes. Given the central role of the PI3K-Akt pathway, further research on inhibitors targeting this pathway may be a promising strategy for treating patients with B-ALL. Additionally, since metabolism and immunity play crucial roles in B-ALL pathogenesis and progression, future studies could explore the interactions between other signaling pathways, metabolism, and immunity, potentially leading to the development of targeted therapies. Recent studies have demonstrated that inhibiting cholesterol biosynthesis with statins can reduce the proliferation and induce apoptosis in CLL, ALL, and multiple myeloma cells31. Additionally, targeting the serine/glycine metabolic pathway effectively suppresses the proliferation of B-ALL cells9. Nevertheless, numerous uncharted strategies remain to be explored and warrant further investigation.
Although this study identified key molecular features of B-ALL in different patient cohorts through multi-omics analysis, the limited sample size may affect the robustness of the statistical analysis and the generalizability of the results. Moreover, the study lacks further functional validation, and future research should verify the specific roles and therapeutic potential of these pathways in B-ALL using experimental approaches such as gene knockout and overexpression studies.
Conclusion
This study highlights molecular differences in B-ALL across various patient cohorts through multi-omics analysis, highlighting the importance of the PI3K-Akt signaling pathway, metabolic reprogramming, and immune microenvironment in disease progression. These findings provide potential therapeutic targets for personalized treatment and offer new directions for future research.
Materials and methods
Clinical sample collection
A patient presented to the Second Xiangya Hospital and was diagnosed with B-ALL. Bone marrow, peripheral blood, and plasma samples were collected from this patient with informed consent. Normal bone marrow cells were also collected from a donor at the Second Xiangya Hospital, Central South University, P.R. China, with informed consent from the volunteer. All consents were obtained in accordance with the Declaration of Helsinki, and the studies were approved by the Human Research Ethics Committee of the Second Xiangya Hospital.
Transcriptomic analysis
Transcriptomic analysis was performed on 24 newly diagnosed cases and 8 relapsed cases, including 15 standard-risk and 9 poor-risk newly diagnosed patients. Adapter sequences were trimmed using Trimmomatic (v0.36), and the trimmed reads were aligned to the reference genome using STAR (v2.6.1d). Gene expression was quantified as fragments per kilobase of exon per million mapped reads (FPKM) using a GTF file for read count conversion. Gene annotations were retrieved from Ensembl using the biomaRt package (org.Hs.eg.db v3.6.0) in R. Student’s t-test was employed to assess the statistical significance of differential gene expression between groups. Volcano plots were generated to visualize p values and fold changes (FC), with p < 0.05 and log2FC > 1 representing upregulated genes, and p < 0.05 and log2FC < − 1 representing downregulated genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted for differentially expressed genes (DEGs), with the top 20 significant pathways highlighted.
Metabolomic analysis
After gradual thawing at 4 °C, serum samples (from 17 normal controls, 20 newly diagnosed cases, and 9 relapsed cases, including 13 standard-risk and 7 poor-risk newly diagnosed patients) were prepared for analysis. For each sample, 30 µL of serum was mixed with pre-cooled methanol/acetonitrile/water solution (2:2:1, v/v), vortexed, sonicated at low temperature for 30 min, and allowed to stand at − 20 °C for 10 min. The samples were centrifuged at 14,000g at 4 °C for 20 min, and the supernatant was dried under vacuum. Dried extracts were reconstituted in 100 µL acetonitrile/water solution (1:1, v/v), vortexed, centrifuged at 14,000g at 4 °C for 15 min, and analyzed by mass spectrometry. Separation was performed using an Agilent 1290 Infinity LC Ultra High-Performance Liquid Chromatography (UHPLC) system equipped with a HILIC column. Samples were analyzed using an AB Triple TOF 6600 mass spectrometer for both MS and MS/MS spectra acquisition.
Raw LC-MS data were processed using XCMS software for baseline correction, peak identification, integration, retention time correction, and peak alignment. The resulting data matrix included retention time, mass-to-charge ratio, and peak intensity. Missing values were handled using the 80% rule, retaining variables only if they had non-zero values in at least 80% of samples within one group. Total ion normalization was applied to correct for sample preparation and instrument variability, followed by log10 transformation of the normalized data. Variables with a relative standard deviation (RSD) > 30% in QC samples were excluded. Metabolites were identified by matching mass spectrometry data with public databases such as HMDB (http://www.hmdb.ca/) and Metlin (https://metlin.scripps.edu/). Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using the ropls package (Version 1.6.2) in R. Significant differential metabolites were identified based on variable importance in projection (VIP) values from the OPLS-DA model and p values from Student’s t-test, with VIP > 1 and p < 0.05 defining significant metabolites. Pathway enrichment and clustering analyses were conducted using MetaboAnalyst 5.0 and the KEGG database (https://www.kegg.jp/kegg/pathway.html) to identify metabolic pathways involving the differential metabolites.
Proteomic analysis
Proteomic analysis was performed on serum samples from five healthy controls and five newly diagnosed patients. Samples were reconstituted in UPLC loading buffer containing 2% acetonitrile (pH 10) and further separated using ACQUITY Ultra Performance Liquid Chromatography (UPLC). Peptide samples were analyzed by online nano-flow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS). The resulting MS/MS raw data were searched against databases using Proteome Discoverer™ (Thermo Scientific, Ver. 2.2). The precursor mass tolerance was set at 20 ppm, and the fragment ion mass tolerance was set at 0.02 Da. Tryptic digestion was allowed with up to two missed cleavages. Carbamidomethylation of cysteine and TMT labeling of peptide N-termini and lysine side chains were set as fixed modifications, while methionine oxidation was considered a dynamic modification. Database searches were performed against NCBI (ftp://ftp.ncbi.nlm.nih.gov/blast/db/) and UniProt (https://www.uniprot.org/) databases. The false discovery rate (FDR) for peptide identification was set at ≤ 0.01, and at least one unique peptide was required for protein identification. PCA was performed using the prcomp function in R, based on expression levels of confidently identified proteins. Differential analysis between the normal and newly diagnosed groups was performed using Student’s t-test. Functional annotation and enrichment of proteins were carried out using the GO and KEGG databases. A significance threshold of P < 0.05 was applied. Protein–protein interaction (PPI) networks were constructed using data from the STRING database (https://string-db.org/). The top 25 proteins with the highest connectivity were selected, and their interactions were visualized using Cytoscape (v3.9.1).
Correlation analysis of transcriptome and metabolome
Paired samples from standard-risk and poor-risk groups were subjected to integrated analysis. Genes and differential metabolites from key pathways were analyzed for Pearson correlation based on one-to-one matching of samples. A p value < 0.05 was considered indicative of a significant correlation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
L.Y and S.Z wrote the main manuscript text and prepared figures. H.P and Z.W conceptualized the entire article and reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers 82070175, 82370128 and 82300208), Hunan Natural Science Foundation of China (Grant Numbers 2023JJ60091, 2024JJ5470 and 2022JJ30830), Ambassador Program for Science and Technology Innovation in Hunan Province (2022RC3074), Hunan Provincial Health and Medical High-Level Talent Support Program.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was approved by the ethics committee of the Second Xiangya Hospital, Central South University, P.R. China. Informed consent was obtained from all patients. This process was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongling Peng and Zhihua Wang contributed equally to this work and should be considered co-corresponding authors.
Contributor Information
Hongling Peng, Email: penghongling@csu.edu.cn.
Zhihua Wang, Email: wangzhihuazsc@csu.edu.cn.
References
- 1.Dores, G. M., Devesa, S. S., Curtis, R. E., Linet, M. S. & Morton, L. M. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood119, 34–43. 10.1182/blood-2011-04-347872 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissel, N. et al. Real-world use of blinatumomab in adult patients with B-cell acute lymphoblastic leukemia in clinical practice: Results from the NEUF study. Blood Cancer J.13, 2. 10.1038/s41408-022-00766-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul, S., Rausch, C. R., Nasnas, P. E., Kantarjian, H. & Jabbour, E. J. Treatment of relapsed/refractory acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol.17, 166–175 (2019). [PubMed] [Google Scholar]
- 4.Lin, C. et al. Integrating RNA-seq and scRNA-seq to explore the biological significance of NAD+ metabolism-related genes in the initial diagnosis and relapse of childhood B-cell acute lymphoblastic leukemia. Front. Immunol.13, 1043111. 10.3389/fimmu.2022.1043111 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jedraszek, K., Malczewska, M., Parysek-Wojcik, K. & Lejman, M. Resistance mechanisms in pediatric B-cell acute lymphoblastic leukemia. Int. J. Mol. Sci.23, 3067. 10.3390/ijms23063067 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan, F., Wong, N. C., Powell, D. R. & Curtis, D. J. A 9-gene score for risk stratification in B-cell acute lymphoblastic leukemia. Leukemia34, 3070–3074. 10.1038/s41375-020-0888-8 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Buchner, M., Swaminathan, S., Chen, Z. & Muschen, M. Mechanisms of pre-B-cell receptor checkpoint control and its oncogenic subversion in acute lymphoblastic leukemia. Immunol. Rev.263, 192–209. 10.1111/imr.12235 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Wang, F. et al. Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia. Nat. Commun.15, 203. 10.1038/s41467-023-44270-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, Y. et al. Lycorine eliminates B-cell acute lymphoblastic leukemia cells by targeting PSAT1 through the serine/glycine metabolic pathway. Eur. J. Pharmacol.961, 176162. 10.1016/j.ejphar.2023.176162 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Yang, K. et al. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics13, 1774–1808. 10.7150/thno.82920 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao, Y. & Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther.221, 107753. 10.1016/j.pharmthera.2020.107753 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida, A. R. M. et al. Interleukin-7 receptor alpha mutational activation can initiate precursor B-cell acute lymphoblastic leukemia. Nat. Commun.12, 7268. 10.1038/s41467-021-27197-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morishita, N. et al. Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatr. Blood Cancer59, 83–89. 10.1002/pbc.24034 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Sarno, J. et al. Dasatinib overcomes glucocorticoid resistance in B-cell acute lymphoblastic leukemia. Nat. Commun.14, 2935. 10.1038/s41467-023-38456-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, L., Zhou, S., Zhou, T., Li, X. & Tang, J. Targeting the lncRNA DUXAP8/miR-29a/PIK3CA network restores doxorubicin chemosensitivity via PI3K-AKT-mTOR signaling and synergizes with inotuzumab ozogamicin in chemotherapy-resistant B-cell acute lymphoblastic leukemia. Front. Oncol.12, 773601. 10.3389/fonc.2022.773601 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Icard, P. et al. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett.471, 125–134. 10.1016/j.canlet.2019.12.010 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Murai, T. & Matsuda, S. Fatty acid metabolites and the tumor microenvironment as potent regulators of cancer stem cell signaling. Metabolites13, 709. 10.3390/metabo13060709 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artico, L. L. et al. IGFBP7 fuels the glycolytic metabolism in B-cell precursor acute lymphoblastic leukemia by sustaining activation of the IGF1R-Akt-GLUT1 axis. Int. J. Mol. Sci.24, 9679. 10.3390/ijms24119679 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minematsu, H. et al. Cancer stem cells induced by chronic stimulation with prostaglandin E2 exhibited constitutively activated PI3K axis. Sci. Rep.12, 15628. 10.1038/s41598-022-19265-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell, J. D., Pollizzi, K. N., Heikamp, E. B. & Horton, M. R. Regulation of immune responses by mTOR. Annu. Rev. Immunol.30, 39–68. 10.1146/annurev-immunol-020711-075024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan, T. et al. Regulation of PI3K signaling in T-cell acute lymphoblastic leukemia: A novel PTEN/Ikaros/miR-26b mechanism reveals a critical targetable role for PIK3CD. Leukemia31, 2355–2364. 10.1038/leu.2017.80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Vivancos, P., de Simone, A., Kiddle, G. & Foyer, C. H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med.89, 1154–1164. 10.1016/j.freeradbiomed.2015.09.023 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Cai, X. et al. XPO1 inhibition displays anti-leukemia efficacy against DNMT3A-mutant acute myeloid leukemia via downregulating glutathione pathway. Ann. Hematol.103, 2311–2322. 10.1007/s00277-024-05706-y (2024). [DOI] [PubMed] [Google Scholar]
- 24.Dixon, S. J. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072. 10.1016/j.cell.2012.03.042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita, K. et al. MiR-7-5p is a key factor that controls radioresistance via intracellular Fe(2+) content in clinically relevant radioresistant cells. Biochem. Biophys. Res. Commun.518, 712–718. 10.1016/j.bbrc.2019.08.117 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun.13, 2206. 10.1038/s41467-022-29905-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, J. et al. Complement and coagulation cascades are associated with prognosis and the immune microenvironment of lower-grade glioma. Transl. Cancer Res.13, 112–136. 10.21037/tcr-23-906 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, X. et al. An immune gene-related five-lncRNA signature for to predict glioma prognosis. Front. Genet.11, 612037. 10.3389/fgene.2020.612037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesselring, R. et al. The complement receptors CD46, CD55 and CD59 are regulated by the tumour microenvironment of head and neck cancer to facilitate escape of complement attack. Eur. J. Cancer50, 2152–2161. 10.1016/j.ejca.2014.05.005 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Laverdiere, I. et al. Complement cascade gene expression defines novel prognostic subgroups of acute myeloid leukemia. Exp. Hematol.44, 1039–1043. 10.1016/j.exphem.2016.07.012 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Lee, J., Mani, A., Shin, M. J. & Krauss, R. M. Leveraging altered lipid metabolism in treating B cell malignancies. Prog. Lipid Res.95, 101288. 10.1016/j.plipres.2024.101288 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.