Abstract
RNA helicases are the largest group of enzymes in eukaryotic RNA metabolism. The DEXD/H-box putative RNA helicases form the helicase superfamily II, whose members are defined by seven highly conserved amino acid motifs, making specific targeting of selected members a challenging pharmacological problem. The translation initiation factor eIF4A is the prototypical DEAD-box RNA helicase that works in conjunction with eIF4B and eIF4H and as a subunit of eIF4F to prepare the mRNA template for ribosome binding, possibly by unwinding the secondary structure proximal to the 5′ m7GpppN cap structure. We report the identification and characterization of a small molecule inhibitor of eukaryotic translation initiation that acts in an unusual manner by stimulating eIF4A-associated activities. Our results suggest that proper control of eIF4A helicase activity is necessary for efficient ribosome binding and demonstrate the feasibility of selectively targeting DEAD-box RNA helicases with small molecules.
Keywords: chemical biology, DEAD-box helicase, pateamine
The ribosome recruitment step of translation initiation is rate-limiting and an important regulatory point whereby cellular environmental cues (e.g., amino acid starvation, mitogenic stimulation, and hypoxia) are linked to the process of translation (1). Two distinct pathways exist for recruitment of the ribosome to the mRNA template. One mechanism is cap-dependent and is facilitated by the presence of the 5′ cap structure (m7GpppN, where N is any nucleotide) on the mRNA. It is catalyzed by the eIF4 class of translation initiation factors and involves the recruitment of ribosomes near the 5′ end of the mRNA template (1). The second mode involves ribosome recruitment in a cap-independent fashion to an internal ribosome entry site (IRES). Initiation factor requirement for internal ribosome binding varies among IRESes, with some not requiring any factors (2).
Preparation of the mRNA template for cap-dependent ribosome recruitment is achieved by eIF4F, eIF4A, eIF4B, eIF4H, and ATP hydrolysis (1). The eIF4F complex is comprised of three subunits: (i) eIF4E, which binds the mRNA cap structure in an ATP-independent fashion; (ii) eIF4A, an RNA helicase that exhibits RNA-dependent ATPase activity and ATP-stimulated RNA binding activity (3); and (iii) eIF4G, a modular scaffold that mediates mRNA binding of the 43S preinitiation complex through interactions with eIF3. eIF4B, and eIF4H cooperate with eIF4A to make its helicase activity more processive (4, 5). eIF4A exists as a free form (referred to herein as eIF4Af) and as a subunit of eIF4F (eIF4Ac) and is thought to cycle through the eIF4F complex during initiation (6-8). When localized in the eIF4F complex, eIF4Ac is ≈20-fold more efficient as an RNA helicase than when found alone (4, 9), leading to the proposal that eIF4Ac is the functional helicase for translation initiation (10). The helicase activity of eIF4F (via eIF4Ac) is thought to unwind local secondary structure in the 5′ UTR of mRNAs to facilitate cap-dependent ribosome recruitment (6-8). In the crystal form, eIF4Af has a distended “dumbbell” structure consisting of two domains (11-13), which undergo conformational changes in response to RNA and ATP binding (14).
There are three eIF4A family members: eIF4AI, eIF4AII, and eIF4AIII. eIF4AI and eIF4AII show 90-95% similarity at the amino acid level, are involved in translation, and appear to have the same biological activity in vitro (15, 16). eIF4AIII is 65% similar to the other isoforms and is implicated in nonsense-mediated decay (17-20). The eIF4A isoforms are members of the DEAD-box putative RNA helicase protein family. These and related DEXD/H (where X is any amino acid) box proteins are characterized by seven highly conserved amino acid sequence motifs implicated in RNA remodeling. These proteins are involved in virtually all aspects of cellular RNA metabolism, including ribosome biogenesis, splicing, translation, and mRNA degradation (for examples, see www.helicase.net). Small molecule targeting of DEXD/H family members would provide mechanistic insight into the properties of these proteins and help define their roles in normal and abnormal cellular and developmental processes. In this report, we identify and characterize a small molecule inhibitor of translation initiation that stimulates the RNA and ATP binding, ATPase, and helicase activities of eIF4Af.
Materials and Methods
Pateamine Isolation and Generation of Affinity Matrix. Pateamine was isolated from Mycale sp., as described (21), and purity was established by NMR to be >95%. For the generation of a pateamine affinity matrix, epoxy-activated Sepharose 6B was prepared according to the manufacturer's instructions and resuspended in 5 volumes of 60 mM pateamine A in methanol containing 66 mM triethylamine. Coupling was performed overnight at 45°C with agitation. After three washes with five volumes of methanol, the resin was lyophilized, resuspended in 1 M diethanolamine, and left overnight at room temperature with agitation to block any remaining epoxide groups. A control resin was generated by incubating the epoxy-activated Sepharose 6B with 1 M aqueous diethanolamine overnight at 4°C. Subsequent wash steps were performed according to the manufacturer's instructions, concluding with three MilliQ water washes. Beads were lyophilized and stored at -20°C until needed.
Protein Purification and Activity Assessment. Mouse eIF4AI and the mutant (with an 76AQSGTGKT to 76VQSGTGKT alteration) cDNAs were subcloned into pET15b. Recombinant proteins were expressed in Escherichia coli BL21 codon +(DE3) and purified by using Ni-NTA agarose and Q Sepharose chromatography. ATPase assays, ATP and RNA crosslinking assays, and helicase assays were performed with recombinant eIF4AI, as described (4, 9, 22, 23). Recombinant Ded1p was expressed in E. coli as a histidine-tagged protein and purified as described (24).
Results
Characterization of an Inhibitor of Eukaryotic Translation. During the course of a high-throughput screening campaign to identify inhibitors of eukaryotic protein synthesis (25), we found a potent inhibitory activity associated with a marine natural product (Fig. 1A). Pateamine is known to be cytotoxic to mammalian cells (21, 26), but its mechanism of action or biological targets are not defined.
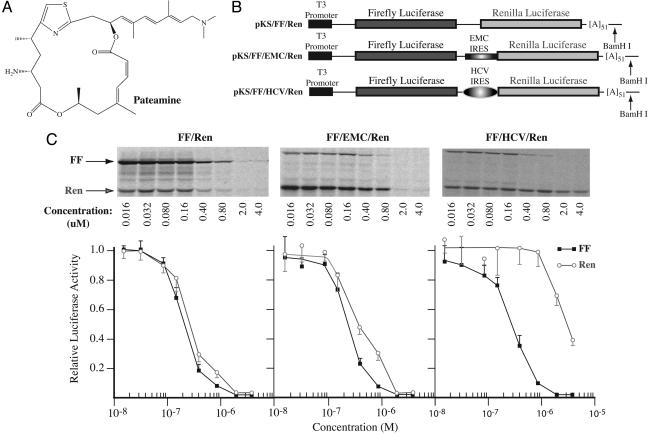
Fig. 1.
Inhibition of eukaryotic translation by pateamine. (A) Chemical structure of pateamine. (B) Schematic diagram of bicistronic constructs used to assess translation inhibition by pateamine. (C) Titration of pateamine in Krebs extracts programmed with bicistronic mRNAs. Translations were performed in the presence of the indicated amounts of pateamine and at a final mRNA and K+ concentration of 5 μg/ml and 100 mM, respectively. Firefly and renilla luciferase activity (RLU) were measured on a Berthold Technologies (Bad Wildbad, Germany) Lumat LB 9507 luminometer. Control translation reactions contained equivalent amounts of DMSO (data not shown). (Upper) Representative autoradiograph from an experiment performed with [35S]methionine. After separation of protein products on 10% polyacrylamide/SDS gels, the gels were treated with EN3Hance (PerkinElmer) dried, and exposed to X-Omat (Kodak) film. (Lower) Graphical representation of the effects of pateamine on translation of bicistronic mRNAs in Krebs extracts. The obtained luciferase activities were normalized to the activity obtained in the absence of compound (which was set at one). Each data point represents the average of three to seven translations, and the standard error of the mean is presented.
A series of dose-response experiments in Krebs translation extracts were performed by using three bicistronic mRNA reporters, two of which contain IRESes (Fig. 1B, FF/EMC/Ren and FF/HCV/Ren). Initiation at the EMC IRES utilizes eIF4G, eIF4A, and eIF4B, whereas these are dispensable for HCV IRES activity (27). For all three reporters, the IC50 for cap-dependent firefly luciferase translation in Krebs extracts was ≈0.2 μM (Fig. 1C). There was moderate renilla luciferase expression from FF/Ren mRNA, presumably due to ribosome reinitiation after termination at the upstream firefly cistron. Inhibition of renilla production from FF/Ren mRNA and from FF/EMC/Ren mRNA (due to internal ribosome recruitment) showed the same IC50 as for cap-dependent translation. However, translation of the renilla ORF from FF/HCV/Ren was ≈10-fold more resistant to inhibition by pateamine (Fig. 1C). No inhibition of prokaryotic translation was observed in E. coli S30 extracts with concentrations up to 10 μM (Fig. 6A, which is published as supporting information on the PNAS web site). These data suggest that at low concentrations (<0.8 μM), pateamine inhibits translation initiation, because expression of only the firefly cistron was decreased when extracts were programmed with FF/HCV/Ren mRNA (Fig. 1C).
Pateamine exerted a similar inhibitory profile on translation of the mRNA reporters in rabbit reticulocyte lysates (Fig. 6B). We therefore used rabbit reticulocyte lysates to address whether pateamine inhibits 48S preinitiation complex formation (Fig. 2 A and B). Complexes were trapped by using the nonhydrolyzable GTP analogue, GMP-PNP, and resolved by sedimentation velocity centrifugation. Pateamine prevented formation of the 48S preinitiation complexes when chloramphenicol acetyl transferase (CAT) mRNA was used as template (Fig. 2 A) but not when the HCV IRES was used (Fig. 2B). These results indicate that pateamine acts before or at the ribosome recruitment step of translation initiation. In addition, they suggest that eIF4A, eIF4B, or eIF4G may be the biological targets of pateamine, because ribosome recruitment to the HCV IRES is independent of these initiation factors (27) and is not affected by pateamine (Fig. 2B).
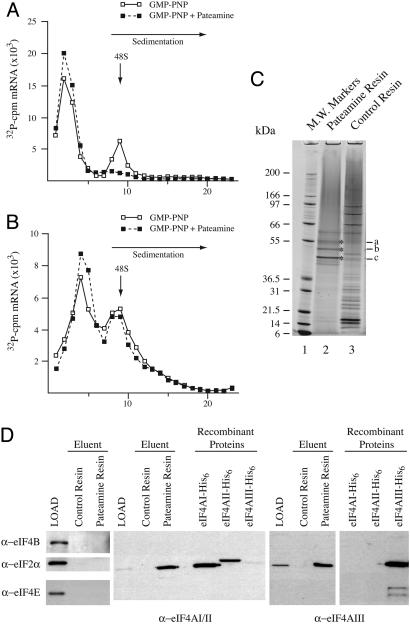
Fig. 2.
Pateamine inhibits ribosome recruitment. (A and B) 32P-labeled CAT and HCV/Ren mRNAs were incubated in rabbit reticulocyte lysate in the presence of 1 mM GMP-PNP or 1 mM GMP-PNP and 10 μM pateamine. After centrifugation (SW40; 39,000 rpm/3.5 h), fractions from each sucrose gradient were collected by using a Brandel (Bethesda, MD) Tube Piercer connected to an ISCO fraction collector and were individually counted. Total counts recovered from each gradient and the percent mRNA bound in 48S complexes were: (A) CAT mRNA/GMP-PNP (64,430 cpm, 21% binding) and CAT mRNA/GMP-PNP+pateamine (62,713 cpm, <0.7% binding), and (B) HCV/Ren mRNA/GMP-PNP (57,844 cpm, 7% binding) and HCV/Ren mRNA/GMP-PNP+pateamine (56,113 cpm, 6% binding). (C) eIF4A is specifically retained on a pateamine-Sepharose affinity matrix. Pateamine was coupled to epoxy-activated Sepharose, and total HL-60 cell extracts were loaded onto pateamine or control resin, washed with 0.1% Triton X-100 in 10 mM PBS, and eluted in SDS/PAGE sample buffer. Proteins were fractionated on a 4-12% polyacrylamide/SDS gel and stained with colloidal Coomassie blue. (D) Immunoblots of HL-60 extract (LOAD) and eluents from control- and pateamine-affinity resins. The antibodies used in the immunoblots are indicated. Recombinant eIF4AI, eIF4AII, and eIF4AIII proteins were used to assess antibody specificity. Recombinant eIF4AII has a higher molecular mass than recombinant eIF4AI due to additional vector-derived sequences.
eIF4A Family Members Are Biological Targets of Pateamine. To identify the biological target(s) of pateamine, total cell extracts were prepared from HL-60 cells and loaded onto an affinity matrix containing immobilized pateamine. After extensive washing, polypeptides specifically retained on the matrix were separated and visualized by using SDS/PAGE. The bands denoted by asterisks were excised from the gel (Fig. 2C, lane 2), as well as the corresponding region of the gel from the control resin (lane 3), and were subjected to trypsin digestion followed by peptide mass fingerprinting (Fig. 2C, indicated with an asterisk and labeled a, b, and c). In silico comparison to peptide fragment masses generated from GenBank identified the proteins specifically retained by pateamine-Sepharose to be cytokeratin (band a), tubulin (band b), and eIF4AI/eIF4AII (band c) (Fig. 7, which is published as supporting information on the PNAS web site, and data not shown).
To assess whether other translation factors were retained on the pateamine affinity resin, we probed the HL-60 extracts (LOAD) and eluents from the control- and pateamine-resins for the presence of eIF4B, eIF2α, and eIF4E (Fig. 2D). None of these factors were recognized by pateamine, because they were not retained on the affinity matrix. In contrast, eIF4AI/eIF4AII and eIF4AIII were specifically retained on the affinity matrix (Fig. 2D). (Note that in this experiment, we cannot distinguish between the eIF4AI and eIF4AII isoforms, due to lack of available isoform-specific antibodies.) These results indicate that members of the eIF4A family are selective targets of pateamine. Given the well documented role of eIF4AI and eIF4AII in translation initiation, and the relatedness of these two proteins, we focused the remainder of this study on characterizing the effects of pateamine on eIF4AI activity.
Pateamine Stimulates eIF4AI Activity. The effect of pateamine on the ATPase activity of eIF4AIf was assessed and surprisingly showed a ≈10-fold increase in initial rate of hydrolysis (Fig. 3A). This effect was RNA-dependent, because no stimulation was observed in the absence of exogenously supplied poly(U) (data not shown). We then assessed whether pateamine affected ATP binding to eIF4AIf. In the absence of poly(U), there was no change in UV-induced crosslinking of ATP to eIF4AIf (Fig. 3B, compare lane 1 with 2). However, in the presence of poly(U), binding of ATP to eIF4AIf was significantly enhanced by pateamine (Fig. 3B, compare lane 3 with 4). The binding of eIF4AIf to RNA has been previously characterized and is stimulated by ATP (Fig. 3C, compare lanes 1 and 3 with 2) (23). Pateamine-stimulated nonsequence-dependent RNA binding of eIF4AIf (Fig. 3C, compare lane 4 with 3) and eliminated the stimulatory requirements of ATP for RNA binding (Fig. 3C, compare lane 6 with 5). We observed the same stimulatory effect on RNA binding by using UV light-induced crosslinking on body-labeled mRNA or using a filter-binding assay in which protein-RNA complexes were trapped on nitrocellulose filters (data not shown).
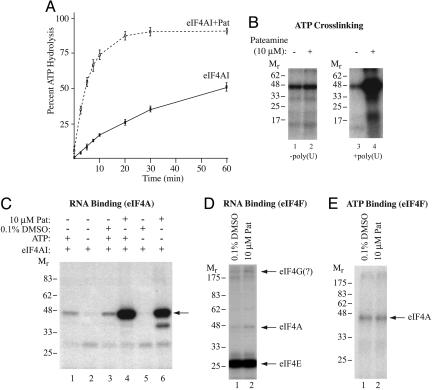
Fig. 3.
Pateamine stimulates eIF4AI activity. (A) Pateamine stimulates eIF4AI-mediated ATP hydrolysis. ATPase assays were performed for the indicated times by using 1 μM γ-[32P]ATP/1 μM poly(U)/3.6 μg of recombinant eIF4AI and monitored by thin-layer chromatography. Quantitations were performed by using Fujix BAS2000 with a Fuji imaging screen, and the data are from a total of three experiments. (B) Pateamine stimulates ATP binding to eIF4AI in the presence of RNA. Crosslinking of ATP was performed with 1 μg of recombinant eIF4AI and 2.5 μCi (Ci = 37 GBq) of α-[32P]ATP (3,000 Ci/mmol) by using UV light and resolved by SDS/PAGE. The presence or absence of 7.5 μM poly(U) is indicated below. The gel was dried and exposed to x-ray film (Kodak) at -80°C for 12 h with an intensifying screen. (C) Pateamine stimulates RNA-binding activity of eIF4AI. 32P-cap-labeled CAT mRNA (105 cpm) was incubated with recombinant eIF4AI (1 μg) for 10 min at 30°C, chemically crosslinked, treated with RNase A, and resolved by SDS/PAGE. Gels were dried and exposed to x-ray film (Kodak) at -80°C for 1 h with an intensifying screen. (D) Chemical crosslinking of eIF4F to 32P-cap labeled oxidized mRNA. The presence of 10 μM pateamine is indicated at the top. Components of the eIF4F complex are labeled to the right. The gel was dried and exposed to x-ray film (Kodak) at -80°C with an intensifying screen. (E) UV light-induced crosslinking of α-[32P]ATP to eIF4Ac. Crosslinking of ATP was performed with 0.74 μg of eIF4F and 2.5 μCi of α-[32P]ATP (3,000 Ci/mmol) by using UV light and resolved by SDS/PAGE. The gel was dried and exposed to x-ray film (Kodak) at -80°C.
We also assessed whether pateamine affected the RNA- and ATP-binding properties of eIF4Ac (Fig. 3 D and E). Toward this end, eIF4F was chemically crosslinked to 32P-cap labeled oxidized mRNA in the absence or presence of pateamine (Fig. 3D). No observable effect on the RNA-binding properties of the eIF4E and eIF4Ac subunits was noted. eIF4E crosslinking was inhibited by m7GDP, and eIF4Ac crosslinking was ATP-dependent (data not shown). Crosslinking of α-[32P]ATP to eIF4Ac was not stimulated by pateamine (Fig. 3E). We conclude that pateamine affects primarily eIF4AIf's ATP- and RNA-binding properties.
The effect of pateamine on the RNA helicase activity of eIF4AIf was assessed (Fig. 4A). As previously documented, eIF4AIf is capable of unwinding an RNA duplex with a ΔG = -17.9 kcal/mol (Fig. 4A, compare lane 3 with 1) (4). This process requires ATP (compare lane 5 with 3) and is defective when an eIF4AIf mutant [in which the ATPase A motif (76AQSGTGKT) is mutated to (76VQSGTGKT)] is used. This mutation abolishes the ability of eIF4AIf to bind and hydrolyze ATP, as well as to unwind RNA duplexes (Fig. 4A, compare lane 7 with 3) (9). Increasing the stability of the RNA duplex impairs eIF4AIf's helicase activity (Fig. 4A, compare lane 10 with 3) (4). Pateamine did not inhibit eIF4AIf's helicase activity when using the RNA-1/RNA-11 duplex as substrate (Fig. 4A, compare lane 4 with 3), nor did it eliminate the ATP requirement for helicase activity (Fig. 4A, compare lane 6 with 4). When we used an RNA duplex with a ΔG = -21.4 kcal/mol as substrate, pateamine stimulated eIF4AIf helicase activity on this more stable duplex (compare lane 11 with 10).
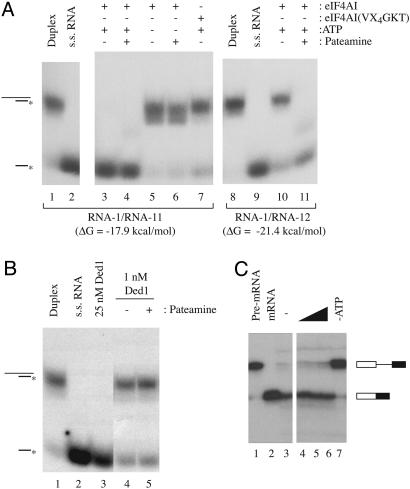
Fig. 4.
Pateamine stimulates eIF4AI helicase activity. (A) Reactions were performed with RNA duplexes RNA-1/RNA-11 or RNA-1/RNA-12 for 15 min at 35°C in the presence of 0.36 μg of recombinant protein. The presence or absence of 1 mM ATP or 10μM pateamine is indicated. Reactions were resolved on 12% native gels, which were dried, and exposed to x-ray film (Kodak) at -80°C for 12 h with an intensifying screen. The position of migration of duplex or radioactive single-strand RNA is indicated to the left. (B) Helicase assays performed with recombinant Ded1p protein in the presence of pateamine. Conditions were similar to those described for eIF4AI. (C) In vitro splicing reactions in the presence of pateamine. In vitro splicing reactions were performed with the AdML pre-mRNA and analyzed, as described (17). Reaction products were separated on a 15% polyacrylamide/8 M urea gel, which was dried, and exposed to X-Omat (Kodak) x-ray film at -80°C for 1 h. The position of migration of the pre-mRNA (lane 1) and spliced mRNA (lane 2) is indicated to the right. Splicing reactions were performed without pateamine (lane 3), in the presence of increasing concentrations of pateamine [lane 4 (0.5 μM), lane 5, (2 μM), and lane 6 (10 μM)], or in the absence of exogenously added ATP (lane 7).
To gauge the selectivity of pateamine for eIF4A, we assessed its effects on another DEAD box family member, Ded1p, implicated in translation initiation in yeast (28, 29). No stimulatory (or inhibitory) effect on the helicase activity of Ded1p was observed when pateamine was present in helicase assays with Ded1p (Fig. 4B, compare lane 5 with 4). Splicing of eukaryotic mRNAs depends on the activity of 7-13 DEXD/H box helicases (30, 31). The addition of pateamine to an in vitro splicing reaction did not inhibit (Fig. 4C) or stimulate (data not shown) processing of the input mRNA template, indicating that pateamine is not a general inhibitor of splicing.
Pateamine Inhibits Cap-Dependent Translation in Vivo. Pateamine is a potent in vivo inhibitor of protein synthesis showing an IC50 of 5 nM in HeLa cells (Fig. 8A, which is published as supporting information on the PNAS web site). Metabolic labeling experiments revealed little effect on DNA or RNA synthesis when HeLa cells were exposed to 1 μM compound for 1 h (Fig. 8B). Whereas cells recovered from a translational block imposed by anisomycin within 1 h after removal from the media, no recovery is observed up to 6 h after removal of pateamine from the media, suggesting that binding of pateamine to its target is either extremely strong or irreversible (Fig. 8C). Analysis of polysomes from pateamine-treated cells revealed that very little translating ribosomes remain mRNA associated after a 20-min exposure to pateamine (Fig. 8D).
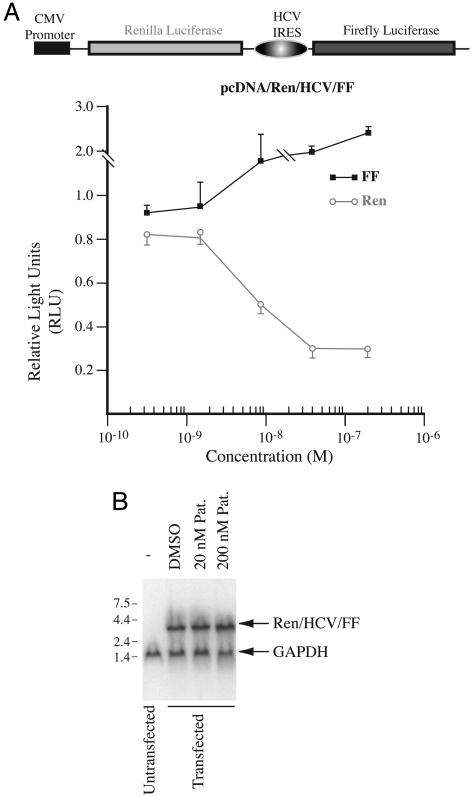
If eIF4A is the relevant biological target of pateamine in mediating its inhibitory effect on translation initiation in vivo, then pateamine should not affect translation initiation mediated by the HCV IRES, under conditions where cap-dependent protein synthesis is inhibited. This was tested by transfecting HeLa cells with the bicistronic reporter, pcDNA/Ren/HCV/FF, and monitoring luciferase production in response to increasing pateamine concentration (Fig. 5A). Expression of renilla luciferase was inhibited in a dose-dependent fashion by pateamine, whereas firefly luciferase expression increased slightly in response to higher levels of pateamine (Fig. 5A). These results are unlikely the consequence of nonspecific mRNA degradation or activation of a cryptic promoter within the HCV IRES, because no evidence of truncated transcripts was observed by Northern blot analysis of the RNA from pcDNA/Ren/HCV/FF-transfected cells (Fig. 5B).
Fig. 5.
Pateamine inhibits cap-dependent protein synthesis in vivo. (A) Dose-response experiment of pateamine on cells transfected with pcDNA/Ren/HCV/FF. HeLa cells were incubated with the indicated concentrations of pateamine for 10 h, at which time luciferase activity was measured in cell extracts. The relative luciferase activity was determined by comparing to the activity obtained in control cells exposed to DMSO. The average of two experiments is presented with the error of the mean. (B) Northern blot of RNA isolated from cells transfected with pcDNA/Ren/HCV/FF. After transfection, cells were incubated with the indicated concentrations of pateamine. RNA was isolated, fractionated on a 1.2% agarose/formaldehyde gel, and transferred to Hybond N+ (Amersham Pharmacia Biosciences). The blot was then probed with radiolabeled cDNA fragments to Ren/HCV/FF and GAPDH (position of migration indicated). Probes were produced with the Readiprime kit using the manufacturer's recommendations (Amersham Pharmacia).
Discussion
Pateamine is a potent small molecule inhibitor of eukaryotic translation. Several lines of evidence indicate that eIF4A family members are the relevant biological targets of pateamine. (i) eIF4AI/II and eIF4AIII are retained on a pateamine affinity resin, whereas eIF4E, eIF2α, and elF4B are not (Fig. 2D). (ii) Initiation from the HCV IRES does not require eIF4A (27) and accordingly is not inhibited by concentrations of pateamine where cap-dependent translation is inhibited, both in vitro and in vivo (Figs. 1C, 2B, and 5A). (iii) Pateamine stimulates several eIF4Af associated activities, including RNA-dependent ATP hydrolysis, ATP and RNA binding, and RNA unwinding (Figs. 3 and 4). (iv) The compound appears selective for eIF4Af, because it failed to affect the helicase activity of the DEAD box family member, Ded1p (Fig. 4B) and of a DNA helicase from Pseudomonas aeruginosa, named dnaB (data not shown). In addition, no effect was observed on in vitro splicing reactions (Fig. 4C). Although it is difficult to assess whether the activity of other cellular helicases is affected by pateamine, none were readily identified by direct binding to a pateamine affinity resin (Fig. 2C).
The mechanistic details of how eIF4A prepares the mRNA template for ribosome recruitment are not well understood. A current model postulates that eIF4F binds to the mRNA 5′ cap structure to deliver eIF4A to the mRNA. This is suggested from experiments showing that a dominant-negative mutant of eIF4A inhibits eIF4F helicase and cap-binding activity by forming a more stable complex with eIF4G, presumably inhibiting eIF4Af recycling through eIF4F (7, 8). In addition, the 20-fold higher helicase activity of eIF4Ac relative to eIF4Af suggests that eIF4Ac is the functional helicase for translation (6, 10). An eIF4A-binding domain within eIF4G (amino acid 737-774) alters the conformation of eIF4A to favor RNA binding (32). Once released on the mRNA, eIF4A (in conjunction with eIF4B and/or eIF4H) is thought to use ATP hydrolysis to melt the secondary structure (4, 6, 10). ATP hydrolysis may also be required for the initial release of eIF4A from the eIF4F complex (8), as well as for the release of eIF4F from the m7G cap structure (6, 23). Each initiation round may require multiple eIF4F-binding events to deposit eIF4A on the mRNA template (7), and eIF4F complexes devoid of the eIF4A subunit no longer recycle (6).
We propose that binding of pateamine to eIF4Af presets the protein in the high-affinity RNA-binding state, suggested by the ability of pateamine to stimulate eIF4Af RNA binding in the absence of ATP (Fig. 3C). This in turn stimulates ATP binding, hydrolysis, and RNA unwinding (Figs. 3 A and B and 4A). There are several models that could explain how pateamine inhibits translation. (i) Pateamine may inhibit channeling of eIF4Af through the eIF4F complex. This would occur if a different conformation than pateamine-bound eIF4Af is required for eIF4G binding or the pateamine-binding site on eIF4A overlaps with or interferes in an allosteric fashion with eIF4G binding. If this model is correct, pateamine could be a useful tool for distinguishing between the pioneer round of translation and subsequent initiation rounds. Pateamine does not seem to alter the ATP- or RNA-binding properties of eIF4Ac (Fig. 3 D and E) and thus is unlikely to bind to eIF4A already present in the eIF4F complex. Consistent with this, eIF4E (Fig. 2D) and eIF4G (data not shown) did not copurify with eIF4A on the pateamine-affinity resin. (ii) Pateamine may cause unscheduled unwinding by eIF4Af on mRNA templates. eIF4Af is not thought to participate directly in translation initiation events but rather to be channeled through eIF4F (described above). Pateamine-stimulated direct binding of eIF4Af to RNA would be nonspecific and the resulting helicase activity likely not useful for translation, potentially interfering with initiation or elongation events. This may explain the inhibition observed on HCV driven translation at higher pateamine concentrations (>4 μM), which does not appear to be a consequence of reduced ribosome recruitment (Fig. 2B). A similar inhibitory effect was observed on CrPV IRES-mediated translation at 10 μM pateamine in Krebs extract (data not shown), an IRES with no eIF dependency for 40S ribosome recruitment (33). This inhibitory effect on HCV-driven translation is not observed in vivo (Fig. 5A), reflecting either limitations of the in vitro translation system or the lower concentrations required to achieve inhibition of cap-dependent translation in vivo. The slight stimulatory effect seen in vivo on HCV IRES-mediated translation at higher concentrations of pateamine may represent a competitive advantage for the HCV IRES (e.g., more available eIF3, eIF2, or ribosomal subunits) when cap-dependent translation is inhibited. (iii) Pateamine may uncouple the coordinated activities between eIF4Af and eIF4B and/or eIF4H. eIF4B, and eIF4H stimulate the ATPase (3, 5, 6, 34), RNA-binding (5, 34, 35), and helicase activities (4, 6, 10, 36) of eIF4Af and eIF4Ac. This model would suggest that tight regulation of eIF4Af activity is essential for optimal translation initiation on cap-dependent mRNA templates, as well as on eIF4A-dependent IRESes (Figs. 1C and 5A). Current efforts are aimed at differentiating among these possibilities, but clearly chemical perturbation of eIF4Af's intrinsically weak helicase activity has catastrophic consequences on protein synthesis.
Pateamine is functionally distinct from other reported inhibitors of eIF4A. A dominant-negative mutant of eIF4A has been described that associates more strongly with eIF4G than wild-type eIF4A and subsequently inhibits eIF4E crosslinking to the cap structure (8). Translation inhibition by this dominant-negative mutant is thought to be a consequence of inhibiting eIF4A recycling (8). RNA aptamers targeting eIF4A have also been described that impair its ATPase activity and inhibit cap-dependent translation (37). Third, the tumor suppressor Pdcd4 is an inhibitor of translation that interacts with eIF4A and independently with eIF4G (38) and is thought to prevent translation by competing with eIF4G for binding to eIF4A and/or inhibiting eIF4A's helicase activity. Fourth, DAP5/p97 functions as a general repressor of translation by forming translationally inactive complexes that include eIF4A and eIF3 but not eIF4E (39). Pateamine is the only small molecule inhibitor of translation that increases eIF4A's helicase activity, and that can readily be used in vivo. The characterization of pateamine demonstrates the feasibility of selectively targeting DEXD/H box RNA helicases with small molecule inhibitors for pharmacological intervention.
Supplementary Material
Acknowledgments
We are grateful to Isabelle Harvey and Anne-Sophie Guenier for their experimental expertise during the course of this work and to Dr. Yuri Svitkin for helpful comments on the manuscript. We are grateful to Dr. Melissa Moore for help with the in vitro splicing reactions. We thank Ann Brasey and Maria Ferraiuolo for advice regarding purification of recombinant eIF4A and anti-eIF4A immunoblotting. We thank the Jean-Louis Lévesque Foundation and Valorisation-Recherche Québec for their contribution and support of the McGill Biochemistry and Cancer Center High Throughput Screening Facility. M.-E.B. was supported by a Canadian Institutes of Health Research (CIHR) Cancer Consortium Training Grant Award and a Fonds de Recherche en Santé du Québec studentship award. This work was supported by National Cancer Institute of Canada Grant 014313 (to J.P.). J.P. is a CIHR Senior Investigator.
Abbreviations: IRES, internal ribosome entry site; CAT, chloramphenicol acetyl transferase.
References
- 1.Hershey, J. W. B. & Merrick, W. C. (2000) Initiation of Protein Synthesis (Cold Spring Habor Lab. Press, Plainview, NY).
- 2.Wilson, J. E., Pestova, T. V., Hellen, C. U. & Sarnow, P. (2000) Cell 102, 511-520. [DOI] [PubMed] [Google Scholar]
- 3.Grifo, J. A., Abramson, R. D., Satler, C. A. & Merrick, W. C. (1984) J. Biol. Chem. 259, 8648-8654. [PubMed] [Google Scholar]
- 4.Rogers, G. W., Jr., Richter, N. J. & Merrick, W. C. (1999) J. Biol. Chem. 274, 12236-12244. [DOI] [PubMed] [Google Scholar]
- 5.Richter-Cook, N. J., Dever, T. E., Hensold, J. O. & Merrick, W. C. (1998) J. Biol. Chem. 273, 7579-7587. [DOI] [PubMed] [Google Scholar]
- 6.Ray, B. K., Lawson, T. G., Kramer, J. C., Cladaras, M. H., Grifo, J. A., Abramson, R. D., Merrick, W. C. & Thach, R. E. (1985) J. Biol. Chem. 260, 7651-7658. [PubMed] [Google Scholar]
- 7.Pause, A., Methot, N., Svitkin, Y., Merrick, W. C. & Sonenberg, N. (1994) EMBO J. 13, 1205-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svitkin, Y. V., Pause, A., Haghighat, A., Pyronnet, S., Witherell, G., Belsham, G. J. & Sonenberg, N. (2001) RNA 7, 382-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pause, A. & Sonenberg, N. (1992) EMBO J. 11, 2643-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozen, F., Edery, I., Meerovitch, K., Dever, T. E., Merrick, W. C. & Sonenberg, N. (1990) Mol. Cell. Biol. 10, 1134-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benz, J., Trachsel, H. & Baumann, U. (1999) Structure Fold. Des. 7, 671-679. [DOI] [PubMed] [Google Scholar]
- 12.Caruthers, J. M., Johnson, E. R. & McKay, D. B. (2000) Proc. Natl. Acad. Sci. USA 97, 13080-13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, E. R. & McKay, D. B. (1999) RNA 5, 1526-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorsch, J. R. & Herschlag, D. (1998) Biochemistry 37, 2194-2206. [DOI] [PubMed] [Google Scholar]
- 15.Conroy, S. C., Dever, T. E., Owens, C. L. & Merrick, W. C. (1990) Arch. Biochem. Biophys. 282, 363-371. [DOI] [PubMed] [Google Scholar]
- 16.Yoder-Hill, J., Pause, A., Sonenberg, N. & Merrick, W. C. (1993) J. Biol. Chem. 268, 5566-5573. [PubMed] [Google Scholar]
- 17.Shibuya, T., Tange, T. O., Sonenberg, N. & Moore, M. J. (2004) Nat. Struct. Mol. Biol. 11, 346-351. [DOI] [PubMed] [Google Scholar]
- 18.Ferraiuolo, M. A., Lee, C. S., Ler, L. W., Hsu, J. L., Costa-Mattioli, M., Luo, M. J., Reed, R. & Sonenberg, N. (2004) Proc. Natl. Acad. Sci. USA 101, 4118-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palacios, I. M., Gatfield, D., St Johnston, D. & Izaurralde, E. (2004) Nature 427, 753-757. [DOI] [PubMed] [Google Scholar]
- 20.Chan, C. C., Dostie, J., Diem, M. D., Feng, W., Mann, M., Rappsilber, J. & Dreyfuss, G. (2004) RNA 10, 200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northcote, P. T., Blunt, J. W. & Munro, M. H. G. (1991) Tetrahedron Lett. 32, 6411-6414. [Google Scholar]
- 22.Lorsch, J. R. & Herschlag, D. (1998) Biochemistry 37, 2180-2193. [DOI] [PubMed] [Google Scholar]
- 23.Abramson, R. D., Dever, T. E., Lawson, T. G., Ray, B. K., Thach, R. E. & Merrick, W. C. (1987) J. Biol. Chem. 262, 3826-3832. [PubMed] [Google Scholar]
- 24.Fairman, M. E., Maroney, P. A., Wang, W., Bowers, H. A., Gollnick, P., Nilsen, T. W. & Jankowsky, E. (2004) Science 304, 730-734. [DOI] [PubMed] [Google Scholar]
- 25.Novac, O., Guenier, A. S. & Pelletier, J. (2004) Nucleic Acids Res. 32, 902-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hood, K. A., West, L. M., Northcote, P. T., Berridge, M. V. & Miller, J. H. (2001) Apoptosis 6, 207-219. [DOI] [PubMed] [Google Scholar]
- 27.Pestova, T. V., Shatsky, I. N., Fletcher, S. P., Jackson, R. J. & Hellen, C. U. (1998) Genes Dev. 12, 67-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang, R. Y., Weaver, P. L., Liu, Z. & Chang, T. H. (1997) Science 275, 1468-1471. [DOI] [PubMed] [Google Scholar]
- 29.de la Cruz, J., Iost, I., Kressler, D. & Linder, P. (1997) Proc. Natl. Acad. Sci. USA 94, 5201-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staley, J. P. & Guthrie, C. (1998) Cell 92, 315-326. [DOI] [PubMed] [Google Scholar]
- 31.Jurica, M. S. & Moore, M. J. (2003) Mol. Cell 12, 5-14. [DOI] [PubMed] [Google Scholar]
- 32.Korneeva, N. L., First, E. A., Benoit, C. A. & Rhoads, R. E. (2005) J. Biol. Chem. 280, 1872-1881. [DOI] [PubMed] [Google Scholar]
- 33.Jan, E. & Sarnow, P. (2002) J. Mol. Biol. 324, 889-902. [DOI] [PubMed] [Google Scholar]
- 34.Richter, N. J., Rogers, G. W., Jr., Hensold, J. O. & Merrick, W. C. (1999) J. Biol. Chem. 274, 35415-35424. [DOI] [PubMed] [Google Scholar]
- 35.Abramson, R. D., Dever, T. E. & Merrick, W. C. (1988) J. Biol. Chem. 263, 6016-6019. [PubMed] [Google Scholar]
- 36.Rogers, G. W., Jr., Richter, N. J., Lima, W. F. & Merrick, W. C. (2001) J. Biol. Chem. 276, 30914-30922. [DOI] [PubMed] [Google Scholar]
- 37.Oguro, A., Ohtsu, T., Svitkin, Y. V., Sonenberg, N. & Nakamura, Y. (2003) RNA 9, 394-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, H. S., Jansen, A. P., Komar, A. A., Zheng, X., Merrick, W. C., Costes, S., Lockett, S. J., Sonenberg, N. & Colburn, N. H. (2003) Mol. Cell. Biol. 23, 26-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imataka, H., Olsen, H. S. & Sonenberg, N. (1997) EMBO J. 16, 817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.