Abstract
Background
Urinary tract infections (UTIs) are a significant global health issue, especially among women, with growing concerns related to antibiotic resistance and adverse effects. The Uromune®, a sublingual, heat-inactivated, polybacterial vaccine, represents a promising therapeutic alternative by enhancing immune responses against uropathogens.
Methods
This pilot retrospective study, conducted at Hospital Universitari de Sant Joan de Reus from January 2018 to August 2022, assessed the association between Uromune® administration and changes in recurrent UTIs. Patients received personalized autovaccines administered as two sublingual puffs daily for three months. Clinical, microbiological, and demographic data were analyzed to assess treatment outcomes and identify recurrence-associated factors.
Results
Forty-nine patients (mean age, 61 years, and 59.2% women) were included in the study. Uromune® treatment decreased UTI episodes from 3.73 ± 0.97 the year before to 0.98 ± 1.36 (p < 0.001) the year after its administration. The number of patients who suffered three or more episodes per year dropped from 43 (87.7%) before the intervention to 7 (14.3%) afterwards. The maximum effectiveness of the autovaccine was observed three months post-administration, with 44 patients not experiencing any UTI episodes. Regression analysis identified having had a urostomy, chronic kidney disease, and being immunosuppressed as predictors of recurrence.
Conclusion
Uromune® autovaccine was associated with a significant reduction in the frequency of recurrent UTIs and related hospitalizations, offering substantial relief to patients. These findings suggest that Uromune® may be a promising option for managing recurrent UTIs, though controlled studies are needed to confirm its efficacy compared to standard treatments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10524-2.
Keywords: Antibiotic resistance, Immunotherapy, Recurrent urinary tract infections, Sublingual bacterial vaccine, Uromune® vaccine
Introduction
Urinary tract infections (UTIs) are a significant global health concern, affecting millions worldwide. These infections are caused by various bacterial species invading the urinary tract, potentially leading to functional or morphological disorders [1]. Recurrent UTIs (rUTIs) profoundly impact the quality of life, influencing mental health, social interactions, work productivity, and sexual well-being [2, 3]. This condition presents a substantial clinical challenge, particularly for women, who are disproportionately affected. The limitations of traditional management strategies, which heavily depend on antibiotics, have become increasingly evident. Growing concerns about antibiotic resistance and the adverse effects of long-term antibiotic use have intensified interest in alternative therapies [4].
Uromune®, a vaccine composed of a glycerinated suspension of heat-inactivated whole bacterial cells, presents a promising alternative to combat rUTIs. The vaccine is administered sublingually, stimulating the mucosal immune system, especially the gut-associated lymphoid tissue, which plays a crucial role in the body’s immune defense [5–7]. By exposing the immune system to these inactivated bacteria, Uromune® aims to promote the production of specific antibodies and bolster the overall immune response, thereby preventing the colonization and infection of the urinary tract. The sublingual route of immunization is increasingly favored for its ability to bypass degradation by gastric enzymes and induce immune responses in various tissues, including the genitourinary tract [8, 9]. Studies have shown that Uromune®, when administered sublingually, triggers both systemic and genitourinary immune responses through T-cell activation and innate immunity. This mechanism supports its use as a sublingual vaccination strategy [10].
Since its initial report in 2013 [11], several studies have reported a reduction in the frequency of rUTIs following Uromune® administration, with good tolerance and minimal side effects. These studies have shown the vaccine’s effectiveness across diverse patient populations, including varying ages, sexes, and underlying conditions [5–7, 12–15]. According to a recent systematic review, this formulation is currently in phase 3 of clinical development as part of the pre-licensing process and is available through named patient and other special access programs in 26 countries [7]. Developed by Immunotek S.L. (Alcalá de Henares, Spain) and commercialized by Q Pharma S.L. (Alicante, Spain), Uromune® was approved by the Spanish Agency for Medicines and Health Products in October 2010 to prevent rUTIs. In March 2023, the European Association of Urology Guidelines on Urological Infections recognized the polybacterial Uromune® as a beneficial immunoprophylactic agent [16]. These guidelines are expected to facilitate the broader and more consistent use of this vaccine globally.
Uromune® offers a hopeful outlook for managing rUTIs with minimal side effects. The vaccine was available in two formulations: (1) a generic formulation, termed MV140, consisting of a mixture of equal amounts of selected strains of Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, and Enterococcus faecalis, and (2) an autovaccine, composed of cultures derived directly from the patient’s urine. Since January 2018, regulatory changes in Spain have mandated that Uromune® be manufactured solely as an autovaccine [5]. Despite its potential, data on the effects of this autovaccine remains limited.
This study exclusively investigated the Uromune® autovaccine. We did not study the MV140 formulation. We aimed to expand global knowledge regarding the association between autovaccine administration and the reduction of rUTI episodes and to explore the influence of comorbidities and other patient-specific factors on treatment outcomes. The findings of our study provide further insights into the potential benefits of this autovaccine in managing recurrent UTIs.
Methods
Patients’ characteristics and study design
We conducted a pilot retrospective study of all patients treated with Uromune® for rUTIs in Hospital Universitari de Sant Joan de Reus, Tarragona, Spain, between January 21, 2018, and August 31, 2022. This hospital, a member of the Hospital Network for Public Use in the Autonomous Community of Catalonia, Spain, has 367 inpatient beds and a 20-bed Intensive Care Unit. Serving a population of over 175,000, the hospital provides care to primary facilities and elderly residences in the region.
The study included cases with complicated or uncomplicated rUTI. Exclusion criteria were being under 18 years of age or having a cognitive impairment that prevented comprehension of instructions or the ability to provide written informed consent. UTI was defined as the presence of 100,000 or more colony-forming units of bacteria in the urinary system, accompanied by symptoms [1]. rUTI was diagnosed in patients with three or more episodes of UTI within one year or two episodes within six months [2]. At baseline, all patients underwent a comprehensive assessment, including anamnesis, abdominal and genital physical examinations, urine cultures, blood tests, and ultrasounds of the urinary tract with postvoid volume measurement. Additional diagnostic procedures such as computed tomography scans, urethra-cystoscopy, urine cytology, or urodynamics were performed for patients showing signs of complicated UTI. As follow-up, anamnesis and urine culture were performed at 3, 6, and 12 months after the end of treatment. “Recurrence” in this study refers to a clinical concept rather than a microbiological one. Patients were considered to have had no recurrence of UTI episodes if they did not report symptoms such as fever or urinary discomfort during follow-up visits, regardless of the presence of microorganisms in urine cultures. This approach emphasizes clinical resolution of symptoms rather than solely microbiological outcomes.
Clinical, microbiological, and demographic data were meticulously gathered from patients’ electronic medical records by the research staff, who individually reviewed each record to ensure thoroughness and accuracy. The McCabe score was calculated to assess clinical prognosis [17], and the Charlson index was used to categorize patient comorbidities [18]. Three urologists and four internists participated in the study.
Personalized preparation and administration protocol for the Uromune® autovaccine
In this study, only the Uromune® autovaccine was administered to patients. This autovaccine was personalized and tailored to each patient based on cultures derived from their own urine. The midstream portion of the patient’s first-morning urine was collected in a sterile container under strict hygienic conditions. This method was chosen to ensure that the vaccine was specifically designed to target the bacteria present in the patient’s urinary tract, thereby enhancing its effectiveness. Patients were given a sample transport kit, including a cotton swab, a plastic tube with a culture medium, and a padded envelope. The swab was used to inoculate the culture tube, which was then securely sealed and placed in the padded envelope for delivery to a pharmacy and subsequent shipment to Q Pharma/Immunotek Laboratories in Alicante, Spain. Based on the culture results, the laboratory prepared an individualized autovaccine, which was then returned to the pharmacy and subsequently delivered to the patient, ensuring safe and secure transportation.
Microorganisms were isolated and identified directly from patients’ urine samples using standard microbiological techniques. These included phenotypic methods, such as selective and differential media to observe colony morphology, color, and hemolytic properties, as well as biochemical tests like catalase, coagulase, oxidase, and the Analytical Profile Index. The identified strains were then used to prepare personalized autovaccines, specifically tailored to target the pathogens present in each patient’s urinary tract.
Only the bacteria isolated from the patient’s sample were used in the formulation; type strains were excluded. For patients with a single uropathogen, a mono-species autovaccine was prepared using the isolated organism. In cases where multiple pathogens were identified, the autovaccine included an equal representation of all isolated species. The pathogens were heat-inactivated and adjusted to a final concentration of 300 Formazin Turbidity Units for sublingual administration, ensuring a precise and individualized treatment approach.
The vaccine was administered as two sublingual puffs at one time, once daily on an empty stomach for three months. Patients were advised to avoid swallowing saliva for one minute after the vaccine administration and to abstain from eating, drinking, or brushing their teeth for 30 min after that to maximize absorption. Treatment was temporarily halted only in the presence of fever, regardless of its cause, to prioritize patient safety and minimize the risk of complications. Once the fever subsided and patients were clinically stable, treatment was promptly resumed, and the full 3-month treatment period was completed as originally planned. Each puff, approximately 100 µL in volume, contained 108 heat-inactivated whole bacteria. The formulation consisted of 100% of the bacteria isolated from the patient’s urine culture; if two bacterial strains were present, each strain was represented at a concentration of 50%.
Statistical analyses
Unless otherwise specified, results are presented as numbers and percentages or as means and standard deviations. The Fisher’s exact test was used to identify differences in categorical data, while the Mann-Whitney U test was applied to compare means between two groups. Analysis of variance (ANOVA) was employed to examine the relationship between a continuous outcome and multiple predictor variables. Statistical significance was defined as p < 0.05. All analyses were conducted using SPSS 25.0 statistical software (SPSS Inc., Chicago, IL, USA), and graphs with GraphPrism version 9 (GraphPad, Boston, MA, USA).
Results
Patient demographics and clinical characteristics
We included 49 patients of Caucasian ethnicity. Seven patients had experienced two UTI episodes within six months, while the remaining 42 had experienced three or more episodes within one year. The mean age at the start of therapy was 61 years, ranging from 14 to 92 years. Twenty-nine (59.2%) of these patients were women. Eleven patients (22.4%) were current smokers, and six (12.2%) regularly consumed alcoholic beverages. Thirty-two patients (65.3%) were treated in the Urology Department, while 17 (34.7%) were treated in the Internal Medicine Department. The most common comorbidities were cardiovascular diseases, including arterial hypertension (67.3%) and dyslipidemia (40.8%). Cystitis was the most prevalent type of urinary infection (59.3%), with most infections caused by Gram-negative bacteria (81.6%).
When patients were analyzed by sex, men exhibited fewer instances of urinary incontinence but a higher incidence of urostomies. In men, rUTIs were commonly associated with orchitis, prostatitis, or urinary catheter infections, whereas in women, they were frequently linked to cystitis. (Table 1). The individual data are shown in Supplementary Table 1.
Table 1.
Demographic and clinical characteristics of included patients
| Variable | Total (n = 49) |
Women (n = 29) |
Men (n = 20) |
p-Value1 | Recurrence2 (n = 24) |
No recurrence2 (n = 25) |
p-Value3 |
|---|---|---|---|---|---|---|---|
| Age, years | 61 (18) | 57 (20) | 66 (13) | 0.226 | 61 (15) | 61 (20) | 0.795 |
| Sex, women | 29 (59.2) | - | - | - | 11 (45.8) | 18 (72.0) | 0.085 |
| Smoking | 11 (22.4) | 6 (21.7) | 5 (25.0) | 0.740 | 7 (29.2) | 4 (16.0) | 0.321 |
| Alcohol intake | 6 (12.2) | 2 (6.9) | 4 (20.0) | 0.210 | 4 (16.7) | 2 (8.0) | 0.417 |
| Recurrence | - | 18 (62.1) | 7 (35.0) | 0.085 | - | - | - |
| Department of admission | 0.017 | 0.769 | |||||
| Urology | 32 (65.3) | 23 (79.3) | 9 (45.0) | 15 (62.5) | 17 (68.0) | ||
| Internal Medicine | 17 (34.7) | 6 (20.7) | 11 (55.0) | 9 (37.5) | 8 (32.0) | ||
| UCC | 13 (26.5) | 7 (24.1) | 6 (30.0) | 0.747 | 6 (25.0) | 7 (28.0) | 1.000 |
| UIP | 21 (42.9) | 18 (62.1) | 3 (15.0) | 0.001 | 8 (33.3) | 13 (52.0) | 0.252 |
| Urostomy | 6 (12.2) | 1 (3.4) | 5 (25.0) | 0.035 | 4 (16.7) | 2 (8.0) | 0.417 |
| Nephrolitiasis | 10 (20.4) | 7 (24.1) | 3 (15.0) | 0.496 | 4 (16.7) | 6 (24.0) | 0.725 |
| Dialysis | 1 (2.0) | 1 (3.4) | 0 (0.0) | 1.000 | 0 (0.0) | 1 (4.0) | 1.000 |
| Neurogenic bladder | 8 (16.3) | 7 (24.1) | 1 (5.0) | 0.119 | 3 (12.5) | 5 (20.0) | 0.702 |
| OUD | 25 (51.0) | 12 (41.4) | 13 (65.0) | 0.148 | 15 (62.5) | 10 (40.0) | 0.156 |
| CKD | 9 (18.4) | 5 (17.2) | 4 (20.0) | 1.000 | 7 (29.2) | 2 (8.0) | 0.074 |
| T2DM | 16 (32.7) | 7 (24.1) | 9 (45.0) | 0.215 | 10 (41.7) | 6 (24.0) | 0.232 |
| CVD | 33 (67.3) | 16 (55.2) | 17 (85.0) | 0.035 | 18 (75.0) | 15 (60.0) | 0.364 |
| HF–IHD | 3 (6.1) | 1 (3.4) | 2 (10.0) | 0.559 | 1 (4.2) | 2 (8.0) | 1.000 |
| CLD | 2 (4.1) | 2 (6.9) | 0 (0.0) | 0.507 | 0 (0.0) | 2 (8.0) | 0.490 |
| CLUD | 5 (10.2) | 3 (10.3) | 2 (10.0) | 1.000 | 3 (12.5) | 2 (8.0) | 0.667 |
| CND | 4 (8.2) | 3 (10.3) | 1 (5.0) | 0.636 | 2 (8.3) | 2 (8.0) | 1.000 |
| Immunodepression | 7 (14.3) | 4 (13.8) | 3 (15.0) | 1.000 | 4 (16.7) | 3 (12.0) | 0.702 |
| Cancer | 3 (6.1) | 1 (3.4) | 2 (10.0) | 0.559 | 2 (8.3) | 1 (4.0) | 0.609 |
| Obesity | 14 (28.6) | 8 (27.6) | 6 (30.0) | 0.931 | 7 (29.2) | 7 (28.0) | 0.932 |
| Dyslipidemia | 20 (40.8) | 13 (44.8) | 7 (35.0) | 0.349 | 10 (41.7) | 10 (40.0) | 1.000 |
| Anxiety-depression | 13 (26.5) | 11 (37.9) | 2 (10.0) | 0.047 | 7 (29.2) | 6 (24.0) | 0.754 |
| CEVD | 3 (6.1) | 2 (6.9) | 1 (5.0) | 1.000 | 1 (4.2) | 2 (8.0) | 1.000 |
| Dementia | 3 (6.1) | 2 (6.9) | 1 (5.0) | 1.000 | 1 (4.2) | 2 (8.0) | 1.000 |
| Antibiotics allergy | 9 (18.4) | 6 (20.7) | 3 (15.0) | 0.720 | 4 (16.7) | 5 (20.0) | 1.000 |
| Menopause | 20 (40.8) | 20 (69.0) | 0 (0.0) | < 0.001 | 7 (29.2) | 13 (52.0) | 0.148 |
| Charlson index | 0.937 | 0.244 | |||||
| 0 | 23 (46.9) | 15 (51.7) | 8 (40.0) | 9 (37.5) | 14 (56.0) | ||
| 1 | 15 (30.6) | 8 27.6) | 7 (35.0) | 8 (33.3) | 7 (28.0) | ||
| 2 | 5 (10.2) | 3 (10.3) | 2 (10.0) | 2 (8.3) | 3 (12.0) | ||
| 3 | 4 (8.2) | 2 (6.9) | 2 (10.0) | 4 (16.7) | 0 (0.0) | ||
| 4 | 2 (4.1) | 1 (3.4) | 1 (5.0) | 1 (4.2) | 1 (4.0) | ||
| Mc Cabe score | 0.422 | 0.419 | |||||
| NFD | 44 (89.8) | 27 (93.1) | 17 (85.0) | 23 (95.8) | 20 (80.0) | ||
| UFD | 3 (6.1) | 1 (3.4) | 2 (10.0) | 1 (4.2) | 3 (12.0) | ||
| RFD | 2 (4.1) | 1 (3.4) | 1 (5.0) | 0 (0.0) | 2 (8.0) | ||
| Type of urinary tract infection | < 0.001 | 0.731 | |||||
| Cystitis | 29 (59.2) | 23 (79.3) | 6 (30.0) | 13 (54.2) | 16 (64.0) | ||
| Pyelonephritis | 9 (18.4) | 6 (20.7) | 3 (15.0) | 4 (16.7)) | 5 (20.0) | ||
| Orchitis | 1 (2.0) | 0 (0.0) | 1 (5.0) | 1 (4.2) | 0 (0.0) | ||
| Prostatitits | 7 (14.3) | 0 (0.0) | 7 (35.0) | 5 (20.8) | 2 (8.0) | ||
| Urostomy infection | 2 (4.1) | 0 (0.0) | 2 (10.0) | 1 (4.2) | 1 (4.0) | ||
| Urinary catheter infection | 1 (2.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 1 (4.0) | ||
| Type of bacteria | 0.468 | 1.000 | |||||
| Gram-positive | 6 (12.2) | 2 (6.9) | 4 (20.0) | 3 (12.5) | 3 (12.0) | ||
| Gram-negative | 40 (81.6) | 25 (86.2) | 15 75.0) | 20 (83.3) | 20 (80.0) | ||
| Mixed | 3 (6.1) | 2 (6.9) | 1 (5.0) | 1 (4.2) | 2 (8.0) |
1 Comparing women vs. men. 2 Columns 6 and 7 display the studied variables, categorized based on whether patients experienced recurrent episodes of urinary tract infection or not. 3 Comparing recurrence vs. no recurrence. Results are shown as numbers and percentages or as means and SD. CEVD: Cerebrovascular disease; CKD: Chronic kidney disease; CLD: Chronic liver disease; CLUD: Chronic lung disease; CND: Chronic neurological or neuromuscular disease; CVD: Cardiovascular disease; HF–IHD: Heart failure– ischemic heart disease; NFD: Non-fatal disease; OUD: Other urological diseases (Polypoid cystitis, intravaginal urethral meatus, scrotal genital edema and phimosis, retroperitoneal adenopathy, benign prostatic hyperplasia, urethral stricture, abscessed pyelonephritis, hydronephrosis, polycystic kidney disease); RFD: Rapidly fatal disease; T2DM: Type 2 diabetes mellitus; UCC: Urinary catheter carrier; UFD: Ultimately fatal disease; UIP: Urinary incontinence– prolapse. The Fisher’s exact test was used to identify differences in categorical data, while the Mann-Whitney U test was applied to compare means between two groups (age). Significant p-values are highlighted to emphasize the key findings
Influence of Uromune® autovaccine administration on UTI recurrence
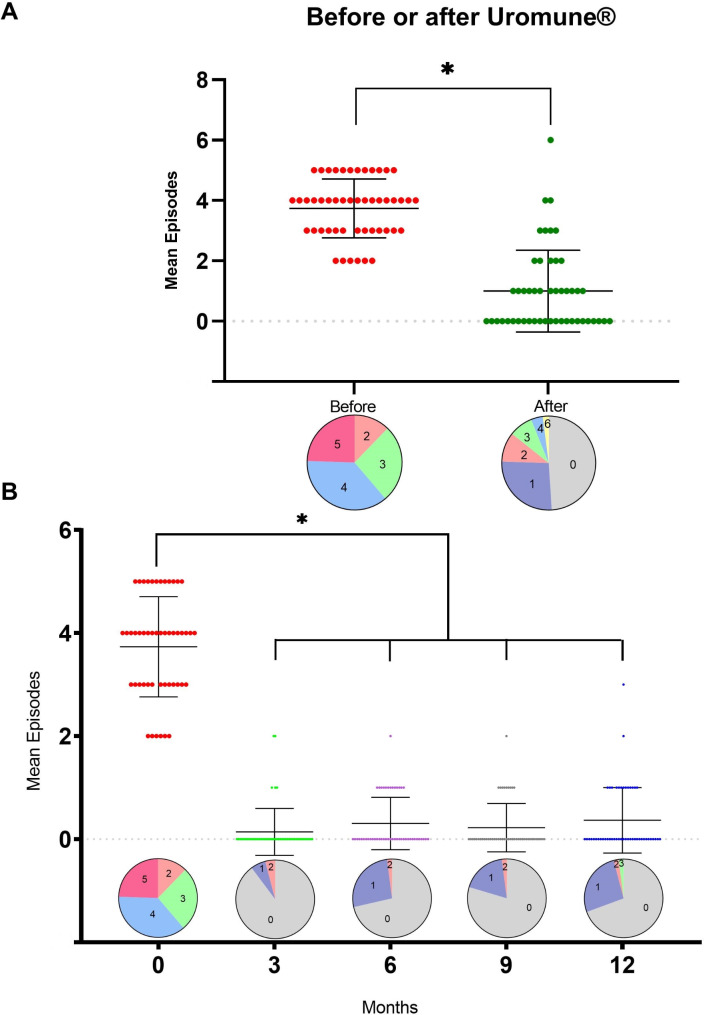
Uromune® treatment was associated with a decrease in UTI episodes, from 3.73 ± 0.97 (mean ± SD) the year before to 0.98 ± 1.36 (p < 0.001) the year after the autovaccine administration. Twenty-four patients did not report any episode of UTI during the first year post-Uromune®. Similarly, the number of patients who suffered three or more episodes per year dropped from 43 (87.7%) before the intervention to 7 (14.3%) afterward (Fig. 1A). The maximum effectiveness of the autovaccine was observed three months post-administration, with 44 patients not experiencing any UTI episode, three patients experiencing one episode, and two patients having two episodes. While there was a slight increase in UTI during the remainder of the follow-up period, the incidence remained considerably lower than before treatment (Fig. 1B).
Fig. 1.
Total number of urinary tract infection (UTI) recurrences, with pie charts comparing the number of UTI episodes in the year before and after Uromune® administration (A). Monthly breakdown of UTI recurrences, with pie charts showing the distribution of episodes by month following treatment (B). The pie charts represent the percentage of patients grouped by the number of UTI recurrences they experienced. The numbers within each segment indicate the actual number of recurrences for that group. The colors are used solely for visual differentiation and do not carry any specific meaning. For example, in panel A, before treatment, 12.2% of patients had 2 recurrences, 26.5% had 3, 36.7% had 4, and 24.5% had 5. After treatment, 49% of cases had no recurrences, 26.5% had 1, 10.2% had 2, 8.2% had 3, 4.1% had 4, and 2.0% had 6. Results are shown as individual values, means and standard deviations. * p < 0.001 by the Mann-Whitney U test
Similarly, the trend in hospital admissions followed that of UTI episodes, decreasing from 0.53 ± 1.24 before treatment to 0.20 ± 0.76 afterward, although differences did not reach statistical significance, probably due to the low number of hospitalized patients. Before treatment, 13 patients required hospitalization; one needed five hospitalizations, and one needed six. After treatment, only six patients required hospitalization, with just one needing five hospitalizations (Fig. 2A). The most substantial reduction in hospitalizations, similar to the decrease in UTI episodes, was observed three months after starting treatment, although the differences did not reach statistical significance (Fig. 2B).
Fig. 2.
Total number of hospital admissions, with pie charts comparing the number of admissions in the year before and after Uromune® administration (A). Monthly breakdown of hospital admissions, with pie charts showing the distribution of admissions by month following treatment (B). The pie charts represent the percentage of patients grouped by the number of hospital admissions. The numbers within each segment indicate the actual number of admissions for that group. The colors are used solely for visual differentiation and do not carry any specific meaning. For example, in panel A, before treatment, 73.5% of patients had no admissions, 18.4% had 1, 4.1% had 3, and 2.0% had 5, and 2.0% had 6. After treatment, 87.8% of cases had no admissions, 10.2% had 1, and 2.0 had 5. Results are shown as individual values, means and standard deviations
Factors influencing UTI recurrence after Uromune® autovaccine administration
We did not find any significant differences in demographic characteristics, department of care, associated diseases, or comorbidities between patients who experienced recurrent infections after autovaccine administration and those who did not (Table 1). However, ANOVA including demographic variables, the most relevant urological complications and comorbidities (those with a frequency > 10%), and the Charlson and McCabe indices, revealed that having undergone a urostomy, having chronic kidney disease or being immunosuppressed were significantly and independently associated with an increased number of recurrences following autovaccine administration (Table 2).
Table 2.
Multiple regression analysis of the variables associated with the number of recurrences
| Variable | B | SD | Beta | p-Value |
|---|---|---|---|---|
| Age | -0.007 | 0.016 | -0.088 | 0.148 |
| Sex, women | 0.123 | 0.609 | 0.045 | 0.841 |
| Smoking | -0.874 | 0.541 | -0.272 | 0.117 |
| Alcohol intake | -1.233 | 0.760 | -0.302 | 0.115 |
| UCC | 0.361 | 0.720 | 0.119 | 0.620 |
| UIP | 0.350 | 0.551 | 0.129 | 0.530 |
| Urostomy | 1.839 | 0.863 | 0.450 | 0.042 |
| Nephrolitiasis | -0.194 | 0.634 | -0.058 | 0.761 |
| NB | -0.090 | 0.811 | -0.025 | 0.913 |
| OUD | -0.822 | 0.443 | -0.307 | 0.068 |
| CKD | -1.393 | 0.576 | -0.403 | 0.022 |
| T2DM | -0.170 | 0.597 | -0.059 | 0.778 |
| CVD | -0.660 | 0.684 | -0.231 | 0.342 |
| CLUD | -1.053 | 0.862 | -0.238 | 0.231 |
| Immunodepression | -1.345 | 0.621 | -0.351 | 0.039 |
| Obesity | -0.130 | 0.265 | -0.078 | 0.628 |
| Dyslipidemia | 0.183 | 0.463 | 0.067 | 0.695 |
| Charlson index | 0.289 | 0.313 | 0.242 | 0.364 |
| Mc Cabe score | 0.841 | 0.465 | 0.301 | 0.081 |
| Constant | 1.570 | 1.057 | 0.148 |
The first column lists the independent variables, and “Beta” represents the standardized regression coefficient, indicating the strength and direction of the relationship with the outcome. Significant p-values are highlighted to emphasize the key findings. Model summary: r = 0.704; p = 0.157. CKD: Chronic kidney disease; CLUD: Chronic lung disease; CVD: Cardiovascular disease; NB: Neurogenic bladder; OUD: Other urological diseases (Polypoid cystitis, intravaginal urethral meatus, scrotal genital edema and phimosis, retroperitoneal adenopathy, benign prostatic hyperplasia, urethral stricture, abscessed pyelonephritis, hydronephrosis, polycystic kidney disease); T2DM: Type 2 diabetes mellitus; UCC: Urinary catheter carrier; UIP: Urinary incontinence– prolapse
Influence of Uromune® autovaccine administration on bacteria isolated from urine cultures
Details of these findings are shown in Table 3. One year after Uromune® administration, bacterial growth was detected in urine cultures from 30 of the 49 patients (61.2%), while fungi of the genus Candida were identified in 2 cases (4.1%). The culture was polymicrobial in 13 cases before treatment and 2 cases after. The most frequently isolated bacterium was Escherichia coli, identified in 25 cases before treatment. It was also the most persistent, being detected in 20 cases despite intervention. This was followed by Klebsiella pneumoniae, with 9 cases before and 7 after. Other common bacteria included Enterococcus faecalis (10 cases before treatment, 2 after) and Proteus mirabilis (8 cases before treatment, none after).
Table 3.
Bacterial species identified in urine cultures before and one year after Uromune® autovaccine administration
| Case | Before Uromune® | After Uromune® | Number of recurrences after Uromune® |
|---|---|---|---|
| 1 | Enterococcus faecalis | Enterococcus faecalis | 2 |
| 2 |
Klebsiella pneumoniae Escherichia coli |
Klebsiella pneumoniae | 4 |
| 3 |
Escherichia coli Proteus mirabilis |
Escherichia coli | 0 |
| 4 | Escherichia coli | Escherichia coli | 1 |
| 5 | Escherichia coli | Escherichia coli | 0 |
| 6 | Escherichia coli | Escherichia coli | 2 |
| 7 | Proteus mirabilis | Klebsiella pneumoniae | 0 |
| 8 | Escherichia coli | - | 0 |
| 9 |
Escherichia coli Klebsiella pneumoniae Enterococcus faecalis |
Escherichia coli Klebsiella pneumoniae |
0 |
| 10 | Escherichia coli | Escherichia coli | 1 |
| 11 | Proteus mirabilis | - | 0 |
| 12 |
Pseudomonas aeruginosa Enterococcus faecalis |
Candida albicans | 0 |
| 13 |
Escherichia coli Enterobacter aerogenes |
Escherichia coli | 2 |
| 14 | Escherichia coli | Escherichia coli | 0 |
| 15 |
Klebsiella pneumonia Escherichia coli Enterococcus faecalis Proteus vulgaris |
Escherichia coli | 0 |
| 16 | Escherichia coli | Escherichia coli | 1 |
| 17 | Klebsiella pneumoniae | Klebsiella pneumoniae | 2 |
| 18 | Proteus mirabilis | Klebsiella oxytoca | 3 |
| 19 | Enterococcus faecalis | Escherichia coli | 3 |
| 20 | Enterococcus faecalis | Escherichia coli | 4 |
| 21 |
Klebsiella pneumoniae Escherichia coli |
Escherichia coli | 0 |
| 22 | Escherichia coli | - | 0 |
| 23 | Escherichia coli | Candida parapsilosis | 1 |
| 24 | Escherichia coli | - | 1 |
| 25 |
Proteus mirabilis Citrobacter koseri |
Klebsiella pneumoniae | 0 |
| 26 |
Pseudomonas aeruginosa Proteus mirabilis |
Klebsiella pneumoniae | 1 |
| 27 | Escherichia coli | - | 3 |
| 28 |
Escherichia coli Enterococcus faecalis |
- | 3 |
| 29 |
Klebsiella pneumoniae Escherichia coli Enterobacter cloacae Proteus vulgaris |
- | 0 |
| 30 | Escherichia coli | Escherichia coli | 2 |
| 31 | Escherichia coli | Escherichia coli | 0 |
| 32 | Enterococcus faecalis | - | 0 |
| 33 |
Morganella morganii Enterococcus faecalis |
- | 1 |
| 34 |
Enterococcus faecalis Staphylococcus sp. |
- | 0 |
| 35 | Escherichia coli | Escherichia coli | 1 |
| 36 | Pseudomonas sp. | Escherichia coli | 0 |
| 37 | Klebsiella pneumoniae | - | 0 |
| 38 |
Escherichia coli Pseudomonas aeruginosa |
- | 0 |
| 39 |
Pseudomonas aeruginosa Klebsiella pneumoniae Enterococcus faecalis |
Klebsiella pneumoniae Proteus vulgaris Morganella morganii |
1 |
| 40 | Citrobacter freundii | - | 0 |
| 41 |
Enterococcus faecalis Serratia marcescens |
Pseudomonas aeruginosa | 6 |
| 42 | Proteus mirabilis | - | 1 |
| 43 | Escherichia coli | Escherichia coli | 1 |
| 44 | Staphylococcus haemolyticus | Escherichia coli | 1 |
| 45 | Pseudomonas aeruginosa | - | 0 |
| 46 | Candida tropicalis | - | 1 |
| 47 |
Klebsiella pneumoniae Proteus mirabilis |
- | 1 |
| 48 | Pseudomonas aeruginosa | Escherichia coli | 0 |
| 49 | Escherichia coli | Enterococcus faecalis | 0 |
Not all patients with a positive urine culture one year after treatment exhibited clinical symptoms, such as fever or urinary discomfort, typically associated with recurrence. Of the 30 patients with positive cultures, 5 were asymptomatic.
No clear correlation was observed between the type of microorganism and a large number of recurrence episodes after treatment. For instance, the only patient who experienced 6 recurrence episodes was initially infected with Enterococcus faecalis and Serratia marcescens before treatment and later with Pseudomonas aeruginosa after treatment. This patient had a history of prostatitis and a urinary catheter infection. Aditionally, two patients experienced 4 recurrence episodes each. Both suffered from cystitis. One patient was initially infected with Escherichia coli and Proteus mirabilis before treatment and later with Escherichia coli after treatment. The other patient was infected with Enterococcus faecalis before treatment and Escherichia coli after.
Urinary catheter status and UTI outcomes
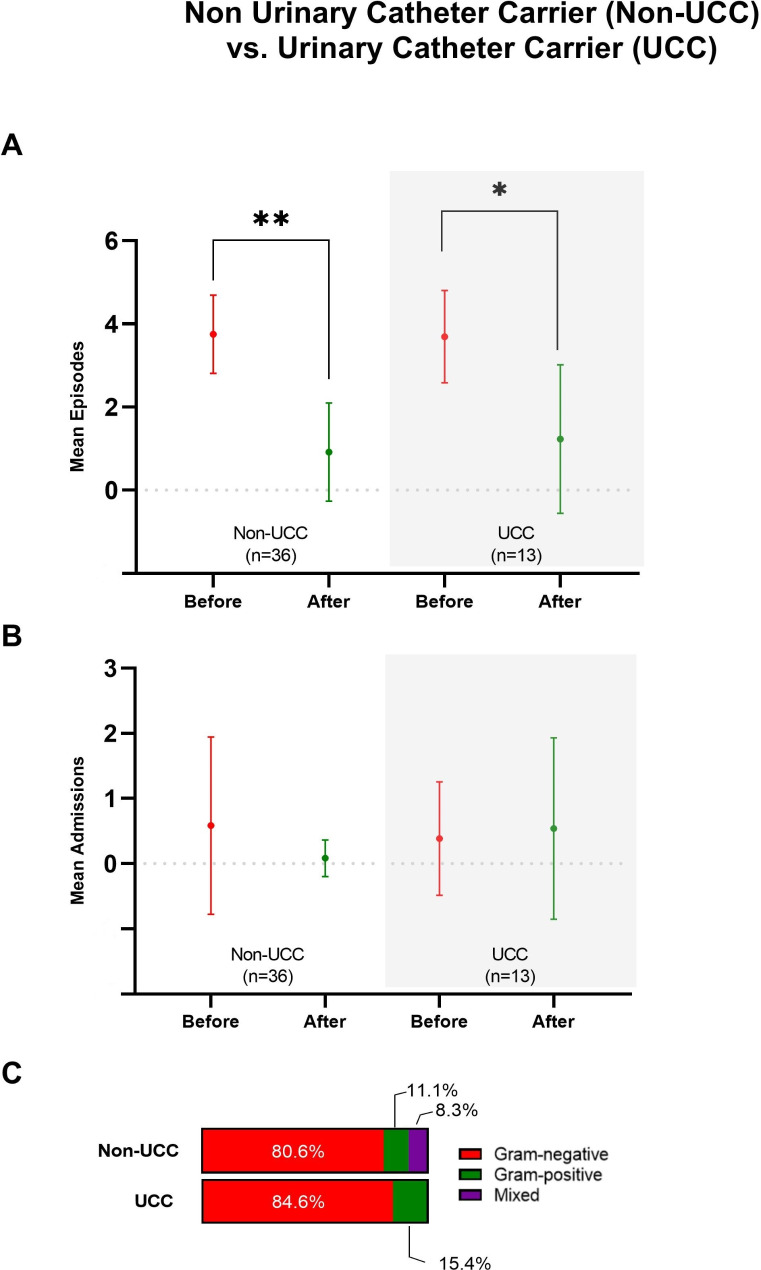
We compared the reduction in UTIs between patients with and without a urinary catheter, acknowledging that catheter-associated infections often have a different etiology than uncomplicated UTIs and that catheter withdrawal may influence the outcomes. Our analysis revealed no significant differences between the two groups in the number of UTI episodes or hospital admissions, either before or after the administration of the autovaccine (Fig. 3A and B).
Fig. 3.
Total episodes of urinary tract infections (A), hospital admissions (B), and types of microorganisms isolated (C) in patients categorized by urinary catheter carrier (UCC) status, before and after Uromune® administration. Results are shown as means and standard deviations. * p < 0.01, ** p < 0.001 by the Mann-Whitney U test
Similarly, no notable differences were observed in the frequency of microorganisms causing the infections. Among patients without a catheter, the most common pathogens before treatment were Escherichia coli, Enterococcus faecalis, and Proteus mirabilis, while Escherichia coli and Klebsiella pneumoniae predominated afterwards. In catheter carriers, Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecalis were the most frequent pathogens before treatment, with Escherichia coli and Klebsiella pneumoniae remaining predominant afterwards (Fig. 3C; Table 4).
Table 4.
Number of urinary tract infection episodes by microorganism in patients with and without urinary catheters, before and after Uromune® administration
| Microorganism | Number of episodes | Frequency (%) |
|---|---|---|
| No urinary catheter carrier, before Uromune ® | ||
| Citrobacter koseri | 1 | 2.8 |
| Enterobacter cloacae | 1 | 2.8 |
| Enterococcus faecalis | 6 | 16.7 |
| Escherichia coli | 17 | 47.2 |
| Klebsiella pneumoniae | 3 | 8.3 |
| Proteus mirabilis | 5 | 13.9 |
| Proteus vulgaris | 2 | 5.6 |
| Pseudomonas aeruginosa | 1 | 2.8 |
| No urinary catheter carrier, after Uromune® | ||
| None | 13 | 36.1 |
| Candida albicans | 1 | 2.8 |
| Enterococcus faecalis | 1 | 2.8 |
| Escherichia coli | 13 | 36.1 |
| Klebsiella pneumoniae | 5 | 13.9 |
| Morganella morganii | 1 | 2.8 |
| Proteus vulgaris | 1 | 2.8 |
| Pseudomonas aeruginosa | 1 | 2.8 |
| Urinary catheter carrier, before Uromune® | ||
| Enterobacter aerogenes | 1 | 7.7 |
| Enterococcus faecalis | 2 | 15.4 |
| Escherichia coli | 4 | 30.8 |
| Klebsiella pneumoniae | 3 | 23.1 |
| Morganella morganii | 1 | 7.7 |
| Proteus mirabilis | 1 | 7.7 |
| Pseudomonas aeruginosa | 1 | 7.7 |
| Urinary catheter carrier, after Uromune® | ||
| None | 4 | 30.7 |
| Candida parapsilosis | 1 | 7.7 |
| Escherichia coli | 5 | 38.5 |
| Klebsiella oxytoca | 1 | 7.7 |
| Klebsiella pneumoniae | 2 | 15.4 |
Adverse effects
No relevant adverse effects were reported during the Uromune® autovaccine administration period, even in patients with comorbidities. While no hospitalizations or admissions were directly attributed to the autovaccine, the possibility of its involvement in certain events, such as fever, cannot be entirely excluded in the absence of a control group.
Discussion
A systematic review reported the potential of Uromune® in treating rUTIs, presenting it as a promising alternative to antibiotics [5]. However, despite the encouraging findings, the application of this vaccine to clinical practice remains limited. The recent inclusion of Uromune® in the European Association of Urology’s Guidelines on Urological Infections [16] has opened up a significant opportunity for widespread adoption. This development presents a hopeful prospect for the management of rUTIs. Most studies to date have focused on MV140 generic formulation, and just a few have reported reported on the utility of the autovaccine [19, 20]. This new endorsement has inspired us to share the experience of our institution with this therapy, further fueling our hope for the future of rUTIs treatment.
Our study evaluated 49 patients with a diverse age range, reflecting a broad spectrum of the population affected by rUTIs. The gender distribution was slightly skewed, with women constituting most of the cohort, aligning with the known higher prevalence of UTIs in females. Lifestyle factors included relevant percentages of current smokers and patients regularly consuming alcohol, both of which can impact immune function and potentially affect susceptibility to infections [21]. The average age in our study is consistent with previous reports [2, 5, 6, 15] and reflects the typical aged profile of patients with UTIs who need to be seen in a hospital consultation. Most patients received treatment in the Urology Department, with the remainder treated in Internal Medicine, indicating that these infections often necessitate specialized care. The high prevalence of urological disorders and urinary incontinence underscores the significant burden of these conditions in our population. Additionally, many patients were using urinary catheters, a known risk factor for UTI. The comorbidity profile revealed a high prevalence of cardiovascular diseases and dyslipidemia, which is expected in older individuals. These comorbid conditions might contribute to the complexity of managing UTI in these patients. The high rate of Gram-negative bacterial infections aligns with common etiological patterns observed in patients with UTI [22].
The observed sex differences in rUTI presentation and management highlight distinct underlying clinical patterns. In men, rUTIs were often associated with conditions such as orchitis and prostatitis. In contrast, rUTIs in women were predominantly linked to cystitis. These differences reflect complex clinical scenarios that may require tailored therapeutic approaches, and underscore the need for sex-specific considerations in the diagnosis and treatment of rUTIs, ensuring that management strategies address the differing underlying causes and clinical circumstances effectively.
We have also analyzed changes in the urinary flora identified through culture during the first year after the administration of Uromune®. Several findings are noteworthy and merit discussion.
Firstly, in several cases, the microorganism identified post-treatment differed from the one isolated pre-treatment. This may reflect the inclusion of complex patients in our study, such as those with neurogenic bladders or urostomies, where the manipulation of catheters or collection bags significantly increases the risk of reinfection or microbiota shifts [22]. In two cases, post-treatment infections were caused by fungi of the genus Candida, likely linked to excessive antibiotic use, which can disrupt the balance of the urinary and gastrointestinal microorganisms [23].
Secondly, our results indicate that Escherichia coli remains not only the most frequently isolated uropathogen but also the most persistent following treatment. This observation aligns with its role as a key component of the human gut microbiota and its ability to act as a reservoir for recurrent infections. The resilience of Escherichia coli is likely due to its adaptive capacity and colonization advantage within the urinary tract [24].
Lastly, the persistence of the same causative organism in 38.8% (19/49) of patients one year after Uromune® administration aligns with the therapeutic nature of the vaccine. Uromune® is designed to modulate the immune response and alleviate clinical symptoms of recurrent UTIs rather than eradicating the causative microorganism. While this persistence may suggest a possibility of further recurrence in these patients, it is important to note that the vaccine has demonstrated significant reductions in the frequency and severity of symptomatic episodes. This observation underscores the vaccine’s primary objective of improving patient quality of life, even in cases where the uropathogen remains detectable. These findings highlight the need for ongoing research to explore the relationship between microbial persistence, immune modulation, and clinical outcomes in autovaccine [25].
Over the past decade, immunoprophylaxis with vaccines has demonstrated to be useful in reducing recurrent UTIs and healthcare costs compared to antibiotic therapy, with a lower risk of bacterial resistance. Studies have shown that both autovaccines and the MV140 vaccine effectively reduce recurrent UTIs, although outcomes vary based on sample size and follow-up duration. Smaller studies have generally reported higher success rates, while larger studies observed reduced effectiveness [2, 12, 15]. Additionally, some studies suggest that autovaccines may be more effective [5, 20]. The present study shows that the administration of Uromune® autovaccine was associated with a substantial reduction in UTI episodes. Twenty-four patients did not report any episodes in the first year post-administration, and the proportion of patients experiencing three or more episodes per year decreased dramatically. This marked improvement was most pronounced three months after initiating the treatment, where 44 patients remained episode-free, indicating the peak effectiveness of the autovaccine. The autovaccine reduced the frequency of UTIs, the severity of symptoms, and the need for antibiotic use. Although there was a slight uptick in the incidence of UTIs over the subsequent months, the overall rates stayed much lower than pre-treatment levels. This slight increase could be due to factors such as the natural progression of the disease or a certain development of resistance to the autovaccine. Our data are consistent with those previously reported with the generic preparation MV140 [2, 5, 6, 13, 15, 20, 26, 27]. A recent randomized placebo-controlled efficacy study demonstrated that treatment with MV140 was associated with reducing the burden of UTI, reducing symptoms and antibiotic use, and significantly improving patients’ quality of life [27].
There is very little data on the effects of autovaccines; however, two reports suggest that, as in our case, they are associated with reduced UTI episodes [19, 20]. We also found that hospital admissions decreased notably in the year following the Uromune® autovaccine administration. Before treatment, 13 patients required hospitalization, with several experiencing multiple hospitalizations. Post-treatment, only six patients needed hospitalization, suggesting a substantial reduction in severe episodes necessitating hospital care. However, while the trend in reduced hospitalizations was clear, it did not achieve statistical significance, potentially due to the limited sample size or variability in patient responses.
The possible mechanism of action of Uromune® has been previously studied in mice and human cells in culture [10]. These studies suggested that the vaccine likely exerts its effects by modulating both local and systemic immune responses through the induction of a balanced Th1/Th17/interleukin-10 T cell profile. Sublingual administration of Uromune® engages human dendritic cells (DCs) via Syk- and MyD88-dependent pathways, leading to the activation of NF-κB and p38 signaling pathways, which are critical for generating Th1 and Th17 responses. These responses may enhance protective immunity by promoting the recruitment and activation of effector T cells capable of controlling uropathogens. Additionally, the production of interleukin-10 by vaccine-stimulated T cells may contribute to limiting excessive inflammation, thereby reducing tissue damage during infections.
In addition, the activation and persistence of tissue-resident memory T (TRM) cells in the bladder mucosa may play a central role. These cells, established in response to antigenic stimulation, provide rapid local immune responses to recurrent infections. Evidence showed that TRM cells can mediate long-term protection against UTI, even in the absence of circulating T cells [28]. By generating a robust local T cell response, Uromune® may enhance the mucosal immune memory required to prevent recurrent infections.
The daily administration of the vaccine is designed to ensure consistent antigen exposure, which is necessary to stimulate and sustain an effective immune response, particularly through the activation and maintenance of T cells in the bladder mucosa. Current evidence suggests that the persistence of antigen is critical for the development of TRM cells, which are thought to mediate long-term protection against UTI. However, as noted, the follow-up period in this study is relatively short, and the long-term effectiveness of the vaccine beyond one year remains unknown. Systematic reviews of vaccines for urinary tract infections have consistently highlighted the limited follow-up periods in published studies, underscoring the need for longer-term evaluations to better understand the durability of immune protection [29, 30].
Although the 2023 European recommendations [16] did not differentiate between complicated and uncomplicated rUTI, they were based on studies conducted primarily in patients with uncomplicated disease. Notably, our study demonstrated a reduction in UTI episodes after Uromune® autovaccine administration, even in patients with severe comorbidities. However, some patients continued to experience recurrent infections despite treatment. Our analysis found no significant differences in demographic characteristics, department of care, associated diseases, comorbidities, or clinical indices (Charlson and McCabe) between those who did and did not experience recurrences, suggesting that these factors alone do not predict the likelihood of recurrence after autovaccine administration. However, multiple regression analysis identified that having a urostomy, a chronic kidney disease, or being immunosuppressed were independent predictors of UTI recurrence post-Uromune® treatment. This finding highlights the need for targeted strategies to manage UTIs in these high-risk groups. These targeted strategies could include frequent monitoring, personalized treatment plans, or additional preventive measures.
Limitations of the study
While this study provides valuable insights into the potential utility of the Uromune® autovaccine in managing rUTIs, several limitations should be considered. As a retrospective study, it is limited in its ability to establish causal relationships, and reliance on historical data from electronic medical records introduces the potential for incomplete or biased information. A prospective study design would have allowed for better control over data collection and confounding factors. Additionally, the small sample size and single-center design limit the generalizability of the findings, particularly for broader rUTI populations, including those with comorbidities. The lack of a control group or comparison to alternative treatments or placebo further limits the ability to attribute observed outcomes solely to the Uromune® autovaccine.
While procedures such as anamnesis, blood tests, physical examinations, and urodynamics were essential for accurately characterizing and classifying the patient population, their results were not directly included in the analysis of correlations or associations. Nevertheless, these assessments were crucial for patient stratification, enabling the identification of key clinical features, comorbidities, and subgroups. Although not part of the statistical analysis, these diagnostic efforts strengthened the study’s robustness by ensuring a well-defined cohort.
Finally, due to the heterogeneity of the patient population and the limited sample size, the potential influence of medications on UTI episodes and hospital admissions could not be assessed. However, we recognize the importance of this factor, as medications may significantly affect patient outcomes.
Overall, while the results suggest promising utility, caution is warranted in generalizing our findings. Further studies with larger, more diverse cohorts and rigorous methodologies are necessary to better assess the clinical utility of Uromune® in rUTI management and to address questions raised by this pilot study.
Conclusion
This pilot study suggests that the administration of Uromune® autovaccine can significantly reduce the frequency of rUTIs and associated hospitalizations, particularly within the first few months of treatment. While autovaccine is broadly effective, patients with urological or renal diseases or immunosuppression may require additional therapeutic strategies to prevent recurrences. The findings of this study underscore the urgent need for further prospective, randomized trials with larger cohorts and extended follow-up periods to confirm these findings and optimize treatment protocols for different patient subgroups. Specifically, more research is needed to explore the efficacy and safety of Uromune® in patients with complicated rUTIs, where treatment outcomes may differ. The urgency and importance of this research cannot be overstated, as it has the potential to significantly improve the management of this disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
Analysis of variance
- CEVD
Cerebrovascular disease
- CKD
Chronic kidney disease
- CLD
Chronic liver disease
- CLUD
Chronic lung disease
- CND
Chronic neurological or neuromuscular disease
- CVD
Cardiovascular disease
- HF–IHD
Heart failure– ischemic heart disease
- NFD
Non-fatal disease
- OUD
Other urological diseases
- RFD
Rapidly fatal disease
- T2DM
Type 2 diabetes mellitus
- UCC
Urinary catheter carrier
- UFD
Ultimately fatal disease
- UIP
Urinary incontinence– prolapse
- UTI
Urinary tract infection
Author contributions
Conceptualization, S.I. and A.F.L.A. Data curation, S.I. and J.C. Formal analysis, J.C. Funding acquisition, S.I. Investigation, S.I., A.F.L.A., L.P.G., A.P.S. and M.P.Q. Methodology, S.I. and P.L.P. Project administration, J.C. Resources, J.J., A.C. and M.P.Q. Software, P.L.P. and X.G.B. Supervision, J.J. and A.C. Validation, S.I. Visualization, S.I. and J.C. Writing– original draft, J.C. Writing– review & editing, S.I., J.C. and M.P.Q. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of HOSPITAL UNIVERSITARI DE SANT JOAN (protocol code 10-12-23/12proj December 12, 2010). Written informed consent was obtained from all subjects involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Antoni Castro and Mercè Pascual-Queralt shared senior coautorship.
References
- 1.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–84. 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrión-López P, Martínez-Ruiz J, Librán-García L, Giménez-Bachs JM, Pastor-Navarro H, Salinas-Sánchez AS. Analysis of the efficacy of a sublingual bacterial vaccine in the prophylaxis of recurrent urinary tract infection. Urol Int. 2020;104. 10.1159/000505162. (3– 4):293– 300. [DOI] [PubMed]
- 3.Newlands AF, Roberts L, Maxwell K, Kramer M, Price JL, Finlay KA. Development and psychometric validation of a patient-reported outcome measure of recurrent urinary tract infection impact: the recurrent UTI impact questionnaire. Qual Life Res. 2023;32(6):1745–58. 10.1007/s11136-023-03348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geerlings SE, Beerepoot MA, Prins JM. Prevention of recurrent urinary tract infections in women: antimicrobial and nonantimicrobial strategies. Infect Dis Clin North Am. 2014;28(1):135–47. 10.1016/j.idc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Ramírez Sevilla C, Gómez Lanza E, Puyol Pallàs M. Immunoactive prophylaxis protocol of uncomplicated recurrent urinary tract infections in a cohort of 1104 women treated with Uromune® vaccine. Life (Basel). 2024;14(4):464. 10.3390/life14040464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramírez Sevilla C, Gómez Lanza E, Manzanera JL, Martín JAR, Sanz MAB. Active immunoprophyilaxis with Uromune® decreases the recurrence of urinary tract infections at three and six months after treatment without relevant secondary effects. BMC Infect Dis. 2019;19(1):901. 10.1186/s12879-019-4541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickel JC, Saz-Leal P, Doiron RC. Could sublingual vaccination be a viable option for the prevention of recurrent urinary tract infection in Canada? A systematic review of the current literature and plans for the future. Can Urol Assoc J. 2020;14(8):281–7. 10.5489/cuaj.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–92. 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Cuburu N, Kweon MN, Anjuere F, Holmgren J. Sublingual vaccination. Hum Vaccin. 2011;7(1):110–4. 10.4161/hv.7.1.13739. https//. [DOI] [PubMed] [Google Scholar]
- 10.Benito-Villalvilla C, Cirauqui C, Diez-Rivero CM, Casanovas M, Subiza JL, Palomares O. MV140, a sublingual polyvalent bacterial preparation to treat recurrent urinary tract infections, licenses human dendritic cells for generating Th1, Th17, and IL-10 responses via syk and MyD88. Mucosal Immunol. 2017;10(4):924–35. 10.1038/mi.2016.112. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo-Gómez MF, Padilla-Fernández B, García-Criado FJ, Mirón-Canelo JA, Gil-Vicente A, Nieto-Huertos A, et al. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. Int Urogynecol J. 2013;24(1):127–34. 10.1007/s00192-012-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo-Gómez MF, Foley S, Nickel JC, García-Cenador MB, Padilla-Fernández BY, González-Casado I, et al. Sublingual MV140 for prevention of recurrent urinary tract infections. NEJM Evid. 2022;1(5):EVIDoa2100018. 10.1056/EVIDoa2100018. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Ramón S, Fernández-Paredes L, Saz-Leal P, Diez-Rivero CM, Ochoa-Grullón J, Morado C. Sublingual bacterial vaccination reduces recurrent infections in pa-tients with autoimmune diseases under immunosuppressant treatment. Front Immunol. 2021;12:675735. 10.3389/fimmu.2021.675735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciudin A, Padulles B, Popescu R, Manasia P. Autovaccine-based immunotherapy: a promising approach for male recurrent urinary tract infections. Life (Basel). 2024;14(1):111. 10.3390/life14010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018;121(2):289–92. 10.1111/bju.14067. [DOI] [PubMed] [Google Scholar]
- 16.Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B et al. October. EAU Guidelines on urological infections. https://uroweb.org/guidelines/urological-infections/chapter/the-guideline. Accessed 14 2024.
- 17.Reilly JS, Coignard B, Price L, Godwin J, Cairns S, Hopkins S, et al. The reliability of the McCabe score as a marker of co-morbidity in healthcare-associated infection point prevalence studies. J Infect Prev. 2016;7(3):127–9. 10.1177/1757177415617245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil-Bona J, Sabaté A, Miguelena Bovadilla JM, Adroer R, Koo M, Jaurrieta E. Valor De Los índices De Charlson Y La Escala De Riesgo quirúrgico en El análisis de la mortalidad operatoria. Cir Esp. 2010;88(3):174–9. 10.1016/j.ciresp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Esteve-Palau E, Martinez Franco EM, Gonzalez-Cuevas A, Capella A, Moreno E, Alvarez MC et al. Individualised autovaccination is a promising strategy for managing recurrent urinary tract infections in women. In: Proceedings of the 30 European Society of Clinical Microbiology and Infectious Diseases Congress. Paris; 2020. p. 506.
- 20.Lorenzo-Gómez MF, Padilla-Fernández B, Flores-Fraile J, Valverde-Martínez S, González-Casado I, Hernández JD, et al. Impact of whole-cell bacterial immunoprophylaxis in the management of recurrent urinary tract infections in the frail elderly. Vaccine. 2021;39(42):6308–14. 10.1016/j.vaccine.2021.08.093. [DOI] [PubMed] [Google Scholar]
- 21.Roseman C, Truedsson L, Kapetanovic MC. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res Ther. 2012;14(4):R170. 10.1186/ar3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21(1):26–59. 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Miao MX, Jia CL, Cao YB, Yan TH, Jiang YY, et al. Interactions between Candida albicans and the resident microbiota. Front Microbiol. 2022;13:930495. 10.3389/fmicb.2022.930495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Elsas JD, Semenov AV, Costa R, Trevors JT. Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 2011;5(2):173–83. 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colgan R, Jaffe GA, Nicolle LE. Asymptomatic bacteriuria. Am Fam Physician. 2020;102(2):99–104. [PubMed] [Google Scholar]
- 26.Shabaka A, López de la Manzanara V, Zapata N, Santiago JL, Pérez-Flores I, de la Moreno MA, et al. Clinical and immunological response to sublingual vaccination for the prevention of recurrent urinary tract infections in kidney transplant patients results after 1 year of follow-up. Transplantation. 2018;10(Suppl 7):S320. 10.1097/01.tp.0000543044.21389.51. [Google Scholar]
- 27.Curtis Nickel J, Foley S, Yang B, Casanovas M, Caballero R, Diez-Rivero CM, et al. Reducing recurrent urinary tract infections in women with MV140 impacts personal burden of disease: secondary analyses of a randomized placebo-controlled efficacy study. Eur Urol Open Sci. 2024;63:96–103. 10.1016/j.euros.2024.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau M, Lacerda Mariano L, Canton T, Ingersoll MA. Tissue-resident memory T cells mediate mucosal immunity to recurrent urinary tract infection. Sci Immunol. 2023;8(83):eabn4332. 10.1126/sciimmunol.abn4332. [DOI] [PubMed] [Google Scholar]
- 29.Prattley S, Geraghty R, Moore M, Somani BK. Role of vaccines for recurrent urinary tract infections: a systematic review. Eur Urol Focus. 2020;6(3):593–604. 10.1016/j.euf.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Mak Q, Greig J, Dasgupta P, Malde S, Raison N. Bacterial vaccines for the management of recurrent urinary tract infections: a systematic review and meta-analysis. Eur Urol Focus. 2024;10(5):761–9. 10.1016/j.euf.2024.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.