Abstract
Background and Objective
Adherence to urate-lowering therapy among people with gout is poor, so it is important to understand which day-to-day medication-taking (‘implementation’) patterns are most likely to lead to suboptimal serum urate concentrations and worsen clinical outcomes. This study aimed to (1) determine the relative forgiveness (RF) of allopurinol with hypothetical and real-life implementation patterns in people with gout, (2) explore the use of RF as a means of identifying suboptimal implementation patterns, (3) assess the impact of suboptimal implementation patterns on clinical outcomes.
Methods
A simulation study was conducted using a pharmacokinetic–pharmacodynamic model for allopurinol and serum urate to determine the RF of allopurinol implementation patterns.
Results
With 100% (‘perfect’) implementation, the probability of adequate urate control (> 90% of days with urate < 0.36 mmol/L over 360 days) for a 300 mg dose of allopurinol was 0.549. Simulations based on real-life individual implementation patterns over a year yielded a median RF of 0.51, indicating that half of the patterns studied were at least 50% less likely to achieve adequate urate control than perfect implementation.
Conclusion
Our study provides evidence that missing one or two doses of allopurinol, even repeatedly over a year, does not significantly impact serum urate target achievement or clinical outcomes. However, people who take repeated drug holidays of more than 3 days in a row (followed by less than 15 consecutive days of dosing) are less than 0.3 times as likely (at least 70% less likely) to achieve adequate urate control than those with perfect implementation and may see an increase in the frequency of gout flares.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-024-01467-z.
Key Points
| Adherence to urate-lowering therapy among people with gout is poor and understanding which day-to-day medication-taking (‘implementation’) patterns are most likely to lead to suboptimal serum urate concentrations can help improve clinical outcomes. |
| Missing one or two doses of allopurinol, even repeatedly over a year, does not significantly impact serum urate target achievement or clinical outcomes. |
| People who take repeated drug holidays of more than 3 days in a row (followed by less than 15 consecutive days of dosing) are at least 70% less likely to achieve adequate urate control than those with perfect implementation. |
Introduction
Adherence to medication is an important determinant of variability in treatment outcomes in people taking long-term pharmacotherapy to treat or prevent diseases [1]. Adherence is described according to three distinct phases: initiation, implementation and discontinuation [2]. The implementation phase can be defined as the extent to which the doses of medication actually taken by a person correspond to their prescribed dosing regimen, from initiation until the last dose (discontinuation) [2]. The high prevalence of chronic diseases worldwide means that patient adherence to long-term medication is an increasingly important issue. Indeed, the World Health Organisation reported that adherence to long-term therapy for chronic illnesses in developed countries was 50% on average, with even lower rates in developing countries [3].
Gout is characterised by periodic episodes of painful, swollen joints (‘gout flares’) caused by the deposition of monosodium urate crystals and is the most common type of inflammatory arthritis in men [4, 5]. The long-term management of gout requires lifelong prescription of urate-lowering therapy (ULT) to maintain serum urate concentrations below 0.36 mmol/L (target) and avoid monosodium urate crystal deposition [6]. However, adherence to ULT among people with gout is only 46–47% [7–9]. This may, in part, explain the poor outcomes in people with gout prescribed ULT [10]. Indeed, improving adherence to ULT significantly increases the likelihood of achieving serum urate target [11–14], which can, in turn, decrease the frequency of gout flares [15].
The benefits of improving adherence to ULT on urate control and clinical symptoms in people with gout highlight the importance of using appropriate measures of adherence and indicators to identify suboptimal adherence. Indirect methods of measuring adherence are most commonly used. Indeed, of 24 studies evaluating adherence to ULT in people with gout [7], one study relied on a Medication Event Monitoring System (MEMS®) [16], another on pill counts [13], while the remaining studies used prescription claims data (n = 16), self-reported data (n = 3) or interviews (n = 3) to measure rates of adherence. Furthermore, all studies, including those using pill counts or MEMS®, relied on summary statistics estimated over an aggregate period. Although there are a growing number of studies that use electronic medication event monitors to measure adherence in conditions such as hypertension [17], inflammatory bowel disease [18], human immunodeficiency virus [19, 20] and gout [16], in most cases the information collected is analysed using summary measures (with some exceptions [21]), such as medication possession ratio or proportion of days covered. Such summary measures provide no information on the timing and sequence of missed doses, and therefore very different patterns of medication-taking may yield the same overall statistic [2]. These differences in patterns are of interest as they may potentially explain variability in clinical outcomes. To this end, electronically compiled dosing histories detect and store the dates and times when the prescribed medication is assumed to be taken and thus enable longitudinal patterns of medication-taking to be captured [22].
During the implementation phase, detailed day-to-day medication-taking patterns recorded longitudinally (henceforth referred to as ‘implementation patterns’) can be characterised by several types of deviations from 100% (or ‘perfect’) implementation, such as variability in the time of ingestion, taking more or less than the prescribed dose, random missed doses (e.g., one or two consecutive missed doses) and drug holidays (defined as at least three consecutive missed doses) [23]. It is important to recognise that interruptions in medication-taking are rarely isolated responses to exceptional situations and constitute an integral part of the landscape of long-term pharmacotherapy for chronic disease [21, 24]. How sensitive the efficacy of a drug is to such deviations in medication-taking behaviour is referred to as forgiveness. Knowing whether a drug is forgiving to occasional missed doses can be an important consideration in guiding early drug development. For instance, Hill-McManus et al. [25] examined hypothetical novel xanthine oxidase inhibitors for gout and showed that compounds with reduced clearance or increased potency were more forgiving to missed doses.
Forgiveness was first defined by Urquhart [26] as the property of a drug, given as a repeated treatment, to forgive the omission of one dose, or several doses in a row, without a loss of efficacy. In quantitative terms, forgiveness (F) = post-dose duration of action (DA) – dose interval (I) [23, 27]. Furthermore, the number of sequentially missed doses that can be missed with minimal loss of efficacy can be defined using the forgiveness index (FI), where FI = (DA − I)/I [23]. Although a simple concept, quantification of drug forgiveness using a single index is challenging [28–32]. This is because one needs to account both for the pharmacokinetic and pharmacodynamic characteristics of the drug (which determine DA) and the variable implementation patterns of individuals (which determine I). Indeed, since patients may not take medication exactly as prescribed, I represents the actual dose intervals and therefore is impacted by medication-taking behaviour. Assawasuwannakit and colleagues [29] developed a criterion termed ‘relative forgiveness’ which incorporates both these sources of variability, as it compares the probability of therapeutic success under perfect (100%) and imperfect (0–99%) implementation. Relative forgiveness in their work was seen as a property of the drug under certain conditions of adherence, with comparisons between drugs enabled by sampling from simulated ‘typical’ imperfect implementation patterns. In this study, we extend the use of relative forgiveness to characterise individual real-life implementation patterns and therefore patient-level medication-taking behaviour, by comparing the probability of therapeutic success under each specific implementation pattern with that under perfect implementation.
This work explores the relationship between implementation patterns, relative forgiveness and clinical outcomes in people with gout taking allopurinol. The aims of this study were (1) to determine the relative forgiveness of allopurinol with hypothetical and real-life implementation patterns in people with gout using simulation, (2) to explore the use of relative forgiveness as a means of identifying types of implementation patterns and (3) to assess the impact of suboptimal implementation patterns on clinical outcomes, such as serum urate levels (time above target), health-related quality of life (HRQoL) and number of gout flares.
Determining the relative forgiveness of various implementation patterns in people with gout prescribed ULT will help to identify which types and frequencies of deviations from perfect adherence are most likely to lead to suboptimal serum urate concentrations and hence potentially worse clinical outcomes for patients. This information is essential for health care providers to be able to give adequate recommendations to patients concerning their medication-taking behaviour.
Methods
Overview of the Methods
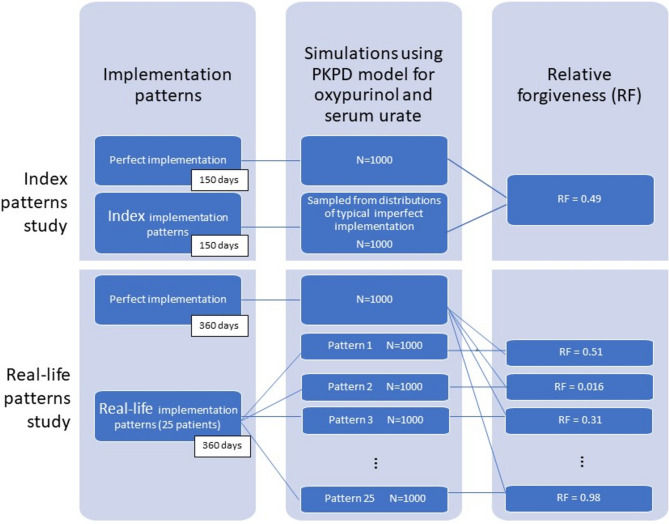
A simulation study was conducted using a published pharmacokinetic-pharmacodynamic model for allopurinol and serum urate [33]. Plasma concentrations of oxypurinol, the active metabolite of allopurinol (which contributes most of the pharmacological effect), and serum urate concentrations every 24 h were simulated for virtual individuals with gout under perfect and imperfect implementation patterns. Using these simulations, the relative forgiveness of different allopurinol implementation patterns was determined. Firstly, we determined the relative forgiveness of allopurinol under the index implementation patterns presented by Assawasuwannakit et al. [29] to enable comparison with their work. Secondly, we used observed real-life implementation patterns from a study on people with gout prescribed allopurinol where clinical outcomes were also collected [34]. Figure 1 provides a flowchart summary of the methodological approach.
Fig. 1.
Flowchart summary of methodological approach showing how relative forgiveness (RF) corresponding to different implementation patterns is obtained via simulation using a pharmacokinetic–pharmacodynamic (PKPD) model (the total number of real-life study patterns is 25, only the first 3 and the last are represented in this flowchart, the remaining 21 patterns with corresponding RF values are presented in Table1)
Relative Forgiveness
The relative forgiveness of allopurinol was determined as defined previously [29]: , where is the relative forgiveness, is the probability of successful attainment of urate target under imperfect implementation, and is the probability of successful attainment of urate target under perfect implementation. The criterion can be interpreted in the same way as an odds ratio, with values close to 1 indicating that the drug is forgiving of deviations to perfect implementation, and values closer to 0 indicating the drug is not forgiving to such deviations.
Index Implementation Patterns
Perfect implementation was defined as taking a single dose of 300 mg of allopurinol every day for 150 days. Steady-state was assumed to start on day 15 on the basis of the half-life of oxypurinol (around 24 h) and the half-life of serum urate (between 1.4 and 3.3 days) for someone with normal renal function [35]. Imperfect implementation patterns were created [29] on the basis of index implementation patterns which contained typical features of deviations from perfect implementation, including random missed doses and drug holidays. We did not assess timing variability in our study owing to the relatively long half-lives of oxypurinol [35] and urate [36] in people with gout (> 24 h) relative to the dosing interval (usually 24 h). Hence variations in time of dose within 24 h are unlikely to have a significant impact on clinical outcomes and those greater than 24 h are recorded as missed doses or drug holidays. Imperfect implementation patterns were then simulated from parametric distributions [for random missed doses: Poisson distribution (mean 13), for drug holidays (always 3 consecutive missed doses occurring 0, 1, 2 or 3 times over the 150-day period with respective likelihoods of 0.42, 0.3, 0.17 and 0.09)].
Real-Life Allopurinol Implementation Patterns
When considering the real-life implementation patterns, perfect implementation was defined as a single dose of allopurinol taken every day for 360 days (as the real-life implementation patterns covered a year). Imperfect implementation patterns corresponded to the true implementation patterns over 1 year of people with gout taking allopurinol obtained from a feasibility study [34] examining the effectiveness of point-of-care urate testing on allopurinol adherence. In brief, all study participants were prescribed allopurinol once daily and followed for 1 year. Allopurinol adherence information was collected using electronic monitoring (MEMS®) where each bottle opening was recorded by a digital device in the cap and uploaded using a mobile phone application. Medication Event Monitoring System (MEMS®) combined with the MEMS Adherence Software (MEMS AS®), AARDEX Group, Belgium, is an integrated system that is used to measure and/or to manage patient adherence to medications. Each bottle opening was assumed to indicate that the prescribed daily dose was taken by the participant. Implementation (i.e. day-to-day medication-taking) data for study participants were derived from the time and date stamp recorded by the MEMS® device. For each participant, every scheduled dose was designated either as a ‘dose missed’ or ‘dose taken’. Overall, five participants (IDs 3, 6, 12, 15 and 19) had allopurinol doses altered by their independent physician during the study so all data prior to the dose change were excluded (days 23, 272, 307, 293 and 180, respectively) to ensure the same dose was used over the whole period where implementation was studied. Only the patterns of the participants with implementation data covering at least 75% of the year and who used the electronic monitoring for the duration of the follow-up were retained (hence IDs 6, 12, 15, 19 and 32 were not included). One participant taking allopurinol every 48 h was excluded owing to difficulties reconciling the implementation data and prescribed therapy. In total, the exact implementation patterns of 25 participants over the first 360 days of the feasibility study [34] were included for the real-life implementation part of our study.
Simulation of Concentration-Time Profiles of Oxypurinol and Serum Urate
A population pharmacokinetic–pharmacodynamic (PKPD) model describing oxypurinol and serum urate concentrations developed previously [33] was used for the simulations. Briefly, this model consisted of a one-compartment first-order absorption pharmacokinetic model linked to a direct effects inhibitory Imax model with a proportional reduction from baseline serum urate. The final pharmacokinetic and pharmacodynamic parameters and corresponding between-subject-variability estimates obtained in this study [33] were used to simulate individual oxypurinol and serum urate concentrations over time by sampling from the corresponding multivariate normal distribution, however residual unexplained variability (measurement error) was set to zero. As the clinical covariates were not available in the gout study [34] from which we derived the real-life implementation patterns, we used the mean covariate values obtained in one of the six sub-studies used to build the model [37].
The simulated profile of serum urate concentrations for each virtual individual was assessed in terms of attainment of the target serum urate concentration (less than 0.36 mmol/L [6]) on each day. Only steady-state profiles were considered (data before day 15 were censored). Adequate urate control for an individual over the whole period was considered to occur when the simulated urate concentration was below 0.36 mmol/L for 90% or more of the days, as per the recommendations of the American College of Rheumatology guidelines for the management of gout [6] and considering biological variation and analytical measurement error.
Using the index implementation patterns, 1000 different imperfect implementation patterns (each with their own individual set of PKPD parameters) were simulated from the parametric distributions described above. In contrast, for the real-life implementation patterns, each of the 25 real-life implementation patterns was used to simulate 1000 individual PKPD profiles, yielding a total of 25,000 simulations. To calculate the relative forgiveness criterion of the index implementation patterns or one of the real-life implementation patterns, the achievement of adequate urate control over the whole period (yes/no, as defined above) was obtained for each simulated individual. The number of successes expressed as a fraction of the 1000 simulations corresponding to that implementation pattern provided the probability of success for either perfect implementation (PP) or imperfect implementation (PIP).
Classifying Implementation Patterns
The quartiles of the relative forgiveness values of the real-life individual implementation patterns were used to classify implementation patterns into two groups according to their forgiveness: least (first quartile) versus more forgiving (other three quartiles). The groups determined using relative forgiveness were then compared with those obtained using an exploratory cluster analysis. The cluster analysis aimed to detect similarities in implementation patterns on the basis of the frequency and duration of consecutive missed doses as well as the frequency and duration of consecutive doses on-therapy (i.e. taking the drug) for each individual standardised to 360 days. Specifically, 15 data items were included; 1, 2, 3, 4, 5 and > 5 consecutive missed doses, 1, 2, 3, 4, 5, 6, 7, 8–15 and > 15 consecutive doses on-therapy. Both hierarchical and K-means cluster analyses were performed [38]. Statistical groupings were assessed using a one-way ANOVA.
Comparison to Outcomes
The groups of implementation patterns identified using relative forgiveness and cluster analysis were then analysed in terms of clinical outcomes. Clinical outcomes reported in the feasibility study [34], such as gout flare occurrence, HRQoL and urate concentrations were also assessed. Gout flares were self-reported using the Gaffo Criteria [39]. Participants completed the EuroQoL EQ-5D-5L questionnaire [40] at baseline; at 3, 6, 9, and 12 month follow-ups; and when they experienced a gout flare to determine their health-related quality of life. A health utility score (ranging from − 0.301 to 1) was generated at each time point using an Australian value set [41]. Urate concentrations were self-monitored by each participant using a point-of-care device (HumaSens2.0plus, Human Diagnostics). Participants were asked to record their urate concentration at least once a month over the study period, although more frequent monitoring was permitted.
The final groups of implementation patterns identified using both relative forgiveness and the cluster analysis were assessed for differences in variables related to the dose of allopurinol, implementation (total number of missed doses, instances of different implementation patterns with total number of missed doses attributed to each pattern, proportion of doses taken) and observed outcomes [days below the urate target, number of gout flares, number of gout flares per person, health utility scores, EuroQoL visual analog scale (EQ-VAS) scores]. Each instance of non-implementation (defined as at least one missed dose) was defined using one of the following implementation patterns on the basis of the modified ‘rule of sixes’ proposed by Urquhart [23]: occasional missed doses (≤ 2 consecutive missed doses followed by ≥ 15 consecutive doses taken as prescribed); repeated missed doses (≤ 2 consecutive missed doses followed by < 15 consecutive doses taken as prescribed); occasional drug holidays (≥ 3 consecutive missed doses followed by ≥ 15 consecutive doses taken as prescribed); repeated drug holidays (≥ 3 consecutive missed doses followed by < 15 consecutive doses taken as prescribed). The percentage of days that the urate was < 0.36 mmol/L throughout the study period was determined for each participant using the measured urate concentrations and a linear interpolation method [42].
Software and Statistical Methods
Statistical analyses were performed using R [43], except the cluster analysis which was conducted using Statistical Package for Social Sciences (SPSS, v. 28.0.0.0, Armonk, NY: IBM Corp). The R package mrgsolve [44] was used to generate the simulated data. In the initial stages of the study, the output and results of our R code were compared with the output obtained using the Matlab code given in the Assawasuwannakit et al. [29] paper to ensure quality control. Unpaired analyses used either a two-tailed Mann–Whitney U test, a Kolmogorov–Smirnov test (for three or more groups if needed) or a χ2 test for a comparison of proportions. Odds ratios for gout flare risk between participants in dichotomous implementation groups were generated using a Fisher’s Exact test. All data were tested for normality using a Kolmogorov–Smirnov test.
Results
Relative Forgiveness of Allopurinol Using Index Implementation Patterns
The simulations using the index implementation patterns gave a probability of adequate urate control (over 90% of days with urate below 0.36 mmol/L) of 0.54 with a perfect implementation pattern (PP) and a probability of adequate urate control of 0.36 with the index imperfect implementation patterns (PIP), yielding a relative forgiveness of 0.49 for allopurinol. This means that therapeutic success (adequate urate control) with allopurinol is 0.49 times as likely (i.e. 51% less likely) with imperfect implementation patterns (defined according to the index implementation patterns) than with perfect implementation. The 5th, 50th and 95th percentile of the simulated oxypurinol and serum urate concentrations over 150 days corresponding to the index implementation patterns are shown in Fig. 2.
Fig. 2.
The 5th, 50th and 95th percentiles of the simulated oxypurinol and serum urate concentrations (n = 1000) over 150 days corresponding to the index implementation patterns (prescribed daily dose of 300 mg of allopurinol), A in case of perfect implementation, B in case of imperfect implementation. The dotted line corresponds to the target serum urate threshold of 0.36 mmol/L
Relative Forgiveness of Allopurinol Using Real-Life Implementation Patterns
Details of the characteristics of patients with gout whose real-life implementation patterns were used are provided elsewhere [34]. In brief, most participants (n = 16) were prescribed a daily allopurinol dose of 300 mg while the remaining participants (n = 9) were prescribed doses ranging between 100 and 600 mg (Table 1). Participants’ dose, implementation, health utility scores, EQ-VAS scores, urate and gout flare data are summarised in Table 2. The median proportion of doses taken was 91% (range 47–100%). There were 1237 missed doses during the study period with a median of 33 missed doses per participant (range 0–180). In total, 675 separate instances of non-implementation with at least one missed dose were identified. Further details regarding the study can be found elsewhere [34].
Table 1.
Relative forgiveness (RF), probability of adequate urate control (over 90% of days with urate below 0.36 mmol/L) with perfect implementation pattern (PP) and imperfect implementation pattern (PIP) obtained from the simulations (n = 1000) using the real-life implementation patterns and doses of 25 patients (designated by their identification number ID) from the feasibility study, classified from the least to the most forgiving pattern
| Pattern | Allopurinol dose (mg) | Probability of adequate urate control | Relative forgiveness (RF) | |
|---|---|---|---|---|
| PIP | PP | |||
| ID21 | 300 | 0 | 0.549 | 0 |
| ID2 | 600 | 0.119 | 0.895 | 0.016 |
| ID16 | 300 | 0.152 | 0.549 | 0.15 |
| ID26 | 300 | 0.171 | 0.549 | 0.17 |
| ID14 | 300 | 0.179 | 0.549 | 0.18 |
| ID3 | 100 | 0.042 | 0.124 | 0.31 |
| ID7 | 300 | 0.278 | 0.549 | 0.32 |
| ID8 | 600 | 0.742 | 0.895 | 0.34 |
| ID9 | 450 | 0.587 | 0.793 | 0.37 |
| ID11 | 500 | 0.658 | 0.836 | 0.38 |
| ID24 | 300 | 0.363 | 0.549 | 0.47 |
| ID30 | 300 | 0.367 | 0.549 | 0.47 |
| ID1 | 300 | 0.385 | 0.549 | 0.51 |
| ID17 | 300 | 0.389 | 0.549 | 0.52 |
| ID29 | 300 | 0.428 | 0.549 | 0.61 |
| ID28 | 200 | 0.243 | 0.321 | 0.68 |
| ID27 | 100 | 0.092 | 0.124 | 0.72 |
| ID4 | 300 | 0.478 | 0.549 | 0.75 |
| ID18 | 300 | 0.480 | 0.549 | 0.76 |
| ID25 | 100 | 0.100 | 0.124 | 0.78 |
| ID13 | 150 | 0.180 | 0.217 | 0.79 |
| ID10 | 300 | 0.498 | 0.549 | 0.81 |
| ID20 | 300 | 0.516 | 0.549 | 0.87 |
| ID31 | 300 | 0.545 | 0.549 | 0.98 |
| ID22 | 300 | 0.549 | 0.549 | 1.00 |
Table 2.
Characteristics of the 25 patients included in the real-life implementation analysis in terms of adherence, relative forgiveness (RF) and outcomes
| All patients (n = 25) |
Group 1 (RF ≥ 0.32) (n = 19) |
Group 2 (RF < 0.32) (n = 6) |
Comparison between the two groups (significance)a | |
|---|---|---|---|---|
| Allopurinol dose (mg)b | 300 (100–600) | 300 (100–600) | 300 (100–600) | 1 |
| Adherence data | ||||
| Total number of missed doses | 1237 | 399 | 838 | < 0.0001 |
| Median number of missed doses per person | 33 | 19 | 118 | |
| Total number of non-implementation eventsd | 675 | 298 | 377 | |
| Occasional missed doses (n) | 108 | 98 | 10 | < 0.0001 |
| Repeated missed doses (n) | 482 | 186 | 296 | < 0.0001 |
| Occasional drug holidays (n) | 5 | 5 | 0 | – |
| Repeated drug holidays (n) | 80 | 9 | 71 | < 0.0001 |
| Proportion of doses taken | 91% | 95% | 67% | < 0.0001 |
| Relative forgiveness of implementation patternsb | ||||
| Relative forgiveness | 0.51 (0–1) | 0.61 (0.32–1) | 0.16 (0–0.26) | 0.0003 |
| Outcomesb | ||||
| Proportion of days at the urate target | 75% | 86% | 41% | 0.001 |
| Flares | ||||
| Total number | 18 | 10 | 8 | OR = 4.3e [95% CI 0.6–30.6] |
| Mean number of flares per person | 0.7 | 0.5 | 1.3 | 0.129 |
| HRQoL utility scores | 0.9459 | 0.9456 | 0.9464 | 0.838 |
| EQ-VAS scores | 80.0 | 80.0 | 81.3 | 0.873 |
RF = relative forgiveness; OR = odds ratio; CI = confidence interval; occasional missed doses = ≤ 2 consecutive missed doses followed by ≥ 15 consecutive doses taken as prescribed; repeated missed doses = ≤ 2 consecutive missed doses followed by < 15 consecutive doses taken as prescribed; occasional drug holidays = ≥ 3 consecutive missed doses followed by ≥ 15 consecutive doses taken as prescribed; repeated drug holidays = ≥ 3 consecutive missed doses followed by < 15 consecutive doses taken as prescribed; HRQoL = health related quality of life; EQ-VAS = EuroQoL-visual analog scale
ap-value of Mann–Whitney test unless otherwise stated
bMedian (range) unless otherwise specified
cp-value of Fisher’s exact test
dA non-adherence event is any instance of an occasional missed dose, repeated missed dose, repeated missed dose or repeated missed dose throughout the study period
eOdds of having at least one flare in subgroup 2
The allopurinol dose prescribed, relative forgiveness, probability of adequate urate control with perfect implementation (PP) and imperfect implementation patterns (PIP) obtained from the simulations using the real-life implementation patterns of the 25 participants in the gout study are provided in Table 1. The probability of adequate urate control with perfect implementation ranged from 0.124 for a daily dose of 100 mg to 0.895 for a daily dose of 600 mg. For the most commonly prescribed dose of 300 mg of allopurinol, this probability was 0.549. Relative forgiveness ranged from 0 for implementation pattern ID21 (meaning that none of the 1000 simulated individuals with that implementation pattern had adequate urate control over 1 year) to 1 for implementation pattern ID22 (a perfect implementation pattern where all 360 daily doses were taken as prescribed).
Using Relative Forgiveness and Cluster Analysis to Classify Implementation Patterns
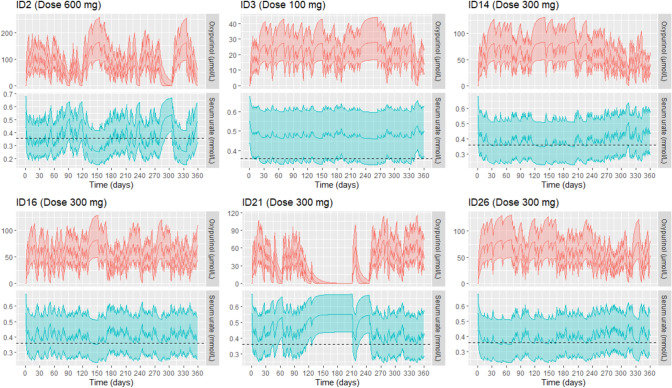
The median relative forgiveness (RF) was 0.51 (interquartile range 0.32–0.76), indicating that 12 out of the 25 individuals in this group had implementation patterns where adequate urate control was at least 50% less likely than under perfect implementation, and 6 of them had implementation patterns where adequate urate control was at least 68% less likely than with perfect implementation (RF < 0.32). These six implementation patterns with relative forgiveness less than the first quartile (RF < 0.32) were identified as patterns of implementation at high risk of inadequate urate control according to our simulation study. These corresponded to individuals ID2, 3, 14, 16, 21 and 26. The 5th, 50th and 95th percentile of the simulated oxypurinol and serum urate concentrations over 360 days corresponding to these latter individuals’ implementation patterns are shown in Fig. 3 and corresponding graphs for the remaining individuals are given in Supplemental Fig. 1.
Fig. 3.
The 5th, 50th and 95th percentile of the simulated oxypurinol and serum urate concentrations (n = 1000) over 360 days corresponding to the implementation patterns of patients ID2, ID3, ID14, ID16, ID21 and ID26 [group 2, with relative forgiveness (RF) in the lower quartile group, RF < 0.32]. The prescribed daily dose of allopurinol for each patient is indicated in brackets. The dotted line corresponds to the target serum urate threshold of 0.36 mmol/L
The exploratory cluster analysis of the 25 real-life implementation patterns identified two groups of implementation patterns; cluster 1 comprised 19 individuals and cluster 2 comprised 6 individuals (ID2, 3, 14, 16, 21 and 26) as shown in the dendrogram (Supplementary Fig. 2). As the individuals in cluster 2 coincided exactly with those in the relative forgiveness lower quartile group (i.e. ID2, 3, 14, 16, 21 and 26), these individuals were named group 2 for the group comparison analysis and compared with the remaining 19 individuals (group 1).
Description of Implementation Pattern Groups and Impact on Clinical Outcomes
The adherence data and the observed implementation patterns for both groups are summarised in Table 1. Individuals in group 2 had significantly more missed doses in total (838 versus 399, p < 0.0001, respectively), and had lower overall implementation (median 67% versus 95%, p < 0.0001, respectively) compared with group 1. Individuals in group 2 took significantly more drug holidays than group 1 (71 versus 14) accounting for nearly 50% of all missed doses in group 2 compared with only 8% in group 1 (p < 0.0001). Conversely, occasional missed doses were infrequent in group 2 compared with group 1 (10 versus 98) and accounted for only 2% of the missed doses compared with 28% in group 1 (p < 0.0001).
In terms of outcomes, individuals in group 2 spent significantly less time at the urate target (median 41% versus 86%, p = 0.001, respectively). No differences were found between both groups in terms of HRQoL utilities, EQ-VAS scores or the frequency of gout flares. Individuals in group 2 experienced about 44% of the recorded flares, a rate of 1.3 flares per person compared with 0.5 flares per person in Group 1, and were 4.3 times as likely as the individuals in group 1 to have at least one flare during the year, although the differences were not statistically significant.
Discussion
To our knowledge, this is the first study to undertake an analysis of allopurinol adherence by quantifying the relative forgiveness of hypothetical and real-life implementation patterns in people with gout using simulations from a PKPD model. While Hill-McManus et al. [45] examined the impact of non-adherence and gout flare resolution on the cost-effectiveness of treatments for gout using a pharmacometrics/pharmacoeconomic model to simulate implementation, they did not examine the notion of forgiveness. We showed that relative forgiveness provides a way of quantifying the decrease in probability of adequate urate control associated with different imperfect implementation patterns. Using this criterion, we were able to identify patterns of implementation most likely to result in suboptimal outcomes in patients with gout taking allopurinol, which is crucial evidence for health care providers as they strive to give relevant and informed counselling to people on the importance of regularly taking their medication.
With perfect implementation, the probability of adequate urate control (over 90% of days with urate below 0.36 mmol/L over 360 days) for a 300 mg dose of allopurinol was only 0.549. Our findings are consistent with clinical trial data (assuming that implementation is higher in clinical trials than in observational studies), indicating that between 50 and 60% of subjects receiving allopurinol 300 mg per day achieve target serum urate concentrations [46, 47]. Our results are also consistent with another simulation study [45] which showed that perfect implementation of 300 mg daily of allopurinol enabled 57% of patients to achieve target serum urate concentrations (< 0.36 mmol/L). In a similar simulation setting, with perfect implementation, warfarin was found to have a probability of therapeutic success of 0.58 (at least 55% of steady state trough values within the INR range of 2–3.5 over a total of 150 days of treatment) [29] and amoxicillin, levofloxacin, moxifloxacin of between 0.86 and 1 (maintaining concentrations above minimum inhibitory concentration during 50% of the administration time interval over a total of 10 days of treatment), depending on the creatinine clearance of the patients [48]. The probability of adequate urate control under perfect implementation varies according to the number of days where target must be reached and the dose of allopurinol administered. Our simulations showed that when the number of days where urate target has been reached was decreased from 90 to 70%, the probability of adequate urate control with perfect implementation (for a dose of 300 mg daily) increased slightly to 0.553. However, this value did not increase any further for lower percentages of target attainment. The probability of adequate urate control under perfect implementation was increased to 0.793 and 0.895 for a daily dose of allopurinol of 450 mg and 600 mg, respectively. This highlights the importance of allopurinol dose optimisation in addition to adequate implementation to achieve target serum urate concentrations.
Under the index imperfect implementation patterns suggested by Assawasuwannakit et al. [29] the relative forgiveness of allopurinol was 0.49. With the same index imperfect implementation patterns, warfarin was found to have a relative forgiveness of 0.78 [29], making it a more forgiving drug than allopurinol. This is likely explained by the fact that warfarin must inhibit the production of clotting factors including thrombin which has a half-life in the systemic circulation of about 60 h [49] compared with that of urate which is around 24–48 h [35]. The median relative forgiveness of allopurinol (0.51) using real-life individual implementation patterns was consistent with that obtained using the index implementation patterns, suggesting that these patterns provide an adequate representation of suboptimal implementation patterns in patients with long-term treatment for asymptomatic chronic conditions. Thus, they could be used in order to obtain an overall estimate of the relative forgiveness of other such drugs if day-to-day implementation data is unavailable.
Our simulation study of real-life daily implementation patterns enabled us to show that implementation patterns where people took repeated drug holidays, with half of all missed doses occurring during breaks in therapy of three or more consecutive doses, had the lowest relative forgiveness and were at high risk of inadequate urate control (serum urate > 0.36 mmol/L). Indeed, the six patients in the feasibility study with the implementation patterns in the lowest quartile of relative forgiveness (RF < 0.32) had a mean overall implementation of 67% and spent only 41% of their time below the target serum urate concentration. This is consistent with another simulation study [45] of allopurinol 300 mg/day which showed that implementation patterns corresponding to an average of 80% implementation led to 41% of patients achieving target serum urate concentrations (< 0.36 mmol/L) and only 19% of patients attained the target for implementation patterns with 50% of doses taken. Conversely, the participants in our study with relative forgiveness in the highest three quartiles achieved target urate 86% of the time with patterns predominantly composed of occasional or repeated missed doses (only missing one or two doses before restarting treatment). Therefore, our estimation of relative forgiveness can adequately predict people who are at high risk of therapeutic failure on the basis of their implementation behaviour.
Although not significant, the rate of gout flares per person was higher in the low relative forgiveness (repeated drug holidays) group than in the other group. Consistent with this, another study [13] showed that although the average serum urate concentrations were significantly higher in non-adherent compared with adherent and persistent ULT-taking patients with gout, the frequency of gout flaresin both groups was similar. An insufficient duration of follow-up might explain these counter-intuitive results, as the effect of urate-lowering treatment on gout flares may best be seen after a year’s adherence to therapy [50]. Indeed, a study [10] with a follow-up of 3 years showed that suboptimal adherence was associated with more severe gout (defined as more hospitalizations or emergency department visits with a main diagnosis code of gout, and prescription episodes for colchicine and prednisone). There is growing evidence that it may be fluctuations in urate concentrations, rather than just the absolute value, which increase the risk of a gout flare [51]. Given that fluctuations in urate are likely to be of greater magnitude in the event of erratic implementation of ULT, the potential clinical impact of repeated drug holidays should be clearly communicated to patients.
Our study has some limitations. Firstly, for the simulations using the PKPD model, we did not have access to the covariate values (creatinine clearance, body weight, ethnicity) of the participants corresponding to each real-life implementation pattern and therefore used fixed mean values which can be considered representative of a standard person with gout. This may have reduced the variability of the simulates obtained. For the index implementation patterns we assumed that implementation was independent of the PKPD parameters, which may not be the case, but is an acceptable simplification [29] given that we are not interested in how patterns of non-implementation arise. Finally, in the real-life implementation patterns, different doses were associated with different implementation patterns and dose-taking was measured on the basis of the recordings of the electronic monitoring device which constitutes an indirect method of measuring implementation. Concerning the latter point, this is not a problem for the simulation part of the study as we simply needed realistic patterns of implementation. While it may potentially constitute a bias when relating the patterns to the clinical outcomes, these results were not significant. Concerning the varying doses, the aim of this work was not to explain the potential association between dose and implementation. Furthermore, when simulations were conducted with the standard dose of 300 mg of allopurinol for all the real-life implementation patterns, the overall median and quartile values of relative forgiveness remained the same.
Conclusions
Using simulations from a PKPD model, this study quantified the relative forgiveness of hypothetical and real-life implementation patterns in people with gout and showed that relative forgiveness can be used to classify implementation patterns in terms of how likely they are to achieve adequate urate control, and hence better clinical outcomes. Indeed, we provide evidence that, for patients with gout taking allopurinol, missing one or two doses, even repeatedly, does not significantly impact serum urate concentration target achievement or clinical outcomes. On the other hand, patients who take repeated drug holidays of more than 3 days in a row (followed by less than 15 consecutive daily doses) are less than 0.3 times as likely (at least 70% less likely) to achieve adequate urate control over a year than a patient with perfect implementation and may see an increase in the frequency of gout flares. Many factors may influence medication-taking behaviour in patients with gout. However, the information from our study could be included in conversations between health care providers and patients to help patients understand the potential impact of not taking their urate lowering therapy at the prescribed frequency and dose on their response to urate control – particularly which deviations from perfect implementation may be more likely to reduce drug effectiveness than others. Such understanding will support patient-led strategies, such as reminder systems, implementation routines and urate self-monitoring [52], which are most likely to achieve consistent long-term implementation and clinical outcomes. Finally, relative forgiveness could be used in future studies as an indicator of the effect of medicine adherence interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Author contributions
Study conception and design: MWK, DFBW, SLS. Data acquisition and preparation: MWK, DFBW, DAH, TJFM, MJC, PA, ROD, SLS. Data analysis: MWK, DFBW, SLS. Simulations: MWK. The first draft of the manuscript was written by MWK, and all authors commented on data interpretation and the manuscript. All authors contributed to and approved the final manuscript.
Funding
No funding was received for conducting this study.
Competing Interests
The authors have no competing financial or non-financial interests to declare that are relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code Availability
All R code used in this study is available as supplementary information.
References
- 1.Robin DiMatteo M, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794. 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Adherence to long-term therapies: evidence for action. WHO; 2003. [Google Scholar]
- 4.Smith E, Hoy D, Cross M, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(8):1470–6. 10.1136/annrheumdis-2013-204647. [DOI] [PubMed] [Google Scholar]
- 5.Wijnands JMA, Viechtbauer W, Thevissen K, et al. Determinants of the prevalence of gout in the general population: a systematic review and meta-regression. Eur J Epidemiol. 2015;30(1):19–33. 10.1007/s10654-014-9927-y. [DOI] [PubMed] [Google Scholar]
- 6.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46. 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin R, Li L, Zhang G, et al. Rate of adherence to urate-lowering therapy among patients with gout: a systematic review and meta-analysis. BMJ Open. 2018;8(4): e017542. 10.1136/bmjopen-2017-017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheepers LEJM, van Onna M, Stehouwer CDA, Singh JA, Arts ICW, Boonen A. Medication adherence among patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;47(5):689–702. 10.1016/j.semarthrit.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 9.De Vera MA, Marcotte G, Rai S, Galo JS, Bhole V. Medication adherence in gout: a systematic review. Arthritis Care Res. 2014;66(10):1551–9. 10.1002/acr.22336. [DOI] [PubMed] [Google Scholar]
- 10.Weisman A, Tomlinson GA, Lipscombe LL, Perkins BA, Hawker GA. Allopurinol adherence, persistence and patterns of use in individuals with diabetes and gout: a retrospective, population-based cohort analysis. Semin Arthritis Rheum. 2021;51(6):1162–9. 10.1016/j.semarthrit.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Halpern R, Mody RR, Fuldeore MJ, Patel PA, Mikuls TR. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin. 2009;25(7):1711–9. 10.1185/03007990903017966. [DOI] [PubMed] [Google Scholar]
- 12.Rashid N, Coburn BW, Wu YL, et al. Modifiable factors associated with allopurinol adherence and outcomes among patients with gout in an integrated healthcare system. J Rheumatol. 2015;42(3):504–12. 10.3899/jrheum.140588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, So MW. Adherence with urate-lowering therapies among male patients with gout in a routine clinical setting. Mod Rheumatol. 2016;26(6):950–5. 10.3109/14397595.2016.1170914. [DOI] [PubMed] [Google Scholar]
- 14.Mikuls TR, Cheetham TC, Levy GD, et al. Adherence and outcomes with urate-lowering therapy: a site-randomized trial. Am J Med. 2019;132(3):354–61. 10.1016/j.amjmed.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–5. 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 16.de Klerk E, van der Heijde D, Landewé R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol. 2003;30(1):44–54. [PubMed] [Google Scholar]
- 17.Márquez-Contreras E, de López García-Ramos L, Martell-Claros N, et al. Validation of the electronic prescription as a method for measuring treatment adherence in hypertension. Patient Educ Couns. 2018;101(9):1654–60. 10.1016/j.pec.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Plevinsky JM, Wojtowicz AA, Miller SA, Greenley RN. Longitudinal barriers to thiopurine adherence in adolescents with inflammatory bowel diseases. J Pediatr Psychol. 2019;44(1):52–60. 10.1093/jpepsy/jsy062. [DOI] [PubMed] [Google Scholar]
- 19.Petersen ML, LeDell E, Schwab J, et al. Super learner analysis of electronic adherence data improves viral prediction and may provide strategies for selective HIV RNA monitoring. J Acquir Immune Defic Syndr 1999. 2015;69(1):109–18. 10.1097/QAI.0000000000000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudhune V, Gvetadze R, Girde S, et al. Correlation of adherence by Pill count, self-report, MEMS and plasma drug levels to treatment response among women receiving ARV therapy for PMTCT in Kenya. AIDS Behav. 2018;22(3):918–28. 10.1007/s10461-017-1724-7. [DOI] [PubMed] [Google Scholar]
- 21.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–7. 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrijens B, Urquhart J, White D. Electronically monitored dosing histories can be used to develop a medication-taking habit and manage patient adherence. Expert Rev Clin Pharmacol. 2014;7(5):633–44. 10.1586/17512433.2014.940896. [DOI] [PubMed] [Google Scholar]
- 23.Urquhart J. Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv Drug Deliv Rev. 1998;33(3):207–19. 10.1016/S0169-409X(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 24.Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–9. 10.1038/clpt.2010.171. [DOI] [PubMed] [Google Scholar]
- 25.Hill-McManus D, Marshall S, Soto E, Hughes DA. Integration of pharmacometrics and pharmacoeconomics to quantify the value of improved forgiveness to nonadherence: a case study of novel xanthine oxidase inhibitors for gout. Clin Pharmacol Ther. 2019;106(3):652–60. 10.1002/cpt.1454. [DOI] [PubMed] [Google Scholar]
- 26.Urquhart J. Role of patient compliance in clinical pharmacokinetics: a review of recent research. Clin Pharmacokinet. 1994;27(3):202–15. 10.2165/00003088-199427030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Urquhart J. The electronic medication event monitor: lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32(5):345–56. 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 28.Nony P, Boissel JP. Use of sensitivity functions to characterise and compare the forgiveness of drugs. Clin Pharmacokinet. 2002;41(5):371–80. 10.2165/00003088-200241050-00004. [DOI] [PubMed] [Google Scholar]
- 29.Assawasuwannakit P, Braund R, Duffull S. Quantification of the forgiveness of drugs to imperfect adherence. CPT Pharmacomet Syst Pharmacol. 2015;4(3):204–11. 10.1002/psp4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assawasuwannakit P, Braund R, Duffull S. A framework for quantifying the influence of adherence and dose individualization. Clin Pharmacol Ther. 2016;99(4):452–9. 10.1002/cpt.268. [DOI] [PubMed] [Google Scholar]
- 31.McAllister NP, Lawley SD. A pharmacokinetic and pharmacodynamic analysis of drug forgiveness. J Pharmacokinet Pharmacodyn. 2022;49(3):363–79. 10.1007/s10928-022-09808-w. [DOI] [PubMed] [Google Scholar]
- 32.Clark ED, Lawley SD. How drug onset rate and duration of action affect drug forgiveness. J Pharmacokinet Pharmacodyn. 2024;51(3):213–26. 10.1007/s10928-023-09897-1. [DOI] [PubMed] [Google Scholar]
- 33.Wright DFB, Hishe HZ, Stocker SL, et al. The development and evaluation of dose-prediction tools for allopurinol therapy (Easy-Allo tools). Br J Clin Pharmacol. 2024. 10.1111/bcp.16005. (Published online February 15, 2024). [DOI] [PubMed] [Google Scholar]
- 34.Michael TJF, Wright DFB, Chan JS, et al. Patient-led urate self-monitoring to improve clinical outcomes in people with gout: a feasibility study. ACR Open Rheumatol. 2024;6(7):403–11. 10.1002/acr2.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet. 2007;46(8):623–44. 10.2165/00003088-200746080-00001. [DOI] [PubMed] [Google Scholar]
- 36.Vitali C, Pasero G, Clerico A, et al. Uric acid turnover in normals, in gout and in chronic renal failure using 14C-uric acid. Adv Exp Med Biol. 1980;122A:27–31. 10.1007/978-1-4615-9140-5_5. [DOI] [PubMed] [Google Scholar]
- 37.Stamp LK, Chapman PT, Barclay ML, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis. 2017;76(9):1522–8. 10.1136/annrheumdis-2016-210872. [DOI] [PubMed] [Google Scholar]
- 38.Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–44. [Google Scholar]
- 39.Gaffo AL, Dalbeth N, Saag KG, et al. Brief report: validation of a definition of flare in patients with established gout. Arthritis Rheumatol Hoboken NJ. 2018;70(3):462–7. 10.1002/art.40381. [DOI] [PubMed] [Google Scholar]
- 40.Buchholz I, Janssen MF, Kohlmann T, Feng YS. A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. Pharmacoeconomics. 2018;36(6):645–61. 10.1007/s40273-018-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norman R, Mulhern B, Lancsar E, et al. The use of a discrete choice experiment including both duration and dead for the development of an EQ-5D-5L value set for Australia. Pharmacoeconomics. 2023;41(4):427–38. 10.1007/s40273-023-01243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9. [PubMed] [Google Scholar]
- 43.R Core Team. R version 4.3.2. Published online 2023. https://www.R-project.org/
- 44.Kyle T Baron. mrgsolve: simulate from ODE-based models. Published online 2024. https://github.com/metrumresearchgroup/mrgsolve
- 45.Hill-McManus D, Marshall S, Soto E, Lane S, Hughes D. Impact of non-adherence and flare resolution on the cost-effectiveness of treatments for gout: application of a linked pharmacometric/pharmacoeconomic model. Value Health. 2018;21(12):1373–81. 10.1016/j.jval.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, Garcia-Erauskin G, Ruiz-Lucea E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57(9):545–9. 10.1136/ard.57.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61. 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 48.Carral N, Lukas JC, Estradé O, Jauregizar N, Morillas H, Suárez E. Non-adherence in adult male patients with community-acquired pneumonia: relative forgiveness of amoxicillin versus respiratory fluoroquinolones. Antibiotics. 2023;12(5):838. 10.3390/antibiotics12050838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.FDA. US food and drug administration. Accessed Jul 15, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/009218s108lbl.pdf
- 50.Stamp LK, Frampton C, Morillon MB, et al. Association between serum urate and flares in people with gout and evidence for surrogate status: a secondary analysis of two randomised controlled trials. Lancet Rheumatol. 2022;4(1):e53–60. 10.1016/S2665-9913(21)00319-2. [DOI] [PubMed] [Google Scholar]
- 51.Uhlig T, Karoliussen LF, Sexton J, et al. Fluctuation and change of serum urate levels and flares in gout: results from the NOR-Gout study. Clin Rheumatol. 2022;41(12):3817–23. 10.1007/s10067-022-06416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spragg JCJ, Michael TJF, Aslani P, et al. Optimizing adherence to allopurinol for gout: patients’ perspectives. Br J Clin Pharmacol. 2023;89(7):1978–91. 10.1111/bcp.15657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.