Abstract
Background/purpose
Peri-implantitis remains a substantial challenge. This study investigated the effect of titanium particles on human oral epithelial cells, focusing on the nucleotide-binding domain and leucine-rich repeat protein (NLRP) 3 inflammasome.
Materials and methods
The Ca9-22 human gingival epithelial cell line was subjected to incubation with titanium particles. To evaluate cell viability, the MTT assay was employed. Total RNA was extracted, and messenger RNA (mRNA) expressions of COX2, TGF-β1, NLRP1, NLPR3, CASP1, and AIM2 were analyzed. The concentration of interleukin (IL)1β in cell supernatants was quantified through enzyme-linked immunosorbent assay. Intracellular reactive oxygen species (ROS) were visualized using an ROS assay Kit.
Results
Ca9-22 cells treated with titanium particles showed >75% cell viability across all concentrations tested, with consistent results. mRNA expressions of inflammation-related genes (COX2 and TGF-β1) significantly increased in a dose-dependent manner. The mRNA expression of NLRP3 and CASP1, as well as the secretion of IL1β, increased after 6-h incubation with titanium particles. Moreover, the ROS assay results showed increased production of ROS after treatment with titanium particles, whereas NLRP3 expression and IL1β secretion reduced after treatment with N-acetyl-l-cysteine (ROS scavenger).
Conclusion
Our findings indicate that titanium particles possess a distinct ability to trigger the NLRP3 inflammasome, partly by producing ROS.
Keywords: Dental implant, Gingival epithelial cells, Inflammasome, Inflammation, Titanium particle
Introduction
The outcomes of dental implant procedures can be classified into three distinct categories: failure, survival, and success.1 To be considered successful, implants must meet specific criteria, including the absence of biological and/or technical complications, attainment of sufficient keratinized mucosa and soft tissue coverage, and overall patient satisfaction.2 Although the survival rate of dental implants is high, achieving success without complications remains a formidable challenge. A considerable threat to the success of implant treatments is peri-implantitis. Peri-implantitis is defined as “a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone”.3,4 Several factors, e.g., patient genetics, microbiome, surgical procedures, and titanium implants, can synergistically activate the immune system, leading to this pathological condition.5, 6, 7 Titanium, which is widely used in dental implants because of its biocompatibility, corrosion resistance, and high tensile strength, contributes to a high survival rate. However, concerns have been raised regarding the potential for titanium to induce inflammatory reactions in host tissues, leading to complications in certain cases. Titanium particles can be released and scattered around titanium implants, such as during implant preparation, insertion, or decontamination, due to the formation of implantation cavities, debridement of peri-implantitis, microgaps, and dissolution by fluoride.8 A previous report detected higher quantities of dissolved titanium in the submucosal biofilm of implants with peri-implantitis than in samples from implants with healthy peri-implant tissues.9 Additionally, studies have found higher concentrations of titanium particles in tissues and cells of peri-implant mucosa compromised by peri-implantitis than in clinically healthy sites.10,11 These findings suggest that the release of titanium particles into tissues may play a role in the pathogenesis of peri-implant diseases.
The periodontium of natural teeth is protected from bacterial invasion by sealing of the junctional epithelium. Attachment of the junctional epithelium to tooth surfaces is mediated by hemidesmosomes, the internal basal lamina, and the cuticle. This structural complex provides frontline defense against infections at the dentogingival junction.12 The epithelium also plays a role in the frontline defense in the peri-implant tissue. Consistent with the epithelial structure and arrangement of natural dentition, the peri-implant mucosa consists of a well-keratinized oral epithelium on the outer surface, which is continuous with the sulcular epithelium lining the lateral aspect of the gingival sulcus.13 One of the important differences between gingival and peri-implant epithelial cells is the exposure to titanium particles.
Inflammation and host immune dysfunction are gaining increasing interest in the pathogenesis of peri-implantitis.14 Over the past decade, a type of large multimolecular complex defined as “inflammasome” has been proven to be critical in the early stages of innate immune response.15 Inflammasomes are intracellular supramolecular protein complexes, comprising an inflammasome sensor molecule, an adapter protein, and a caspase-1 (CASP1) protein, that orchestrate host response mechanisms against physiological aberrations, infectious agents, and external elements, e.g., metallic debris. Several inflammasome sensor molecules have been documented, including the nucleotide-binding domain and leucine-rich repeat protein (NLRP) family members (NLRP1, NLRP3, and NLRC4), as well as absent in melanoma 2 (AIM2) and Pyrin.16 Among these, NLRP3 is the most characterized inflammasome-forming NLR member, notable for its distinct and highly efficient assembly features.16 NLRP3 also responds to common cellular events triggered by various activators, such as the reactive oxygen species (ROS).16
Titanium particles are reported to activate inflammasomes in T-cells16 or macrophages;17 however, whether peri-implant epithelial cells would show a similar reaction to titanium treatment remains elusive. In the present study, the effects of titanium particles on gingival epithelial cells were investigated with a specific focus on the NLRP3 inflammasome and its activation by ROS.
Materials and methods
Cell culture
Considering that peri-implant epithelial cells are the first barrier to titanium particles, the present study was conducted using a well-characterized human oral epithelial cell line Ca9-22 (Riken BioResource Research Center, Tsukuba, Japan). The cells were cultured in Dulbecco's modified Eagle medium (Wako Pure Chemical Industries, Osaka, Japan) containing 10% (v/v) fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) and maintained at 37 °C in a 5% carbon dioxide atmospheric condition.
Cell culture with titanium particles
Commercial titanium particles with a nitride phase were used (Osaka Titanium Technologies, Hyogo, Japan). According to the manufacturer, the average particle diameter is 25.6 μm (range: 0–45 μm).18 Titanium particles were adjusted in a medium mix to obtain low (30 μg/mL), medium (70 μg/mL), and high concentrations (100 μg/mL) based on a previous study.17 The cells were seeded at a density of 1 × 105 cells/mL in 6-well plates and incubated for 12 h. Then, the cells were incubated for 6 h with or without titanium particles in the medium and used for each experiment.
Cell viability assay
Initially, we assessed the cytotoxicity of the titanium particles on Ca9-22 cells. After 6-h incubation with or without titanium particles, the Cell Quanti-MTT cell viability assay kit (BioAssay Systems, Hayward, CA, USA) was used to determine the viability of the remaining cells in each well. Briefly, 15 μL of the MTT reagent was added to 80 μL of the culture medium in each well. After 4 h of incubation, the MTT solubilization solution (100 μL) was added to each well, and plates were placed on a shaker for 1 h. Then, absorbance was measured at 570 nm using a plate reader (Molecular Devices, San Jose, CA, USA).
mRNA expression of inflammation-related genes
The expression of inflammation-related genes was assessed using quantitative real-time polymerase chain reaction (qPCR). After 6 h of exposure to titanium, total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). Following RNA concentration measurement, complementary DNA (cDNA) was synthesized from DNase-treated total RNA using the PrimeScript™ RT Master Mix (Takara Bio, Shiga, Japan). The resulting cDNA was analyzed via qPCR using TB Green® Premix Ex Taq™ II (Takara Bio). PCR was performed using the Thermal Cycler Dice Real-Time System II (Takara Bio). The target mRNA levels for COX2 and TGF-β1 were determined. All qPCR experiments were performed in triplicate, and the specificity of the amplified product was verified using melting curve analysis and calculated using the ΔCt method. GAPDH was used as the internal control. The primer sequences are listed in Table 1.
Table 1.
Quantitative real-time polymerase chain reaction primer sequences.
| Gene | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| GAPDH | GTCTTCACCACCATGGAGAAG | GTTGTCATGGATGACCTTGGC |
| COX2 | CACAGGCTTCCATTGACCAGA | GTGCTCCAACTTCTACCATGG |
| TGF-β1 | CTCGGAGCTCTGATGTGTTGAA | CACCCGCGTGCTAATGGT |
| NLRP3 | GATCTTCGCTGCGATCAACA | GATCTTCGCTGCGATCAACA |
| CASP1 | GCCTGTTCCTGTGATGTGGAG | TGCCCACAGACATTCATACAGTTTC |
| NLRP1 | GCCTGTGGCTACTGAGGTAGTTGA | AGGAACTGGTCCCACACACAGA |
| AIM2 | CTGCAGTGATGAAGACCATTCGTA | GGTGCAGCACGTTGCTTTG |
“GAPDH”: glyceraldehyde-3-phosphate dehydrogenase; “COX2”: cyclooxygenase2; “TGF-β1”: transforming growth factor-β1; “NLRP 3”: the nucleotide-binding domain and leucine-rich repeat protein3; “CASP1”: caspase1; “NLRP1”: the nucleotide-binding domain and leucine-rich repeat protein1; “AIM2”: absent in melanoma 2.
Reactive oxygen species quenching with N-acetyl-l-cysteine)
Of note, phagocytes and antigen-presenting cells exhibit a response to titanium particles.19,20 Moreover, the reactivity of epithelial cells through toll-like receptors in the presence of microbial pathogens is well established. Nevertheless, when investigating the response of epithelial cells to titanium particles, it is crucial to explore alternative mechanisms. In the present study, we explored the effects of inflammasomes. We assessed the gene expressions of NLRP1, NLPR3, CASP1, and AIM2 using qPCR. The primer sequences are listed in Table 1.
ROS is one of the critical mediators of NLRP3 inflammasome activation. To assess the potential contribution of ROS to NLRP3 inflammasome activation, cells were treated with N-acetyl-l-cysteine (NAC) (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), a potent scavenger known for its ability to effectively neutralize ROS in vitro. Throughout the 12-h incubation prior to exposure to titanium particles, cells were treated with or without 5 μmol/L NAC.16 Subsequently, the cells were treated with three different medium mixes of titanium particles: 30 μg/mL, 70 μg/mL, and 100 μg/mL mRNA expression of inflammasome-related genes was assessed using qPCR. In this assessment, cells treated with or without NAC were used. The target mRNA levels were determined for NLRP3 and CASP1, as described above. The primer sequences are listed in Table 1.
Enzyme-linked immunosorbent assay
The interleukin (IL)1β concentration in the cell supernatants was quantified using enzyme-linked immunosorbent assay (ELISA). The cells were seeded at a density of 3 × 105 cells/well in 6-well plates and incubated for 12 h with or without NAC. Following a 6-h exposure to different concentrations of titanium or a medium, cell supernatants were collected. Subsequently, the collected supernatants were processed using the IL-1 beta Human ELISA Kit (Invitrogen, Waltham, MA, USA). IL1β production was detected at 450 nm using a plate reader (SpectraMax ABS Plus; Molecular Devices, San Jose, CA, USA).
Imaging of intracellular reactive oxygen species
To investigate the mechanism of NLRP3 inflammasome activation, intracellular ROS were visualized using an ROS Assay Kit (Dojin Molecular Technologies, München, Germany). The cells at a density of 3 × 105 in 6-well plates were seeded for 12 h with or without NAC. After 6 h of exposure to titanium, the cells were washed thrice with Hanks balanced salt solution (HBSS) (FUJIFILM Wako Pure Chemical Corporation). Cells were incubated with the highly sensitive dichlorodihydrofluorescein diacetate dye working solution for 30 min. Following two washes with HBSS, cells were treated with hydrogen peroxide diluted in HBSS (100 μmol/L) for an additional 30 min. Imaging was performed using a microscope (KEYENCE, Itasca, IL, USA) equipped with a 20 × objective lens. For each sample, six random regions of interest were imaged, and fluorescence-positive areas were calculated using Fiji (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data represent the results of three independent experiments with samples tested in triplicate. Data are expressed as mean and standard deviation. Differences among the titanium concentration groups were evaluated using one-way analysis of variance, followed by Tukey's multiple comparison test or an unpaired t-test. Comparison of pre-treatment and post-treatment with NAC was evaluated using paired t-test. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed using GraphPad Prism Version 10.2.3 (GraphPad Software, Boston, MA, USA).
Results
Cell viability and inflammation-related genes expression with titanium particles
Initially, we assessed the cytotoxicity of the titanium particles on Ca9-22 cells by evaluating cell viability. Incubation with each of the four concentrations of titanium particles resulted in >75% viability of the Ca9-22 cells (Fig. 1a). Notably, there were no statistically significant differences in cell viability between any of the two groups, indicating consistent results at all concentrations. The expression levels of COX2 and TGF-β1 were evaluated by qPCR in varying concentrations of titanium particles. Elevated expression was observed for both genes (Fig. 1b). These findings were consistent with the dose-dependent pattern, reinforcing the effect of titanium concentration on the upregulation of these genes. Furthermore, we assessed the IL1β concentration in cell supernatants using an ELISA assay, which revealed a significant increase in IL1β levels at three different concentrations compared with that in the control group (Fig. 1c).
Figure 1.
Inflammation progression in Ca9-22 cells following titanium particle exposure (a) MTT assay conducted 6 h after exposure to titanium particles shows no statistically significant differences among the groups. (b) Expression of inflammation-related genes (COX2, TGF-β1) assessed via quantitative real-time polymerase chain reaction in groups exposed to high (100 μg/mL) and low (30 μg/mL) concentrations of titanium particles, compared to the control group (0 μg/mL). (c) Secretion of IL1β in Ca9-22 cells evaluated using enzyme-linked immunosorbent assay in groups exposed to high (100 μg/mL), medium (70 μg/mL), and low (30 μg/mL) concentrations of titanium particles, compared to the control group (0 μg/mL). “COX2”: cyclooxygenase2; “TGF-β1”: transforming growth factor-β1; “IL1β”: interleukin1β; “-”: cells cultured in medium without titanium particles; “L”: cells cultured in a medium with low (30 μg/mL) concentration of titanium particles; “M”: cells cultured in a medium with medium (70 μg/mL) concentration of titanium particles; “H”: cells cultured in a medium with high (100 μg/mL) concentration of titanium particles. Statistically significant differences between groups are indicated by the same symbol, with a threshold of P < 0.05.
Effect of titanium particles on NLRP3 inflammasome activation
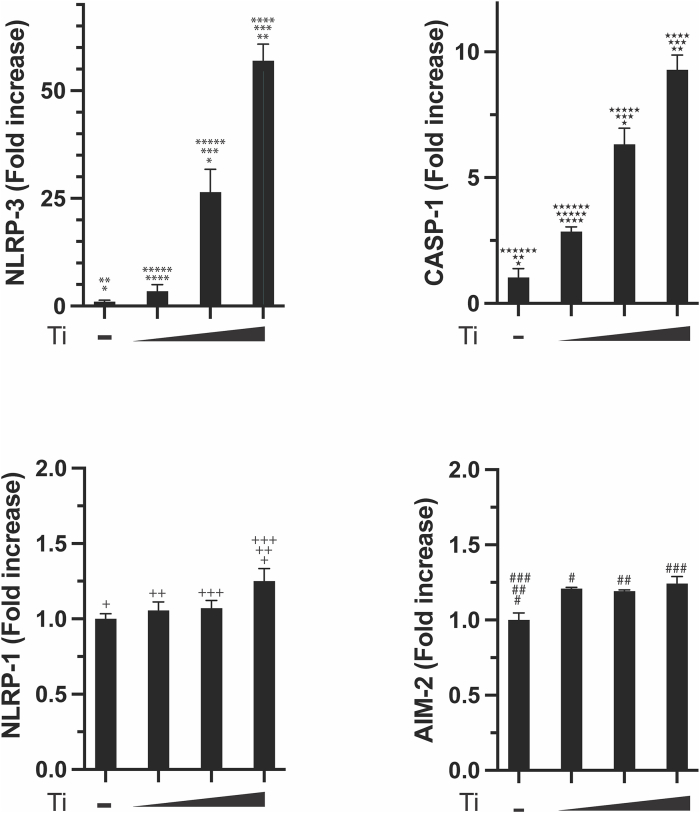
To assess whether the NLRP3 inflammasome could be activated by the titanium treatment in Ca9-22 cells, we measured the mRNA levels of NLRP1, NLRP3, CASP1, and AIM2 across three concentrations of titanium particles and in a control group using qPCR. We observed a concentration-dependent increase in mRNA levels of NLRP3 and CASP1 (Fig. 2). However, the relative increase in NLRP1 and AIM2 gene expressions was minimal, achieving statistical significance in only a few comparisons (Fig. 2).
Figure 2.
Expression of inflammasome-related genes (NLRP1, NLRP3, CASP1, and AIM2) in quantitative real-time polymerase chain reaction. The experimental groups were cultured with high- (100 μg/mL), medium- (70 μg/mL), and low- (30 μg/mL) concentration titanium particles, as well as the control (0 μg/mL). “NLRP1”: the nucleotide-binding domain and leucine-rich repeat protein1; “NLRP3”: the nucleotide-binding domain and leucine-rich repeat protein3; “CASP1”: caspase1; “AIM2”: absent in melanoma 2; “-”: cells cultured in a medium without titanium particles; “L”: cells cultured in a medium with low (30 μg/mL) concentration of titanium particles; “M”: cells cultured in a medium with medium (70 μg/mL) concentration of titanium particles; “H”: cells cultured in a medium with high (100 μg/mL) concentration of titanium particles. Statistically significant differences between groups are indicated by the same symbol, with a threshold of P < 0.05.
Titanium particles promote reactive oxygen species production
As reported previously that titanium particles could induce ROS production in many types of cells,16,21 intracellular ROS was detected using a specific dye. The images of epithelial cells incubated with titanium particles clearly depicted elevated ROS production in comparison with the control group (Fig. 3a). Quantitative analysis of the positive areas revealed a concentration-dependent increase in the ROS-positive areas (Fig. 3b). Treatment with the ROS scavenger (NAC) effectively mitigated the increase in ROS production (Fig. 3a and b).
Figure 3.
Imaging of intracellular reactive oxygen species (ROS) in Ca9-22 cells with or without N-acetyl-l-cysteine (NAC) treatment followed by 6-h exposure to titanium particles. (a) visual images, (b) quantitative data. The experimental groups were cultured with high- (100 μg/mL), medium- (70 μg/mL), and low- (30 μg/mL) concentration titanium particles and the control (0 μg/mL). “L”: cells cultured in a medium with low (30 μg/mL) concentration of titanium particles; “M”: cells cultured in a medium with medium (70 μg/mL) concentration of titanium particles; “H”: cells cultured in a medium with high (100 μg/mL) concentration of titanium particles; “NAC”: N-acetyl-l-cysteine. Statistically significant differences between groups are indicated by the same symbol, with a threshold of P < 0.05.
Reactive oxygen species quenching decreases state of NLRP-3 inflammasome
To verify whether the elevated ROS was involved in the activation of NLRP3 inflammasome, we evaluated the impact of a ROS scavenger on the enhanced expression of the NLRP3 gene (Fig. 4a). The results indicated reduced expression of both NLRP3 and CASP1. In the ELISA, IL1β levels in the supernatant were also reduced following treatment with the ROS scavenger (NAC) at three different concentrations compared with those in the control group (Fig. 4b).
Figure 4.
Influence of reactive oxygen species (ROS) quenching on NLRP3 inflammasome activity: (a) Expression of NLRP3-related genes (NLRP3, CASP1) assessed via quantitative real-time polymerase chain reaction, both with and without N-acetyl-l-cysteine (NAC). (b) Interleukin1β secretion in Ca9-22 cells evaluated using enzyme-linked immunosorbent assay, with or without NAC. Experimental groups were exposed to high (100 μg/mL), medium (70 μg/mL), and low (30 μg/mL) concentrations of titanium particles, as well as a control group (0 μg/mL). “NLRP3”: the nucleotide-binding domain and leucine-rich repeat protein3; “CASP1”: caspase1; “IL1β”: interleukin1β; “-”: cells cultured in a medium without titanium particles; “L”: cells cultured in a medium with low (30 μg/mL) concentration of titanium particles; “M”: cells cultured in a medium with medium (70 μg/mL) concentration of titanium particles; “H”: cells cultured in a medium with high (100 μg/mL) concentration of titanium particles; “NAC”: N-acetyl-l-cysteine. Statistically significant differences between groups are indicated by the same symbol, with a threshold of P < 0.05.
Discussion
The current research has demonstrated that titanium particles possess a distinct ability to trigger the NLRP3 inflammasome in epithelium cells, partly by producing ROS. Inflammasomes are large multiprotein complexes situated in the cell cytoplasm that are responsible for the maturation of proinflammatory cytokines and the initiation of inflammatory cell death, i.e., pyroptosis.22 Interestingly, in our study, the epithelial cells treated with titanium particles exhibited high levels of viability, which is considered to be influenced by both the duration and concentration of exposure to the titanium particles.
Our study showed that the NLRP3 inflammasome is the underlying mechanism of titanium particle-induced inflammation in epithelial cells, providing valuable insights into the pathogenesis of peri-implantitis.23 Recent perspectives have proposed foreign–body reactions as explanatory factors for the breakdown of peri-implant tissues,24 and the activation of the titanium particle-induced NLRP3 inflammasome could emerge as a compelling background mechanism in this scenario. The activation of IL1β release by the inflammasome is triggered by both pathogen-associated molecular pattern molecules (PAMPs) and damage-associated molecular pattern molecules (DAMPs).25 During periodontal pathogenesis, bacterial lipopolysaccharides acts as PAMPs in inflammasomes.25 Conversely, our current findings elucidate the fact that titanium particles can be recognized as DAMPs by epithelial cells in the pathogenesis of peri-implantitis, thereby intensifying the proinflammatory signaling cascade.26 This recognition might lead to an increase in ROS production, possibly due to mitochondrial disruption or NADPH oxidase activation. Consequently, the elevated ROS may directly activate the NLRP3 inflammasome by modifying its proteins or promoting the release of mitochondrial DNA, which could amplify the activation signal. Once activated, the NLRP3 inflammasome is suggested to trigger the release of pro-inflammatory cytokines, such as IL1β, leading to inflammation.
Galindo-Moreno et al. analyzed the presence of inflammasomes NLRP3 and AIM2 in human tissue samples obtained from patients with active peri-implantitis.27 Increased expression of IL1β mediated by caspase-1 was also observed in these lesions compared to samples obtained from clinically healthy supracrestal soft tissue. Our in vitro findings align seamlessly with observations in clinical specimens.
A previous report demonstrated the induction of DNA damage response (DDR) in titanium-treated oral epithelial cells.28 The activity or expression levels of DDR markers, CHK2 and BRCA1, were augmented in the cells cultured with titanium particles extracted from commercially available dental implants.28 Recently, researchers have highlighted the intricate interplay between inflammasomes and DDR.29, 30, 31 Thus, it is plausible that the activation of inflammasomes and DDR in epithelial cells by titanium particles represents a series of interconnected phenomena.
The current trajectory of dental implant research is a transformative shift from emphasis on osseointegration to soft tissue integration.32 Remarkable advancements in implant surface characteristics have resulted in a high osseointegration rate. However, the prevalence of peri-implantitis remains high, highlighting the critical importance of establishing an attachment between the soft tissues and implants at the mucosal margin. Our study provides significant insights into the effect of titanium particles on the activation of inflammasomes in epithelial cells.
This study has certain limitations. First, our analysis focused on the impact of micron-sized titanium particles (0–45 μm). In an orthopedic study involving THP-1 monocytes, the effects of nano-sized (<100 nm) and micro-sized (<5 μm) titanium particles were evaluated.33 These findings indicated that the particle size plays a crucial role in influencing the biological response of macrophages. Another research project related to dental implants examined the effects of titanium ions on inflammasomes in T cells16 and concluded that titanium ions could activate the NLRP3 inflammasome. Consequently, further research is required to assess the effects of titanium of various shapes and characteristics. Second, owing to the multiple roles of ROS in the innate immune response, it is challenging to definitively determine the role of ROS in NLRP3 inflammasome activation. Previous studies have identified ROS as vital intermediaries in activating the NLRP3 inflammasome,34 indicating that ROS modulate activation through the NF-κB signaling pathway.35 However, the specific mechanistic role of ROS in mediating NLRP3 inflammasome activation by titanium particles remains unclear. Further investigation of the signaling pathways involved is imperative for future studies.
Our findings indicate that titanium particles significantly influence cellular biological responses by triggering the NLRP3 inflammasome through ROS activation within the epithelial cells, thereby enhancing IL1β production. This result suggests that titanium particles may act as synergistic activators, amplifying inflammatory responses in epithelial cells. Understanding the effects of titanium particles of various shapes and characteristics is essential for the further application of titanium implants and prevention of peri-implantitis, necessitating further research.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was financially supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (number 22K10042 to A.H.). This work was also partly supported by Nihon University via a 2022 Overseas Researcher grant.
Contributor Information
Akira Hasuike, Email: hasuike.akira@nihon-u.ac.jp.
Kyoko Fujiwara, Email: fujiwara.kyoko@nihon-u.ac.jp.
References
- 1.Da Silva J.D., Kazimiroff J., Papas A., et al. Outcomes of implants and restorations placed in general dental practices: a retrospective study by the Practitioners Engaged in Applied Research and Learning (PEARL) Network. J Am Dent Assoc. 2014;145:704–713. doi: 10.14219/jada.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasuike A., Ueno D., Nagashima H., et al. Methodological quality and risk-of-bias assessments in systematic reviews of treatments for peri-implantitis. J Periodontal Res. 2019;54:374–387. doi: 10.1111/jre.12638. [DOI] [PubMed] [Google Scholar]
- 3.Berglundh T., Armitage G., Araujo M.G., et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz F., Derks J., Monje A., Wang H.L. Peri-implantitis. J Periodontol. 2018;89(Suppl 1):S267–S290. doi: 10.1002/JPER.16-0350. [DOI] [PubMed] [Google Scholar]
- 5.Salvi G.E., Stähli A., Imber J.C., Sculean A., Roccuzzo A. Physiopathology of peri-implant diseases. Clin Implant Dent Relat Res. 2023;25:629–639. doi: 10.1111/cid.13167. [DOI] [PubMed] [Google Scholar]
- 6.Seki K., Hasuike A., Iwano Y., Hagiwara Y. Influence of antihypertensive medications on the clinical parameters of anodized dental implants: a retrospective cohort study. Int. J. Implant Dent. 2020;6:32. doi: 10.1186/s40729-020-00231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki K., Hasuike A., Hagiwara Y. Clinical evaluation of the relationship between systemic disease and the time of onset of peri-implantitis: a retrospective cohort study. J Oral Implantol. 2023;49:55–61. doi: 10.1563/aaid-joi-D-21-00186. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Tong Z., Luo H., Qu Y., Gu X., Si M. Titanium particles in peri-implantitis: distribution, pathogenesis and prospects. Int J Oral Sci. 2023;15:49. doi: 10.1038/s41368-023-00256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safioti L.M., Kotsakis G.A., Pozhitkov A.E., Chung W.O., Daubert D.M. Increased levels of dissolved titanium are associated with peri-implantitis - a cross-sectional study. J Periodontol. 2017;88:436–442. doi: 10.1902/jop.2016.160524. [DOI] [PubMed] [Google Scholar]
- 10.Olmedo D.G., Nalli G., Verdú S., Paparella M.L., Cabrini R.L. Exfoliative cytology and titanium dental implants: a pilot study. J Periodontol. 2013;84:78–83. doi: 10.1902/jop.2012.110757. [DOI] [PubMed] [Google Scholar]
- 11.Wilson TG Jr, Valderrama P., Burbano M., et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86:9–15. doi: 10.1902/jop.2014.140363. [DOI] [PubMed] [Google Scholar]
- 12.Theodoro L.H., Garcia V.G., Ervolino E., Holcroft J., McCulloch C.A., Ganss B. Role of junctional epithelium in maintaining dento-gingival adhesion and periodontal health. Front Dent Med. 2023;4 [Google Scholar]
- 13.Listgarten M.A., Lang N.P., Schroeder H.E., Schroeder A. Periodontal tissues and their counterparts around endosseous implants [corrected and republished with original paging, article orginally printed in Clin Oral Implants Res 1991 Jan-Mar;2(1):1-19] Clin Oral Implants Res. 1991;2:1–19. doi: 10.1034/j.1600-0501.1991.020309.x. [DOI] [PubMed] [Google Scholar]
- 14.Alves C.H., Russi K.L., Rocha N.C., et al. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J Transl Med. 2022;20:425. doi: 10.1186/s12967-022-03636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogura Y., Sutterwala F.S., Flavell R.A. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Tang L., Ye Myat Thu, Chen D. Titanium ions play a synergistic role in the activation of NLRP3 inflammasome in Jurkat T cells. Inflammation. 2020;43:1269–1278. doi: 10.1007/s10753-020-01206-z. [DOI] [PubMed] [Google Scholar]
- 17.Ramenzoni L.L., Flückiger L.B., Attin T., Schmidlin P.R. Effect of titanium and zirconium oxide microparticles on pro-inflammatory response in human macrophages under induced sterile inflammation: an in vitro study. Materials. 2021;14:4166. doi: 10.3390/ma14154166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K., Watanabe M., Mitsuishi K., Iakoubovskii K., Kuroda S. Impact bonding and rebounding between kinetically sprayed titanium particle and steel substrate revealed by high-resolution electron microscopy. J Phys D Appl Phys. 2009;42 [Google Scholar]
- 19.Rakic M., Radunovic M., Petkovic-Curcin A., Tatic Z., Basta-Jovanovic G., Sanz M. Study on the immunopathological effect of titanium particles in peri-implantitis granulation tissue: a case-control study. Clin Oral Implants Res. 2022;33:656–666. doi: 10.1111/clr.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taira M., Kagiya T., Harada H., et al. Microscopic observations and inflammatory cytokine productions of human macrophage phagocytising submicron titanium particles. J Mater Sci Mater Med. 2010;21:267–275. doi: 10.1007/s10856-009-3834-x. [DOI] [PubMed] [Google Scholar]
- 21.Cao J., Ma X., Liu L., et al. Cortistatin attenuates titanium particle-induced osteolysis through regulation of TNFR1-ROS-caspase-3 signaling in osteoblasts. Ann N Y Acad Sci. 2022;1513:140–152. doi: 10.1111/nyas.14774. [DOI] [PubMed] [Google Scholar]
- 22.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchesan J.T., Girnary M.S., Moss K., et al. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol. 2000 2020;82:93–114. doi: 10.1111/prd.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albrektsson T., Tengvall P., Amengual L., Coli P., Kotsakis G.A., Cochran D. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front Immunol. 2023;13 doi: 10.3389/fimmu.2022.1056914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X., Huang H., Zhao L. PAMPs and DAMPs as the bridge between periodontitis and atherosclerosis: the potential therapeutic targets. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.856118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insua A., Galindo-Moreno P., Miron R.J., Wang H.L., Monje A. Emerging factors affecting peri-implant bone metabolism. Periodontol. 2000 2024;94:27–78. doi: 10.1111/prd.12532. [DOI] [PubMed] [Google Scholar]
- 27.Galindo-Moreno P., Montalvo-Acosta S., Martín-Morales N., et al. Inflammasomes NLRP3 and AIM2 in peri-implantitis: a cross-sectional study. Clin Oral Implants Res. 2023;34:1342–1353. doi: 10.1111/clr.14174. [DOI] [PubMed] [Google Scholar]
- 28.Suárez-López Del Amo F., Rudek I., Wagner V.P., et al. Titanium activates the DNA damage response pathway in oral epithelial cells: a pilot study. Int J Oral Maxillofac Implants. 2017;32:1413–1420. doi: 10.11607/jomi.6077. [DOI] [PubMed] [Google Scholar]
- 29.Burlet D., Huber A.L., Tissier A., Petrilli V. Crosstalk between inflammasome sensors and DNA damage response pathways. FEBS J. 2024 doi: 10.1111/febs.17060. [DOI] [PubMed] [Google Scholar]
- 30.Pezone A., Olivieri F., Napoli M.V., Procopio A., Avvedimento E.V., Gabrielli A. Inflammation and DNA damage: cause, effect or both. Nat Rev Rheumatol. 2023;19:200–211. doi: 10.1038/s41584-022-00905-1. [DOI] [PubMed] [Google Scholar]
- 31.Klapp V., Álvarez-Abril B., Leuzzi G., Kroemer G., Ciccia A., Galluzzi L. The DNA damage response and inflammation in cancer. Cancer Discov. 2023;13:1521–1545. doi: 10.1158/2159-8290.CD-22-1220. [DOI] [PubMed] [Google Scholar]
- 32.Touati B., Rompen E., Van Dooren E. A new concept for optimizing soft tissue integration. Pract Proced Aesthetic Dent PPAD. 2005;17:711–715. [PubMed] [Google Scholar]
- 33.Schoenenberger A.D., Schipanski A., Malheiro V., et al. Macrophage polarization by titanium dioxide (TiO2) particles: size matters. ACS Biomater Sci Eng. 2016;2:908–919. doi: 10.1021/acsbiomaterials.6b00006. [DOI] [PubMed] [Google Scholar]
- 34.Dick M.S., Sborgi L., Rühl S., Hiller S., Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2016;7 doi: 10.1038/ncomms11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abais J.M., Xia M., Zhang Y., Boini K.M., Li P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxidants Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]