Abstract
Tongue squamous cell carcinoma (TSCC) is a common malignant oral cancer characterized by substantial invasion, a high rate of lymph node and distant metastasis, and a high recurrence rate. This study aims to provide new ideas for the diagnosis and treatment of TSCC patients by exploring the related mechanisms that affect the migration and invasion of TSCC and inhibit the migration and spread of cancer cells. The results indicated the rate of high expression of IL-17 in cancer tissues was greater than that in tongue tissues, and the expression of IL-17 was related to the TNM stage. The expression of IL-17 in Cal-27 cells was greater than that in HOEC. With increasing IL-17 concentration, cell proliferation, migration, and invasion increased, and the apoptosis rate decreased. After adding the IL-17 inhibitor, the cell proliferation, invasion, and migration abilities decreased, the apoptosis rate increased, and the expression of JAK1and p-STAT3 decreased.IL-17 is highly expressed in oral tongue squamous cell carcinoma and is involved in the occurrence and development of TSCC, possibly through the JAK‒Stat signaling pathway. This study provides a new target and theoretical basis for treating tongue squamous cell carcinoma.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87637-w.
Subject terms: Diseases, Medical research, Oncology
Introduction
Oral cancer is one of the ten most common malignant tumors in the world, with rapid progress, extensive invasion, and distant metastasis in advanced stages, which seriously threatens the life and health of patients1. Squamous cell carcinoma is the most common pathological type of oral cancer, accounting for more than 90% of cases. Oral squamous cell carcinoma (OSCC) is characterized by high malignancy, invasive growth, and early regional lymph node metastasis2. Tongue squamous cell carcinoma (TSCC) is a common malignant oral squamous cell carcinoma. It is characterized by invasion, a high lymph node and distant metastasis rate, and a high recurrence rate, leading to a low quality of life and poor patient prognosis3,4. Although many breakthroughs have been made in diagnosing and treating TSCC, the 5-year survival rate of patients is only approximately 50%5. In recent years, immunotherapy has become an effective treatment option for OSCC, and the discovery of mechanisms that promote malignant transformation, tumor progression, invasion, and metastasis is the basis for finding new targeted therapies. Therefore, exploring the related mechanisms affecting the migration and invasion of TSCC and inhibiting the migration and spread of cancer cells is conducive to providing new ideas for diagnosing and treating TSCC patients.
Interleukin-17 is mainly secreted by T helper 17 cells and participates in innate and acquired immunity. As an important part of the tumor inflammatory microenvironment, its essential role in various malignant tumors has been confirmed6,7. IL-17 has two roles in carcinogenesis: it can promote tumor cell proliferation, metastasis, neovascularization and drug resistance, induce anti-tumor immunity, and improve the clearance ability of CTLs (Cytotoxic T Lymphocytes) to inhibit tumor growth8–10. The abnormal expression and role of IL-17 in oral squamous cell carcinoma are also controversial. IL-17 is highly expressed in oral squamous cell carcinoma. It induces the release of cytokines, which play important roles in forming an inflammatory microenvironment and promote the growth, migration, and invasion of tumor cells11–13. It has also been reported that the level of IL-17 F in the serum of OSCC patients is lower than that in the serum of healthy controls14, and the anti-tumor effect of IL-17 F on OSCC cells was also observed in vitro. IL-17 A is mediated by promoting TH17 differentiation related to tumor growth inhibition in OSCC models transplanted into nude mice15,16, thus playing a protective role in OSCC. The expression and clinical significance of IL-17 in tongue squamous cell carcinoma is unclear, and relevant studies have not been reported. Therefore, further understanding the regulatory mechanism of IL-17 in the progression of oral tongue squamous cell carcinoma is highly important for the clinical judgment of tumor prognosis and treatment strategies.
In this study, the expression of IL-17 in patients with tongue squamous cell carcinoma was detected by immunohistochemistry, and its relationship with clinicopathological parameters was analyzed. Then, the expression of IL-17 in human tongue squamous cell carcinoma cells was detected, the effects of IL-17 on the proliferation, migration, invasion, and apoptosis of human tongue squamous cell carcinoma cells were detected, and its mechanism was studied. To clarify the role of IL-17 in tongue squamous cell carcinoma, we aimed to provide a theoretical basis and new treatment ideas for the clinical diagnosis and targeted therapy of TSCC.
Results
IL-17 expression in oral tongue squamous cell carcinoma and para-cancerous tissues
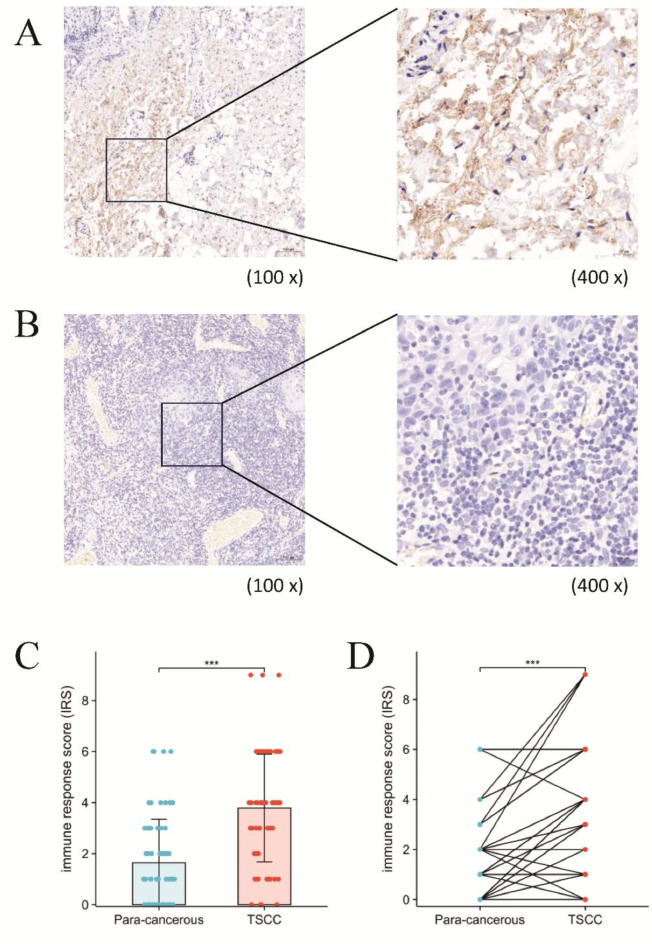
Immunohistochemical staining for IL-17 in oral tongue squamous cell carcinoma and adjacent tissues revealed that positive staining for IL-17 was located mainly in the cytoplasm and was brown-yellow, as shown in Fig. 1A and B. According to the immune response score, the results indicated that the protein expression levels of IL-17 were markedly higher in TSCC tissues than relative to the para-cancerous tissues (Fig. 1C). Furthermore, as illustrated in Fig. 1D, the IL-17 protein level was found to be significantly higher in TSCC tissues as compared to the paired para-cancerous tissues. Additionally, the high expression rate of IL-17 in cancer tissues was 81.43%, while in adjacent non-cancerous tissues it was 27.14%. The rate of high expression of IL-17 in cancer tissues was greater than that in adjacent tissues, and the difference was statistically significant (P < 0.05), as shown in Supplementary Table S1.
Fig. 1.
Immunohistochemical analysis of IL-17 in tongue squamous cell carcinoma (TSCC). (A) An example of IHC image of IL-17 protein expression in TSCC tumor tissue. (B) An example of IHC image of IL-17 protein expression in paired para-cancerous tissue. (C) Box-plot showed differences in protein levels of IL-17 between TSCC tissues and para-cancerous tissues. (D) The IL-17 protein level was significantly higher in TSCC tissue compared to the paired para-cancerous tissue gene.
Relationships between IL-17 expression and clinicopathological parameters in oral tongue squamous cell carcinoma patients
Statistical analysis of clinical and pathological parameters concerning IL-17 expression revealed that IL-17 expression was associated with TNM staging, degree of differentiation, lymph node metastasis, the number of neutrophils, and the neutrophil-to-lymphocyte ratio (NLR) (P < 0.05). However, it was not related to age or gender (P > 0.05), as shown in Table 1.
Table 1.
Relationship between IL-17 protein expression and clinicopathological features in tongue squamous cell carcinoma tissues.
| Characteristics | High-expression | Low-expression | P value |
|---|---|---|---|
| n | 54 | 16 | |
| Gender, n (%) | 0.830 | ||
| Male | 32 (45.7%) | 9 (12.9%) | |
| Female | 22 (31.4%) | 7 (10%) | |
| Age, n (%) | 0.096 | ||
| < 60 | 15 (21.4%) | 8 (11.4%) | |
| ≥ 60 | 39 (55.7%) | 8 (11.4%) | |
| Smoke, n (%) | 0.653 | ||
| No | 37 (52.9%) | 10 (14.3%) | |
| Yes | 17 (24.3%) | 6 (8.6%) | |
| Drink, n (%) | 0.313 | ||
| No | 26 (37.1%) | 10 (14.3%) | |
| Yes | 28 (40%) | 6 (8.6%) | |
| TNM, n (%) | 0.036 | ||
| I-II stage | 28 (40%) | 13 (18.6%) | |
| III-IV stage | 26 (37.1%) | 3 (4.3%) | |
| Metastasis, n (%) | 0.004 | ||
| No | 29 (41.4%) | 15 (21.4%) | |
| Yes | 25 (35.7%) | 1 (1.4%) | |
| Differentiated, n (%) | 0.027 | ||
| Well/moderately | 27 (38.6%) | 13 (18.6%) | |
| Poorly | 27 (38.6%) | 3 (4.3%) | |
| Neutrophil, n (%) | 0.031 | ||
| ≤ 3.74 | 12 (17.1%) | 8 (11.4%) | |
| > 3.74 | 42 (60.0%) | 8 (11.4%) | |
| Lymphocyte, n (%) | 0.335 | ||
| ≤ 1.71 | 23 (32.9%) | 9 (12.9%) | |
| > 1.71 | 31 (44.3%) | 7 (10.0%) | |
| N/L, n (%) | 0.023 | ||
| ≤ 2.13 | 9 (12.9%) | 7 (10.0%) | |
| > 2.13 | 45 (64.3%) | 9 (12.9%) |
Expression of IL-17 in normal oral epithelial cells and tongue squamous cell carcinoma cells
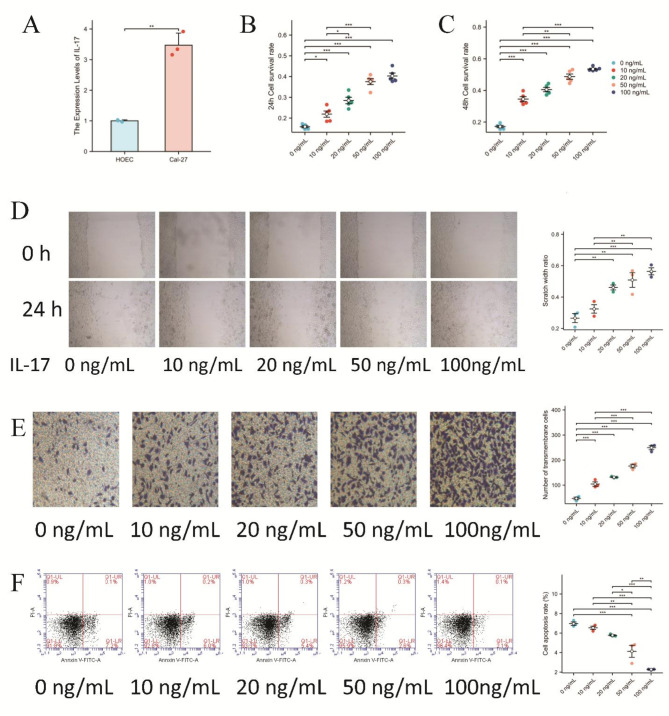
Human normal oral epithelial (HOEC) cells and Cal-27 tongue squamous cell carcinoma cells were collected, and total RNA was extracted. The differences in expression between HOEC and Cal-27 cells were detected via qRT-PCR. The results revealed that the expression of IL-17 in Cal-27 cells was significantly higher than that in HOEC cells (P < 0.01) (Fig. 2A).
Fig. 2.
Effects of IL-17 expression on proliferation, migration, invasion, and apoptosis of Cal-27 cells. (A) The IL-17 mRNA expression level was higher in Cal-2 cells than in HOEC (human normal oral epithelial cell). (B) The effect of different concentrations of IL-17 on the proliferation of Cal-27 cells was assessed after a 24-hour incubation period using the CCK-8 assay. (C) The effect of different concentrations of IL-17 on the proliferation of Cal-27 cells was assessed after a 48-hour incubation period using the CCK-8 assay. (D) Cellular migration evaluated through wound healing assays. (E) Transwell assay (containing matrix gel) to detect cell invasion ability. (F) Annexin V- PI flow cytometry for cell apoptosis.
Effects of IL-17 on the proliferation of the human tongue squamous cell carcinoma cell line Cal-27
To detect the effect of IL-17 on the proliferation of the human tongue squamous carcinoma cell line Cal-27, Cal-27 cells were cultured with different concentrations of IL-17 (0, 10, 20, 50, or 100 ng/mL) for 24 h (Fig. 2B) and 48 h (Fig. 2C), and the absorbance values were detected. The results revealed that at the two-time points of culture, the proliferation ability of the human tongue squamous cell carcinoma cell line Cal-27 gradually increased with increasing IL-17 concentration (P < 0.01).
Effects of IL-17 on the biological activity of the human tongue squamous cell carcinoma cell line Cal-27
To investigate the effect of IL-17 on the biological activity of the human tongue squamous cell carcinoma cell line Cal-27, wound healing, and transwell cell invasion assays were used to detect the effects of IL-17 on the migration and invasion of Cal-27 cells. The results of observation and quantitative analysis revealed that, compared with those in the control group, the relative healing area of the scratch and the migration rate of the cells increased with increasing IL-17 concentration (P < 0.05) (Fig. 2D). In the transwell experiments, with increasing IL-17 concentration, the number of cells that penetrated the membrane gradually increased, and the invasion ability increased (P < 0.05) (Fig. 2E).These results indicated that IL-17 could enhance the migratory and invasive capabilities of Cal-27 cells.
Effects of IL-17 on apoptosis in the human tongue squamous cell carcinoma cell line Cal-27
Cal-27 cells were cultured with different concentrations of IL-17 for 24 h, and Cal-27 cell apoptosis was detected via flow cytometry. The results revealed that the percentage of apoptotic cells decreased gradually with increasing IL-17 concentration, indicating that IL-17 could inhibit Cal-27 cell apoptosis (Fig. 2F).
Effects of an IL-17 inhibitor on the proliferation, migration, invasion, and apoptosis of tongue squamous cell carcinoma Cal-27 cells
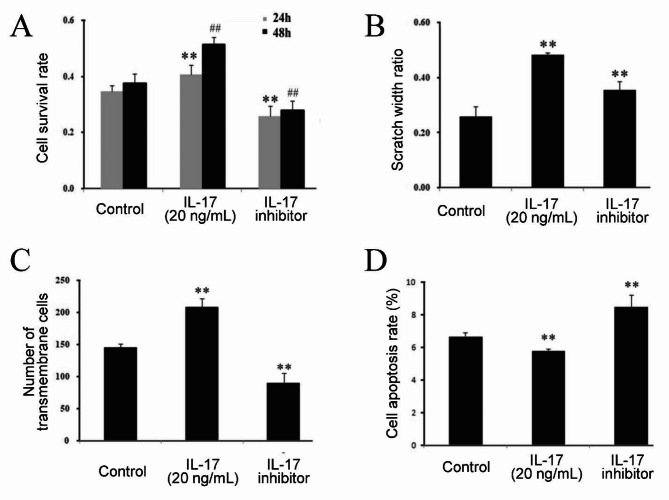
Cal-27 cells were treated with an IL-17 inhibitor or 20 ng/mL IL-17, and changes in the proliferation, migration, invasion, and apoptosis of Cal-27 cells were detected. The results showed that after the addition of the IL-17 inhibitor, the proliferation, invasion, and migration ability of Cal-27 cells decreased, and the apoptosis rate increased (Fig. 3A–D).
Fig. 3.
Effects of IL-17 inhibitor on proliferation, migration, invasion, and apoptosis of Cal-27 cells. IL-17 inhibitor suppressedproliferation (A), migration (B), and invasion (C) of Cal-27 cells while enhancedcell apoptosis rate (D).
Effects of an IL-17 inhibitor on the protein expression of JAK1, STAT3 and p-STAT3 in tongue squamous cell carcinoma cells
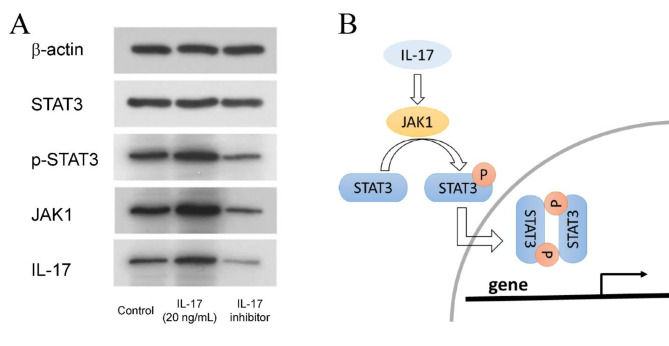
To investigate the effects of IL-17 on the proliferation, migration, invasion and apoptosis of tongue squamous cell carcinoma cells, Cal-27 cells were cultured with IL-17(20 ng/ml)or IL-17 inhibitor. Western blotting was utilized to detect the protein expression of JAK1, STAT3, and p-STAT3. Compared with those in the control group, the expression of JAK1 and p-STAT3 in the IL-17 group was upregulated. The expression of JAK1, STAT3, and p-STAT3 in the IL-17 inhibitor group was down-regulated (Fig. 4A). Based on the results of the Western Blot analysis and the findings reported in the literature, a correlation among IL-17, JAK1, STAT3, and p-STAT3 has been established. The associated pathway was illustrated in Fig. 4B.
Fig. 4.
Effects of IL-17 inhibitor on the expression of JAK1, STAT3, and p-STAT3 in Cal-27 cells. (A) Results of western blot. (B) A correlation among IL-17, JAK1, STAT3, and p-STAT3.
Discussion
As a major type of oral cancer, TSCC is one of the head and neck cancers with the highest mortality rate. Studies have revealed that TSCC has no characteristic obvious symptoms when it progresses from a state before deterioration to invasive cancer, and increased lymph node metastasis is found at the time of initial diagnosis, leading to a poor prognosis. Compared with squamous cell carcinoma in other parts of the oral cavity, TSCC is more aggressive17,18. Clarifying the molecular mechanism of TSCC and identifying new diagnostic and therapeutic targets can provide biomarkers and targets for improving the early diagnosis and targeted therapy of TSCC. Studies have confirmed that IL-17 plays various roles in the inflammatory response, immune response, autoimmune diseases, and malignant tumors.
On the one hand, IL-17 has a significant tumor-promoting effect. For example, the content of IL-17 is significantly increased in malignant tumors such as esophageal cancer, gastric cancer, hepatocellular carcinoma, breast cancer, prostate cancer, leukemia, and multiple myeloma19–23. The study reported that the number of characteristic Th17 cells expressing IL-17 in ovarian cancer tissues was significantly greater than in normal tissues24. Another study reported that the high level of IL-17 expression in breast cancer tissues was related to tumor-associated macrophages25. Hepatocellular carcinoma patients with high expression of IL-17 have increased microvessel density and lymphocyte infiltration, accompanied by high mortality and a low survival rate26. IL-17 can promote the development of pancreatic tuft cells and the stem cell function of adenocarcinoma cells, thereby accelerating the development of pancreatic tumors27. However, it has also been noted that IL-17 can inhibit the occurrence and development of tumors. For example, IL-17 may be protective in patients with colon, ovarian, and acute leukemia28,29.
In this study, we explored the role of IL-17 in developing oral tongue squamous cell carcinoma (TSCC) through immunohistochemical analysis. Our findings showed that the expression of IL-17 in cancerous tissue from TSCC patients was significantly higher than in adjacent non-cancerous tissue. It suggests that IL-17 plays a critical role in the pathogenesis and progression of tongue squamous carcinoma.Further analysis of clinical pathological parameters indicated a correlation between IL-17 expression levels and TNM staging, differentiation grade, and lymph node metastasis. Specifically, elevated IL-17 expression appeared to be a prognostic factor affecting patient survival30. Consequently, assessing IL-17 levels could be a tumor marker for postoperative prognosis in TSCC patients. These results align with previous literature on oral squamous cell carcinoma, indicating that IL-17 levels are associated with disease severity. Neutrophils can promote tumor cell proliferation and progression by releasing neutrophil extracellular traps (NETs)31. Evidence indicates significant neutrophil infiltration within the tumor microenvironment during the development of oral cancer. The study conducted a retrospective study revealing a correlation between extensive neutrophil infiltration in the microenvironment of head and neck squamous cell carcinoma and poor patient prognosis32. In our study, we compared the counts of neutrophils and lymphocytes in the peripheral blood of patients and found that IL-17 expression is associated with the ratio of these two cell types. This suggests a potential link to the inflammatory response in the tumor microenvironment of oral squamous cell carcinoma. We hypothesize that neutrophils may be recruited to the tumor microenvironment through cytokine-mediated inflammation, transforming into tumor-associated neutrophils (TANs). These TANs may then contribute to angiogenesis and enhance tumor cell proliferation, thereby increasing the migration and invasiveness of oral squamous cell carcinoma cells, ultimately impacting patient prognosis. The specific mechanisms underlying these processes require further investigation.
In this study, we collected human normal oral epithelial HOEC cells and tongue squamous cell carcinoma Cal-27 cells in vitro. We detected differences in their expression between HOEC and Cal-27 cells via qRT-PCR. The results revealed that Cal-27 cells expressed more IL-17 than HOEC did. We treated Cal-27 cells with different concentrations of IL-17. CCK-8 assays revealed that IL-17 promoted the proliferation of Cal-27 cells, and scratch repair and transwell invasion assays revealed that IL-17 promoted the migration and invasion of Cal-27 cells. An apoptosis assay revealed that IL-17 inhibited Cal-27 cell apoptosis dose-dependently. An IL-17 inhibitor was used to treat tongue squamous cell carcinoma Cal-27 cells. The results revealed that the proliferation, migration, and invasion abilities of Cal-27 cells decreased, and the apoptosis rate increased. These results suggest that IL-17 is involved in the occurrence and development of oral tongue squamous cell carcinoma and plays a role in tumor promotion.
Several studies have shown that IL-17 can induce the activation of STAT3 by stimulating tumor cells and stromal cells to secrete cytokines and that the expression level of IL-17 is positively correlated with the degree of activation of the Stat3 signal transduction pathway. IL-17 expression and Stat3 pathway activation are related to tumor cell proliferation, invasion, metastasis, apoptosis, and the prognosis of patients and constitute important mechanisms involved in promoting the occurrence and development of tumors33. It has been noted that activation of the JAK-Stat3 signal transduction pathway in the liver can aggravate metabolic stress-induced inflammation in obese mice, thereby accelerating the occurrence of hepatocellular carcinoma34. JAK and Stat3 are activated in myeloma cells, leading to tumor cell survival through upregulating anti-apoptotic genes35. In summary, IL-17 can lead to the upregulation of antiapoptotic and angiogenic factors by promoting the activation of the Stat3 signaling pathway in tumor cells. IL-17 can also activate the Stat3 signaling pathway through the intermediary IL-6. It can upregulate the expression of prosurvival and proangiogenic genes, thereby promoting the growth of tumors36. To investigate whether IL-17 promotes the occurrence and progress of oral tongue squamous cell carcinoma through the JAK-Stat3 pathway, we used an IL-17 inhibitor to detect the protein expression of JAK1, STAT3, and p-STAT3 via Western blotting. Compared with those in the control group, the expression of JAK1 and p-STAT3 was upregulated in the IL-17 group and down-regulated in the IL-17 inhibitor group. Our results showed that IL-17 activated JAK and promoted the translocation of STAT3 into the nucleus as it activated p-STAT3 to recognize target genes. It is speculated that activation of the JAK-Stat signaling pathway may induce abnormal expression of key genes closely related to cell proliferation, differentiation, and apoptosis by regulating antiapoptotic genes, thus promoting cell proliferation and deterioration, hindering apoptosis, and exerting carcinogenic effects.
This study clarified the expression and clinical significance of IL-17 in oral tongue squamous cell carcinoma; confirmed that IL-17 can promote proliferation, migration, and invasion and inhibit apoptosis in tongue squamous cell carcinoma; and explored the molecular mechanism involving the JAK-Stat signaling pathway, which is expected to provide a new target and theoretical basis for the treatment of tongue squamous cell carcinoma. Since the process and mechanism of distant metastasis of tongue squamous cell carcinoma are complex, more relevant experiments are needed.
Materials and methods
Study population
Seventy patients with oral tongue squamous cell carcinoma admitted to the First Affiliated Hospital of Bengbu Medical University from September 2019 to December 2023 were recruited, including 41 males and 29 females aged 38–89 years. All trial personnel provided written informed consent. The inclusion criteria for patients were: (1) oral tongue squamous cell carcinoma confirmed by postoperative pathology; and (2) all patients in the experimental group were the first primary cases and had not received radiotherapy, chemotherapy, or immunotherapy before surgery. The exclusion criteria were as follows: (1) non-squamous cell carcinoma confirmed by postoperative pathology; (2) liver and kidney diseases combined with autoimmune diseases or tumors in other parts of the body; and (3) patients who were not initially diagnosed with a primary tumor or who received treatment for other tumors. This study involving human tissue specimens was approved by the Ethics Committee of Bengbu Medical University (No. 2020LK075), and was conducted following the ethical guidelines of the Declaration of Helsinki.
Immunohistochemical staining
Immunohistochemical staining was performed via the incision method, with PBS as a negative control and known TSCC tissues as a positive control. After the paraffin sections were hydrated with gradient alcohol, they were rinsed with PBS and incubated with PBS buffer containing hydrogen peroxide for 20 min, and an anti-IL-17 antibody (Source of antibody: ab-mart; Item No. : TD6127) was added overnight. After washing with PBS, secondary antibodies were added, followed by color counterstaining and observation under a microscope. The number of positive cells and the total number of cells in the three counting areas of each photograph were measured via ImageJ software, and the number of positive cells in the three counting areas of each picture was calculated. The percentage of positive cells was calculated and scored as follows: 0 points for no positive cells, 1 point for 1-25%, 2 points for 26-50%, 3 points for 51-75%, and 4 points for ≥ 76%. Finally, the immune response score (IRS) was calculated as the percentage of positive cells × cell staining intensity. According to the IRS results, ≦3 was considered a low expression, and > 3 was regarded as a high expression. The patients were grouped according to age, sex, TNM stage, presence of lymph node metastasis, etc. The relationships between the IL-17 expression level and clinicopathological parameters were analyzed.
Cell culture
The human tongue squamous cell carcinoma cell line Cal-27 (Purchased from Fenghui Biological Co., LTD; Item No. : CL0061)and the human oral epithelial cells line HOEC (Purchased from Procell Life Technology Co., LTD; Item No. : CP-H203) were cultured in DMEM containing 10% fetal bovine serum and 100 U/mlpenicillin-streptomycin at 37 °C and 5% CO2. The cells were subcultured every three days, and when the cells were in a good growth state and the logarithmic growth phase, they were digested with trypsin, centrifuged, and resuspended in a medium to obtain a single-cell suspension.
Real-time PCR
Total RNA was extracted from the human tongue squamous cell carcinoma cell line Cal-27 and the human normal oral epithelial cell line HOEC via the Trizol reagent, and the RNA was subsequently reverse transcribed into cDNA via the ReverAid RT reverse transcription kit. qRT‒PCR was performed via an SYBR Green real-time PCR premix with a qRT‒PCR instrument (Applied Biosystems™ 7500, USA). GAPDH was used as the internal reference gene. The following primers were used for expression analysis: IL-17A-fw: 5’-TAGCAAACTCAGCTCTTC-3’, IL-17 A-rev: 5’-TTCTGTCTACAGCATTGG-3’; GAPDH-fw: 5’-CACCCACTCCTCCACCTTTG-3’; GAPDH-rev: 5’-CCACCACCCTGTTGCTGTAG-3’. Relative gene expression level was measured by 2-ΔΔCt relative quantitative method.
CCK-8 assay
Cal-27 cells (2 × 103 cells/well) were seeded into 96-well plates and incubated with different concentrations of IL-17 (0, 10, 20, 50, or 100 ng/ml) for 24–48 h. After incubation, 10 µL of CCK-8 solution was added, and the mixture was incubated at room temperature for 4 h before the absorbance at 450 nm was measured and recorded via a microplate reader.
Scratch repair experiment
Cal-27 cells were seeded in 24-well culture plates (5 × 104 cells/well), and the cell surface was scratched with a 200 µL sterile pipette in a straight line. Fresh serum-free medium containing IL-17 (10, 20, 50, or 100 ng/ml) was added, and the samples were photographed for recording. The cells were cultured in a 37 °C 5% CO2 incubator for 48 h and then removed and photographed to record scratch repair. The data were analyzed via ImageJ software.
Transwell cell invasion assay
The matrigel was evenly spread in the transwell chamber and incubated at room temperature for 30 min, after which the cell density was adjusted to 5 × 104/ml. One hundred microliters of single-cell suspensions were seeded in the upper chambers of transwell chambers coated with Matrigel, and the lower chambers were filled with a complete medium supplemented with or without IL-17 (10, 20, 50, or 100 ng/ml). After 48 h, the cells were fixed in 4% paraformaldehyde solution, stained with crystal violet, observed under a microscope, photographed, and counted.
Detection of apoptosis
Cal-27 cells were cultured with or without IL-17 (10, 20, 50, or 100 ng/ml) for 24 h. After trypsin digestion, the cells were collected, and 1 × 106 cells were centrifuged to discard the supernatant. Annexin V-FITC and PI solutions were added to the precipitate for staining. The cells were incubated at room temperature in the dark for 20 min and then analyzed by flow cytometry.
Effects of IL-17 inhibitors on cell proliferation, migration, invasion, and apoptosis
Cal-27 cells were cultured without IL-17 or in the presence of IL-17 (20 ng/ml) and IL-17 inhibitor (IL-17 A modulator 2, Purchased from MCE Corporation; Item No. : HY-145429) for 24 h, after which the effects of the IL-17 inhibitor on cell proliferation, migration, invasion, and the apoptosis rate were detected.
Western blot analysis
Cal-27 cells were cultured without IL-17 or in the presence of IL-17 (20 ng/ml) and the IL-17 modulator 2 for 24 h. The cells were lysed, the supernatant was collected, and the protein concentration was determined with a BCA protein quantification kit. After adding the loading buffer, the mixture was heated with boiling water for 5 min. After cooling, the samples were divided into aliquots and stored at -80 °C for later use. Proteins were separated via 10% SDS‒PAGE and transferred to PVDF membranes, which were blocked with PBST containing 5% BSA for 2 h at room temperature, and then incubated with the primary antibodies of JAK1, p-STAT3, STAT3 (Source of antibody: CST; Item No. : #3344, #9145, #30835) overnight at 4 °C. After rinsing with PBST buffer for 10 min× three times, the cells were incubated with secondary antibodies for 2 h at room temperature, and an enhanced chemiluminescence (ECL) chromogenic kit was used for color development and image recording.
Statistical analysis
Statistical analysis was performed by using R software (version 4.0.3). And the data are expressed as mean ± SD values. The relationships between the expression level and clinicopathological parameters were tested via the χ2 test. The qRT-PCR was utilized to compare proliferation, migration, apoptosis, and protein expression among multiple groups via one-way ANOVA, and pairwise comparisons between groups were performed via the SNK-q test. P < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conception and design: L.J.; Data acquisition: Z.M. and L.S.; Statistical analysis and interpretation of data: C.Z. and L.J.; Drafting the manuscript: L.J.; Figures and tables: J.L. and H.L. All authors reviewed the manuscript.
Funding
This work was supported by Key Special Projects in Translational Medicine of Bengbu Medical College (BYTM 2019005), and the University Cooperative Research and Public Health Collaborative Innovation Project of Anhui Province(GXXT-2020–021).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bawaskar, H. S. & Bawaskar, P. H. Oral diseases: a global public health challenge. Lancet395, 185–186. 10.1016/S0140-6736(19)33016-8 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Funk, G. F. et al. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 24, 165–180. 10.1002/hed.10004 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Noi, M. et al. Expressions of ezrin, ERK, STAT3, and AKT in tongue cancer and association with tumor characteristics and patient survival. Clin. Experimental Dent. Res.6, 420–427. 10.1002/cre2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen, A. P. et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am. J. Surg.180, 139–143. 10.1016/s0002-9610(00)00433-5 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Goto, M. et al. Prognostic factors and outcomes for salvage surgery in patients with recurrent squamous cell carcinoma of the tongue. Asia-Pac. J. Clin. Oncol.12, e141–e148. 10.1111/ajco.12087 (2016). [DOI] [PubMed] [Google Scholar]
- 6.McGeachy, M. J. et al. The IL-17 family of cytokines in health and disease. Immunity50, 892–906. 10.1016/j.immuni.2019.03.021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian, X. et al. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine89, 34–44. 10.1016/j.cyto.2015.09.011 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Amatya, N., Garg, A. V. & Gaffen, S. L. IL-17 signaling: the Yin and the Yang. Trends Immunol.38, 310–322. 10.1016/j.it.2017.01.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, M. H. et al. Interleukin 17 and peripheral IL-17-expressing T cells are negatively correlated with the overall survival of head and neck cancer patients. Oncotarget. 9, 9825–9837 10.18632/oncotarget.23934 (2018). [DOI] [PMC free article] [PubMed]
- 10.Gaur, P., Singh, A. K., Shukla, N. K. & Das, S. N. Inter-relation of Th1, Th2, Th17 and Treg cytokines in oral cancer patients and their clinical significance. Hum. Immunol.75, 330–337. 10.1016/j.humimm.2014.01.011 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Xiaonan, H. Expression levels of BDNF, VEGF, IL-17 and IL-17F in oral and maxillofacial squamous cell carcinoma and their clinicopathological features. Acta Med. Mediterr.35, 1225–1231 (2019). [Google Scholar]
- 12.Ding, L. et al. Serum IL-17F combined with VEGF as potential diagnostic biomarkers for oral squamous cell carcinoma. Tumour Biol.36, 2523–2529. 10.1007/s13277-014-2867-z (2015). [DOI] [PubMed] [Google Scholar]
- 13.Zielińska, K. et al. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J. Immunol. Res.. 3928504. 10.1155/2020/3928504 (2020). [DOI] [PMC free article] [PubMed]
- 14.Almahmoudi, R. et al. Extracellular interleukin-17F has a protective effect in oral tongue squamous cell carcinoma. Head Neck. 40, 2155–2165. 10.1002/hed.25207 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Almahmoudi, R. et al. Interleukin-17F has anti-tumor effects in oral tongue cancer. Cancers11, 650. 10.3390/cancers11050650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao, Y., Lin, W. & Zhang, Y. Fabrication of tongue extracellular matrix and reconstitution of tongue squamous cell carcinoma in vitro. J. Vis. Exp.5723510.3791/57235 (2018). [DOI] [PMC free article] [PubMed]
- 17.Elseragy, A. et al. Emerging histopathologic markers in early-stage oral tongue cancer:a systematic review and meta-analysis. Head neck. 44, 1481–1491. 10.1002/hed.27022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, R. et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 13, 59. 10.1186/1471-2407-13-59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, F. M. et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol. Cancer. 10, 150. 10.1186/1476-4598-10-150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, R. et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology54, 900–909. 10.1002/hep.24486 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Wang, L. et al. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res.70, 10112–10120. 10.1158/0008-5472.CAN-10-0775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian, G. & Zhao, W. Y. IL-17, an important prognostic factor and potential therapeutic target for breast cancer? Eur. J. Immunol.44, 604–605. 10.1002/eji.201343875 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Miyahara, Y. et al. Generation and regulation of human CD4 + IL-17-producing T cells in ovarian cancer. Proc. Natl. Acad. Sci. USA. 105, 15505–15510. 10.1073/pnas.0710686105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu, X. et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Research: BCR. 10, R95. 10.1186/bcr2195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllaster, J. D. & Cohen, M. S. Role of the lymphatics in cancer metastasis and chemotherapy applications. Adv. Drug Deliv. Rev.63, 867–875. 10.1016/j.addr.2011.05.014 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Y. et al. Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology155, 210–223. .e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abousamra, N. K., El-Din, S., Helal, R. & M., & Prognostic value of Th17 cells in acute leukemia. Med. Oncol.30, 732. 10.1007/s12032-013-0732-3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan, C. et al. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res.352, 351–359. 10.1007/s00441-013-1567-0 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Dawson, H. et al. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology64, 577–584. 10.1111/his.12294 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Wang, L. et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med.206, 1457–1464. 10.1084/jem.20090207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohme, S. et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res.76, 1367–1380. 10.1158/0008-5472.CAN-15-1591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trellakis, S. et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer. 129, 2183–2193. 10.1002/ijc.25892 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Ashrafizadeh, M. et al. .STAT3 pathway in gastric cancer: signaling, therapeutic targeting and future prospects. Biology9, 126. 10.3390/biology9060126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, Y. et al. Dual role for inositol-requiring enzyme 1α in promoting the development of hepatocellular carcinoma during diet-induced obesity in mice. Hepatology68, 533–546. 10.1002/hep.29871 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Bar-Natan, M., Nelson, E. A., Xiang, M. & Frank, D. A. STAT signaling in the pathogenesis and treatment of myeloid malignancies. JAK-STAT1, 55–64. 10.4161/jkst.20006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan, B. et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep.5, 16053. 10.1038/srep16053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.