Abstract
Background
/purpose: The metabolic by-product butyric acid of Gram-negative anaerobic bacteria can invoke pathological effects on periodontal cells resulting in inflammation and further destruction of periodontium. However, limited researches on the effects of butyric acid on cementoblasts were reported. Therefore, this study aimed to investigate the type of cell death in murine cementoblast (OCCM.30) caused by adding the different concentrations of sodium butyrate to the cell culture.
Materials and methods
OCCM.30 cells were exposed to sodium butyrate (0, 2, 4, 8, 16 mM) for 48 h. Cell viability was determined by microculture tetrazolium assay. Cell cycle distribution and cell death were analyzed by flow cytometry. Caspase-mediated apoptotic cascade was evaluated by Western blot.
Results
The concentrations of sodium butyrate≧4 mM were found to inhibit cell viability of OCCM.30 cells in a dose-dependent manner (P < 0.05). Sodium butyrate elevated sub-G1 cell population which exhibited cell apoptosis in OCCM.30 cells (P < 0.05). In addition, early and later apoptotic cells were found in sodium butyrate-induced cell death. Sodium butyrate significantly stimulated the degradation of procaspases-3, -8, and -9 levels, respectively (P < 0.05). Simultaneously, sodium butyrate corresponded to augment the levels of cleaved forms of caspases-3, -8, and -9, respectively (P < 0.05).
Conclusion
Taken together, sodium butyrate is a cytotoxic agent and can induce apoptosis on cementoblasts. The pathway involved in apoptosis is activated by caspase family signaling pathways. These evidences may provide a new mechanistic insight into the mechanism of damage of cementoblasts during the development and progression of periodontitis.
Keywords: Periodontitis, Short-chain fatty acids, Sodium butyrate, Cementoblast, Apoptosis
Introduction
Periodontitis is an infectious and chronic inflammatory disease that can cause periodontal supporting tissues breakdown as the result of anaerobic bacteria specific interaction and host's immune-inflammatory response.1,2 It is usually overlooked the initial symptoms leading to tooth loss and even connection to the occurrence of systemic diseases.3 In Taiwan, the prevalence of periodontitis was reported to significantly increase over past 17 years.4
Butyrate, one of the short chain fatty acids, is the extracellular metabolite from periodontal pathogens such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Tannerella forsythia widely distributed within periodontal pocket.5,6 These periodontopathic bacteria can attack and invade adjacent tissues or release toxic metabolites including butyrate that could evoke further cellular responses. Previous study has shown that the concentrations of butyrate in gingival crevicular fluid (GCF) were correlated with gingival inflammation and periodontal pocket depth.7 The concentrations of butyrate in GCF of patients with chronic periodontitis8 and generalized aggressive periodontitis9 were found to decrease significantly to the levels found in healthy control group at 2 wk post-treatment, respectively. In addition, the levels of butyrate in GCF obtained from generalized aggressive periodontitis were significantly higher in P. gingivalis-, T. denticola-, P. intermedia- or F. nucleatum-positive sites compared with those in negative sites.8 Taken together, butyrate may act as an indicator in the initiation and progression of periodontal diseases.
To respond to the drastic changes in extracellular environment, cells in periodontal niche may undergo inflammatory responses or cell death to alert the immune defense system.10 Apoptosis is a type of programmed cell death characterized by a series of biochemical events that lead to a variety of morphological changes such as cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation.11 Previous report with in situ detection in human gingival tissues revealed that the process of apoptosis could be relevant in the control of inflammation in periodontal disease.12 Furthermore, the clinical significance of periodontal ligament fibroblast apoptosis has been suggested by its linkage to collagen destruction and attachment loss in rat gingival sulci.13 Taken together, apoptosis modulated by anaerobic bacteria may play an important role in pathogenesis of periodontal diseases.
Local production of butyrate from periodontal pathogens in GCF may contribute to periodontal inflammation and tissue breakdown through apoptosis. However, the detailed effects of butyrate on sequential apoptosis processes of periodontal cells such as cemenotblast still remain to be elucidated. The aim of this study was to investigate sodium butyrate on murine immortalized cementoblast cell line (OCCM.30) by measuring cell viability, type of cell death, and caspase-mediated apoptotic cascade signaling pathways.
Materials and methods
Cell culture
Immortalized murine cementoblasts (OCCM.30) derived from root surface of the first mandibular molar of osteocalcin large T-antigen transgenic mice were used for this study.14,15 OCCM.30 cells were grown in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Gaithersburg, MD, USA), supplemented with 10 % fetal calf serum (FCS) and antibiotics (Gibco BRL). Cultures were maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air.
Cytotoxicity assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma–Aldrich, St. Louis, MO, USA) solution prepared in 5 mg/mL phosphate-buffered saline just was use for the cytotoxicity of sodium butyrate (Sigma–Aldrich) on OCCM.30 cells. Briefly, 5 × 104 cells were seeded on a 96-well plate in 100 mL medium and left overnight to attach. Cells were incubated with different concentrations (0, 2, 4, 8, 16 mM) of sodium butyrate for 48 h, respectively. Detailed experimental procedures were performed according to previous studies.16,17
Cell cycle distribution
5 × 105 OCCM.30 cells in six-well culture dishes were cultured and treated with different concentrations sodium butyrate (0, 2, 4, 8, 16 mM) for 48 h. After treatment, cells were washed with phosphate-buffered saline (PBS) and fixed with ice-cold 75 % ethanol at −20 °C for 12 h. Cells were then incubated with 0.5 mL PI/RNase staining buffer for 15 min in the dark followed by filtration through a 40 μm nylon mesh (Falcon, San Jose, CA, USA). As detail described previously,18 DNA contents of stained cells were detected by a BD Accuri C6 flow cytometer (Becton Dickinson, Worldwide Inc., San-Jose, CA, USA) for fluorescence-activated cell sorting analysis of the cell cycle distribution. The percentage of cells in the sub-G0/G1, G0/G1, S and G2/M phases were determined by their accompanied software (Becton Dickinson).
Annexin V-FITC apoptosis staining assay
5 × 105 OCCM.30 cells in six-well culture dishes were cultured and treated with different concentrations sodium butyrate (0, 2, 4, 8, 16 mM) for 48 h. Subsequently, OCCM.30 cells were harvested with trypsinization together with floating non-viable cells. Apoptotic cell death induced by sodium butyrate was determined following the manufacturer's guidelines of the FITC Annexin V Apoptosis Detection Kit I (no. 556547; BD Biosciences, San Jose, CA, USA) as described previously.18 Combined with PI staining, annexin V-FITC apoptosis staining was performed to differentiate apoptosis from necrosis. The qualitative analysis was evaluated by their accompanied software (Becton Dickinson).
Western blot
To investigate the molecular mechanism further, the initiator and effector caspases and signaling pathways were detected using Western blot analysis. Nearly confluent monolayers of cells were washed with serum-free DMEM and immediately thereafter exposed different concentrations sodium butyrate (2, 4, 8, 16 mM) for 48 h. Cultures without FCS were used as negative control. Cell lysates were collected and the extraction of proteins from cells and immunoblotting analysis. Antibodies against pro-caspase-3 was obtained from BD Biosciences. Antibodies against pro-caspase-8, pro-caspase-9, cleaved caspase-8, cleaved caspase-9, cleaved caspase-3, and active caspase-3-mediated cleavage of polymerase (PARP) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-β-actin antibody was obtained from Abcam (Cambridge, MA, USA).
Statistical analysis
Triplicate experiments were performed throughout this study. All assays were repeated three times to ensure reproducibility. The significance of the results obtained from control and treated groups was statistically analyzed by paired Student t test. A P value of <0.05 was considered statistically significant.
Results
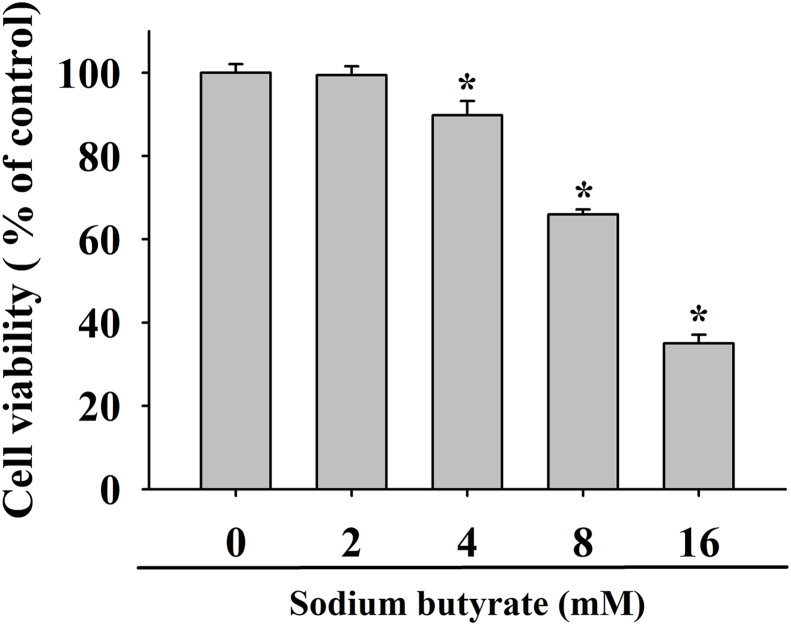
As shown in Fig. 1, the effect of sodium butyrate on OCCM.30 cells were measured by MTT assay for 48 h. The concentrations of sodium butyrate ≧ 4 mM exhibited cytotoxic effect to OCCM.30 cells in a concentration-dependent manner (P < 0.05). At concentrations of 2 mM, 4 mM, 8 mM, and 16 mM sodium butyrate were found to reduce cell viability about 99.4 %, 89.8 %, 66.0 %, and 35.0 % as compared with control, respectively.
Figure 1.
Effects of sodium butyrate on the cell viability of OCCM.30 cells. Data are expressed as the mean ± standard deviation of three independent experiments. ∗ Represents significant difference from without sodium butyrate control values with P < 0.05.
To investigate how sodium butyrate can attenuate the number of viable OCCM.30 cells. Flow cytometry was used to determine the effect of sodium butyrate on the distribution of cell-cycle in OCCM.30 cells (Fig. 2A). As illustrated in Fig. 2B, it demonstrated that the sub-G1 apoptotic fraction was dramatically increased about 8.89 %, 30.24 %, 43.77 %, and 48.41 % in the concentrations of 2, 4, 8, and 16 mM sodium butyrate, respectively (P < 0.05). However, the percentage of cells in G0/G1, S, and G2/M was demonstrated in a decreased pattern (Fig. 2B).
Figure 2.
Effects of the distribution of cell-cycle phase in sodium butyrate-treated OCCM.30 cells. (A) Cells were treated with different concentrations of sodium butyrate (0, 2, 4, 8,16 mM) for 48 h. The distribution of cell-cycle phases and sub-G1 phase (apoptosis) were analyzed by FACS after propidium iodide staining. (B) The percentages of cell population distributed in sub-G1, G0/G1, S, and G2M phases, respectively. Data are expressed as the mean ± standard deviation of three independent experiments. ∗ Represents significant difference from without sodium butyrate control values with P < 0.05.
Apoptosis triggered by sodium butyrate was further confirmed by Annexin V-FITC/PI double-staining. Results were expressed as percentages of necrotic cells (UL), viable cells (LL), later apoptotic cells (UR), and early apoptotic cells (LR) in each panel (Fig. 3A). The concentrations of sodium butyrate higher than 2 mM significantly induced apoptosis of OCCM.30 cells. As shown in Fig. 3B, the proportion of early apoptotic cells dramatically increased about 5.54 %, 18.96 %, 32.18 %, and 51.11 % after treating OCCM.30 cells in the concentrations of 2, 4, 8, and 16 mM sodium butyrate, respectively (P < 0.05). The proportion of late apoptotic cells was also increased in the dose-dependent manner when the concentrations of sodium butyrate ≧ 4 mM (P < 0.05). However, exposure of cells to sodium butyrate slightly dose-dependent increased cell necrosis without statistically significant (P > 0.05).
Figure 3.
Annexin-V/PI double-staining flow cytometry analysis was used to quantify apoptotic cells in OCCM.30 cells treated with sodium butyrate (0, 2, 4, 8,16 mM) for 48 h. (A) Cells in early apoptosis are in lower right quadrant (LR), late apoptosis cells are located in upper right quadrant (UR). Upper left (UL) quarter represents the necrotic cells. Lower left quarter (LL) represents the viable cells. (B) The percentages of cell population distributed in each quarter. Data are expressed as the mean ± standard deviation of three independent experiments. ∗ Represents significant difference from without sodium butyrate control values with P < 0.05.
To investigate the underlying mechanism of sodium butyrate-induced apoptosis, the activation of initiator of an intrinsic pathway (caspase-9), an extrinsic pathway (caspase-8), and the final executioner (caspase-3) were detected in OCCM.30 cells. The results showed that sodium butyrate concentration-dependently induced the degradation of procaspases-8, -9, -3, and PARP in Fig. 4A. The quantitative results of these protein levels were illustrated in Fig. 4B. The levels of procaspases-3 were 0.56, 0.72, 0.81, and 0.19 fold at the concentrations of 2, 4, 8, and 16 mM sodium butyrate as compared with control (P < 0.05). As shown in Fig. 4C, the relative expressions of cleaved caspase-8, -9, -3, and PARP revealed an increased pattern with sodium butyrate as compared with control. The quantitative results of these protein levels were illustrated in Fig. 4D. The levels of cleaved caspase-3 were 1.17, 1.96, 20.67, and 35.35 fold at the concentrations of 2, 4, 8, and 16 mM sodium butyrate as compared with control (P < 0.05).
Figure 4.
OCCM.30 cells were treated with indicated concentrations of sodium butyrate for 48 h, and the protein levels of pro- and cleaved caspases-3, -8, and -9, and PARP were determined by Western blot in panel (A) and (C). The quantitative results of these protein levels were adjusted by β-actin levels illustrated in (B) and (D) Values are presented as the mean ± standard deviation from three independent experiments. ∗ Represents significant difference from without sodium butyrate control values with P < 0.05.
Discussion
Cementum is synthesized by cementoblasts during tooth root formation. Cementoblasts play an important role in the periodontal wound healing. To the best of our knowledge, this is the first study that demonstrated short-chain fatty acid butyrate is cytotoxic to OCCM.30 cells. Our results were in agreement with previous studies that butyrate exhibited cytotoxicity on human gingival epithelial Ca9-22 cells,19, 20, 21 human gingival fibroblasts,21, 22, 23 human osteoblast MG63 cells,24 human vascular endothelial cells,25 and human pulp fibroblasts.26 Taken together, the cytotoxicity of sodium butyrate is non-cell type specific.
Cell-cycle checkpoints and cell-death signals are activated to stop cell growth and to eliminate multiplication of the generally altered cells. In this study, sodium butyrate induced apoptotic effect on OCCM.30 cells by the highly elevation of sub-G1 cell population. In addition, annexin V-FITC apoptosis assay also demonstrated two type of early and later apoptotic cells noted in sodium butyrate-induced cell death. Similar findings were found on inflamed gingival fibroblasts27 and human vascular endothelial cells25 that the number of apoptotic cells increased linearly in a dose-dependent manner. However, butyrate was reported no marked increasing the numbers of apoptotic cells in human gingival fibroblasts22,23 and MG63 osteoblastic cells.24 Taken together, the responses of cell cycle and death pathways to sodium butyrate are varied in different cells.
Caspases, cysteine-aspartic proteases, are proteolytic enzymes largely known for their role in controlling cell death and inflammation. Caspase-3 is a central player in the orchestration of apoptotic cell death as the final executioner. Caspase-8 is an initiator of death-receptor-induced apoptosis in extrinsic pathway. Caspase-9 is the initiator of the mitochondrial apoptotic pathway. To the best of our current knowledge, sodium butyrate was first found to decrease pro-caspase-3, -8, and -9 as well as stimulate cleaved caspase-3, -8, and -9 expression in OCCM.30 cells. In addition to the effector caspase 3, sodium butyrate-induced cell apoptosis was found to activate both extrinsic and intrinsic apoptotic processes in OCCM.30 cells. Among them, the protein that increased the most in quantity was cleaved caspase 3, suggesting that the effector caspase 3 is responsible for the actual dismantling of OCCM.30 cells. In agreement of our findings, previous studies have shown that butyrate could stimulate caspase-3 activity on gingival epithelial Ca9-22 cells by colorimetric assay.19,28 Taken together, caspase apoptotic pathway may act the important pivotal routine in butyrate-induced cell death.
The limitations of this in vitro need to be addressed. It is very difficult, if not impossible, to determine how much sodium butyrate can act on cementoblasts clinically. Therefore, the lower concentrations of sodium butyrate and a longer incubation period are required to further investigate sodium butyrate-induced cell death in OCCM.30 cells.
In summary, the effects of sodium butyrate on cementoblasts cell viability were due to the induction of cell cycle arrest, apoptosis, and necrosis in this study. Sodium butyrate activated both extrinsic and intrinsic apoptotic pathways in OCCM.30 cells. These results indicate that sodium butyrate is a cytotoxic agent to cementoblasts. These detrimental effects of sodium butyrate may also occur in vivo and thus might impair the reparative and regenerative potential of periodontal tissues of persons who can not maintain their oral hygiene properly.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study did not receive any external funding resources.
References
- 1.Ji S., Choi Y.S., Choi Y. Bacterial invasion and persistence: critical events in the pathogenesis of periodontal diseases? J Periodontal Res. 2015;50:570–585. doi: 10.1111/jre.12248. [DOI] [PubMed] [Google Scholar]
- 2.Chen T.P., Yu H.C., Lin W.Y., Chang Y.C. The role of microbiome in the pathogenesis of oral-gut-liver axis between periodontitis and nonalcoholic fatty liver disease. J Dent Sci. 2023;18:972–975. doi: 10.1016/j.jds.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L.C., Yu H.C., Chang Y.C. The recent new findings of periodontal-systemic connection from Taiwan's National Health Insurance Research Database. J Dent Sci. 2021;16:789–790. doi: 10.1016/j.jds.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H.C., Su N.Y., Huang J.Y., Lee S.S., Chang Y.C. Trends in the prevalence of periodontitis in Taiwan from 1997 to 2013: a nationwide population-based retrospective study. Medicine. 2017;96:8585. doi: 10.1097/MD.0000000000008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uematsu H., Sato N., Hossain M.Z., Ikeda T., Hoshino E. Degradation of arginine and other amino acids by butyrate-producing as accharolytic anaerobic Gram positive rods in periodontal pockets. Arch Oral Biol. 2003;48:423–429. doi: 10.1016/s0003-9969(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 6.Johansen H., Olsen I., Kerkes K. Differentiation between Bacteriodes gingivalis, Bacteroides endodontalis, and Bacteroides asaccharolytics by means of HPLC analysis of non-derivatized free metabolic acid. Oral Microbiol Immunol. 1988;3:42–45. doi: 10.1111/j.1399-302x.1988.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 7.Niederman R., Buyle-Bodin Y., Lu B.Y., Robinson P., Naleway C. Short-chain carboxylic acid concentration in human gingival crevicular fluid. J Dent Res. 1997;76:575–579. doi: 10.1177/00220345970760010801. [DOI] [PubMed] [Google Scholar]
- 8.Qiqiang L., Huanxin M., Xuejun G., et al. Longitudinal study of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J Periodontal Res. 2012;47:740–749. doi: 10.1111/j.1600-0765.2012.01489.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu R., Meng H., Gao X., et al. Effect of non-surgical periodontal treatment on short chain fatty acid levels in gingival crevicular fluid of patients with generalized aggressive periodontitis. J Periodontal Res. 2014;49:574–583. doi: 10.1111/jre.12137. [DOI] [PubMed] [Google Scholar]
- 10.Song B., Zhou T., Yang W.L., Liu J., Shao L.Q. Programmed cell death in periodontitis: recent advances and future perspectives. Oral Dis. 2017;23:609–619. doi: 10.1111/odi.12574. [DOI] [PubMed] [Google Scholar]
- 11.Earnshaw W.C. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 12.Gamonal J., Bascones A., Acevedo A., Blanco E., Silva A. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001;72:517–525. doi: 10.1902/jop.2001.72.4.517. [DOI] [PubMed] [Google Scholar]
- 13.Ekuni D., Tomofuji T., Yamanaka R., Tachibana K., Yamamoto T., Watanabe T. Initial apical migration of junctional epithelium in rats following application of lipopolysaccharide and proteases. J Periodontol. 2005;76:43–48. doi: 10.1902/jop.2005.76.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Chen C.S., Lee S.S., Yu H.C., Huang F.M., Chang Y.C. Effects of nicotine on cell growth, migration, and production of inflammatory cytokines and reactive oxygen species by cementoblasts. J Dent Sci. 2015;10:154–160. [Google Scholar]
- 15.Huang F.M., Kuan Y.H., Lee S.S., Chang Y.C. Caspase activation by a zinc-oxide eugenol-based root-canal sealer in cementoblasts. J Dent Sci. 2015;10:338–340. [Google Scholar]
- 16.Chen Y.J., Lee S.S., Huang F.M., Yu H.C., Tsai C.C., Chang Y.C. Effects of arecoline on cell growth, migration, and differentiation in cementoblasts. J Dent Sci. 2015;10:388–393. [Google Scholar]
- 17.Chen Y.J., Lee S.S., Huang F.M., Chang Y.C. Effects of nicotine on differentiation, prostaglandin E2 and nitric oxide production in cementoblasts. J Dent Sci. 2015;10:431–436. [Google Scholar]
- 18.Hsiao P.C., Chang J.H., Lee W.J., et al. The curcumin analogue, EF-24, triggers p38 MAPK-mediated apoptotic cell death via inducing PP2A-modulated ERK deactivation in human acute myeloid leukemia cells. Cancers. 2020;12:2163. doi: 10.3390/cancers12082163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuda H., Ochiai K., Suzuki N., Otsuka K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epithelial cells. J Periodontal Res. 2010;45:626–634. doi: 10.1111/j.1600-0765.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- 20.Evans M., Murofushi T., Tsuda H., et al. Combined effects of starvation and butyrate on autophagy-dependent gingival epithelial cell death. J Periodontal Res. 2017;52:522–531. doi: 10.1111/jre.12418. [DOI] [PubMed] [Google Scholar]
- 21.Kurosawa Y., Yamaguchi H., Uemichi K., Shinozuka K., Kirihara Y., Tsuda H. Butyrate-treatment induces gingival epithelial cell death in a three-dimensional gingival-connective tissue hybrid co-culture system. J Dent Sci. 2023;18:893–897. doi: 10.1016/j.jds.2022.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeng J.H., Chan C.P., Ho Y.S., Lan W.H., Hsieh C.C., Chang M.C. Effects of butyrate and propionate on the adhesion, growth, cell cycle kinetics, and protein synthesis of cultured human gingival fibroblasts. J Periodontol. 1999;70:1435–1442. doi: 10.1902/jop.1999.70.12.1435. [DOI] [PubMed] [Google Scholar]
- 23.Chang M.C., Tsai Y.L., Chen Y.W., et al. Butyrate induces reactive oxygen species production and affects cell cycle progression in human gingival fibroblasts. J Periodontal Res. 2013;48:66–73. doi: 10.1111/j.1600-0765.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang M.C., Chen Y.Y., Lian Y.C., et al. Butyrate stimulates histone H3 acetylation, 8-isoprostane production, RANKL expression, and regulated osteoprotegerin expression/secretion in MG-63 osteoblastic cells. Int J Mol Sci. 2018;19:4071. doi: 10.3390/ijms19124071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang M.C., Wang T.M., Chien H.H., et al. Effect of butyrate, a bacterial by-product, on the viability and ICAM-1 expression/production of human vascular endothelial cells: role in infectious pulpal/periapical diseases. Int Endod J. 2022;55:38–53. doi: 10.1111/iej.13614. [DOI] [PubMed] [Google Scholar]
- 26.Ho Y.C., Chang Y.C. Effects of a bacterial lipid byproduct on human pulp fibroblasts in vitro. J Endod. 2007;33:437–441. doi: 10.1016/j.joen.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Kurita-Ochiai T., Seto S., Suzuki N., et al. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87:51–55. doi: 10.1177/154405910808700108. [DOI] [PubMed] [Google Scholar]
- 28.Evans M., Murofushi T., Tsuda H., et al. Combined effects of starvation and butyrate on autophagy-dependent gingival epithelial cell death. J Periodontal Res. 2017;52:522–531. doi: 10.1111/jre.12418. [DOI] [PubMed] [Google Scholar]