Abstract
Background/purpose
Revascularization procedures are used over apexification to treat teeth with necrotic pulp tissues and incomplete root formation. Clinically, inducing proliferation, migration, matrix deposition, and differentiation of stem cells from apical papilla (SCAPs) are critical for pulp regeneration. The study aimed to elucidate the impact of bone morphogenetic protein-4 (BMP-4) on plasminogen activation molecules and the osteogenic/odontogenic differentiation of SCAPs, as well as understand the related signaling mechanisms.
Materials and methods
SCAPs were exposed to BMP-4 with or without signal transduction inhibitors. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. mRNA levels were quantified using real-time PCR. Protein expression in SCAPs was analyzed through immunofluorescent staining or western blotting. Cellular protein production was measured with enzyme-linked immunosorbent assay.
Results
BMP-4 induced suppressor of mother against decapentaplegic (Smad)1/5/8 and Smad2/3 phosphorylation and activation. It also promoted higher expression of osteogenic and odontogenic markers, including Osterix, N-cadherin, and secreted protein acidic and rich in cysteine (SPARC), in SCAPs. Additionally, BMP-4 stimulated connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 (PAI-1), and urokinase plasminogen activator receptor (uPAR) expression, but inhibited uPA expression and production in SCAPs, indicating its role in matrix remodeling and cell migration. Inhibition of Smad2/3 with SB431542 and Smad1/5/8 with LDN193189 attenuated the BMP-4-induced expression Osx, N-cadherin, CTGF, SPARC, uPAR and PAI-1.
Conclusion
These results indicate that BMP-4 stimulates the osteogenic and odontogenic differentiation of SCAPs by regulating matrix turnover and mineralization-related proteins. Furthermore, these processes are associated with the induction of Smad2/3 and Smad1/5/8 of SCAPs by BMP-4.
Keywords: BMP-4, Osteogenic and odontogenic differentiation, Plasminogen, Smad, Stem cells from apical papilla
Introduction
Infection, inflammation, and early pulp necrosis in young permanent teeth with incomplete root formation are commonly reported in the teeth of patients with dens evaginatus, dental trauma, or dental caries.1 Historically, apexification procedures, such as root canal debridement to control infection, induction of apical root dentin barrier formation by Ca(OH)2 or mineralized trioxide aggregate, and root canal obturation, have been the preferred methods.1 However, apexification is time-consuming and the affected teeth are prone to crown or root fractures due to the weakened remaining tooth structures and a reduced crown-to-root ratio.1
Recently, pulp regeneration using revascularization procedures has been successfully developed to induce apexogenesis in the necrotic pulp of immature permanent teeth with periapical abscesses.2, 3, 4 The procedures include an initial root canal disinfection, inflammation control, induced bleeding, and blood clot formation in the pulp chamber and root canal, with or without scaffolds such as platelet-rich fibrin, collagen plugs, or platelet-rich plasma.5 The proliferation and migration of stem cells from apical papilla (SCAPs) into the root canals, their generation of extracellular matrix, and differentiation into odontoblasts or other mineralized tissue-like cells are considered crucial for clinical success and regenerative endodontics.6 Various factors, including root development stages, the genetic background of donors, and dental inflammation, can affect the function of SCAPs. Multiple factors, including extracellular matrix, basic fibroblast growth factor (bFGF), insulin-like growth factor, and transforming growthfactor beta (TGF-β) superfamily are known to regulate SCAPs.7,8 Additionally, various extracellular matrix components play a significant role in the differentiation of the mineralized tissue-forming cells such as periodontal ligament and dental pulp stem cells.9,10 However, more studies are necessary to understand the effect of bone morphogenetic proteins (BMPs) on SCAPs in pulp and root regeneration.
The apical papilla is the soft tissue around the apical region of an incompletely formed root. Studies on the developing tooth germs of mini-pigs have shown that the removal of the apical papilla from halted root formation, even when the pulp tissue was preserved.11 Sonoyama et al. isolated SCAPs and demonstrated their mesenchymal stem cell characteristics, suggesting that SCAPs are the dental mesenchymal cells responsible for root generation.12 Compared to dental pulp stem cells, SCAPs exhibit better cell migration, quicker population doubling, and more Stro-1-positive cells.11,13 Clinically, stem cells residing in the apical papilla are crucial for the success of pulp regeneration procedures. Similar to dental pulp stem cells, SCAPs express odontogenic and osteogenic markers, such as alkaline phosphatase, dentin sialophosphoprotein, and bone sialoprotein, but with better dentinogenic potential.13 SCAPs are thereby useful for dentin repair, pulpal and root regeneration, and even bioroot tissue engineering when combined with scaffolds and different growth factors.14,15
BMPs, part of the TGF-β superfamily proteins, play pivotal roles in embryogenesis, adult tissue replacement, and wound repair.16,17 In the early stage of tooth development, BMP-4 is the signaling molecule driving the transition from the bud stage to the cap stage.18 During root formation, BMP-4 is expressed in the mesenchyme surrounding Hertwig's epithelial root sheath, whereas other BMPs are barely detectable.19 A recent study further demonstrated that BMP-4 is expressed in ameloblasts, odontoblasts, osteoblasts and preodontoblasts around the developing root.20 These findings suggest that BMP-4 is crucial for root development through cellular differentiation induction. In induced pluripotent stem cell-derived neural crest-like cells, exogenous BMP-4 enhanced the gene expression of msh homeobox 1, dentin matrix protein 1, and dentin sialophosphoprotein, implicating an induction of odontoblast differentiation.21 Furthermore, treatment by BMP-4 enhanced the osteogenic differentiation of SCAPs, which may result from up-regulation of Distal-less homeobox 2, osterix (Osx, Sp7), and Meis homeobox 2 expression.22,23 To our knowledge, the effects of BMP-4 on matrix turnover and the odontogenic differentiation of SCAPs have not yet been investigated. Compared to other growth factors, BMP-4 is known to be a critical regulator of crown and root development.18, 19, 20 The interaction between BMP-4 and SCAPs is an emerging area of research that could significantly impact pulp regeneration therapy.
BMPs primarily form heteromeric receptor complexes with transmembrane type I and II receptors, which then phosphorylate and activate the kinase activities of type I receptors, initiating subsequent signal transduction pathways.16,17,24 BMP-4 has been shown to bind favorably to activin receptor-like kinase 3 (ALK3) and ALK6, but not ALK2.25 The formation of the BMP-4-receptor complex activates the downstream signaling effectors through both non-canonical Smad-independent and canonical Smad-dependent pathways. Suppressor of mother against decapentaplegic (Smad)1, 5 and 8 (also known as Smad9) are considered receptor-regulated Smads that mediate the Smad-dependent pathway for BMPs. BMPs are part of the TGF-β superfamily, comprising over 30 members, and exhibit considerable ligand-receptor signaling promiscuity via 7 type I and 5 type II receptors.25 While BMPs stimulate mainly canonical ALK3 or ALK6 and Smad1, 5, 8 signaling pathways, they also activate non-canonical pathways including ALK5, Smad2/3 and mitogen-activated protein kinases such as ERK, JNK, p38 and PI3K/Akt to regulate the proliferation and differentiation in different kind of dental mesenchymal stem cells.24, 25, 26, 27, 28 The effective concentrations of BMPs are about 1–100 ng/ml in granulosa cells, trophoblasts or other cells.27,28 However, the effects of BMP-4 on ALK5 and Smad2/3 signaling in SCAPs remain poorly understood. More studies are warranted to investigate the potential application of BMP-4 in combination with SCAPs for pulpal regeneration.
BMP-4 is essential for human tooth development and plays a critical role in root formation.18, 19, 20 SCAPs are thought to be precursors of root odontoblasts and potent cell sources for dental tissue regeneration.13,29, 30, 31 During clinical revascularization procedures, BMPs can be found in blood clots or serum (3.2–44 pg/ml),32 released from the dentin matrix of the root canal,33 or added exogenously such as 1.5 mg/ml in collagen sponge or others for tissue engineering.33,34 We hypothesized that BMP-4 might potentially influence the turnover of the extracellular matrix and the differentiation of osteoblast and odontoblast from SCAPs, thereby contributing to the pulpal revascularization and regeneration. Therefore, the current study aims to further explore the influence of BMP-4 on the differentiation (Osx, N-cadherin, secreted protein acidic and rich in cysteine [SPARC, osteonectin] and others), connective tissue growth factor (CTGF) and plasminogen activation system molecules (urokinase plasminogen activator [uPA], urokinase plasminogen activator receptor [uPAR], and plasminogen activator inhibitor-1 [PAI-1]) that are crucial for matrix metabolism and turnover. Additionally, the involvement of Smad-dependent signaling in BMP-4-induced events will be explored. The findings of this study can help us understand these processes and develop effective methods to increase the success of clinical revascularization and pulpal regeneration procedures.
Materials and methods
Materials
Recombinant BMP-4 was obtained from PeproTech (PeproTech Inc. Rocky Hill, NJ, USA). NucleoSpin RNA II and RNA isolation kits were obtained from Macherey-Nagle (Macherey-Nagle Inc, Easton, PA, USA). Cell culture reagents, including glutamine, Dulbecco's modified Eagle medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) were obtained from Life Technologies (Thermo Fisher Scientific Ltd., Waltham, MA, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma–Aldrich Company (St. Louis, MO, USA). Primers for real-time PCR were synthesized by Genemed (Genemed Biotechnologies, Inc., San Francisco, CA, USA). Western blotting luminal reagents and mouse-anti-human-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Other antibodies used in western blotting (Smad2/3, Smad1/5/8, p-Smad2, p-Smad3, p-Smad1/5/8, Osx, N-cadherin, CTGF, SPARC) were obtained from Cell Signaling Technology (Danvers, MA, USA) or Genetex Biotechnology. The 4-(5-benzol[1,3]dioxol-5-yl-4-pyfldin-s-yl-1h-imidazole-2-yl-)-benzamide hydrate (SB431542) (an ALK5/Smad2/3 inhibitor) and 4-[6-[4-(1-Piperazinyl)phenyl] pyrazolo[1,5-a]pyrimidin-3-yl]quinoline dihydrochloride (LDN193189) (a Smad1/5/8 inhibitor) were obtained from Tocris Bioscience Company (St. Louis, MO, USA). The enzyme-linked immunosorbent assay (ELISA) kits for uPA, soluble uPAR (suPAR) and PAI-1 were obtained from R & D Systems (Minneapolis, MN, USA).
Culture and characterization of SCAPs
Ethics approval was obtained from the Ethics Committee of National Taiwan University Hospital. Informed consent was obtained from all participants. The SCAPs used in this study were obtained due to orthodontic demand or tooth impaction. Phosphate-buffered saline was used to wash the teeth, and the apical papilla tissue was separated from the root apex with a scalpel blade and minced into small pieces. The tissue explant method, which has been previously described, was used to culture SCAPs.35, 36, 37 Briefly, these tissues were cultivated with DMEM comprising 10 % FBS, 1 % glutamate, 100 ug/ml streptomycin, and 1 % penicillin in a humidified atmosphere with 95 % air and 5 % CO2 at 37 °C. When the outgrowing cells reached confluence, SCAPS were subcultured at a ratio of 1:3. The 3rd to 8th cell passages were utilized for this investigation.22 Flow cytometric analysis confirmed that our cultured SCAPs expressed mesenchymal stem cell markers such as CD105, CD90 and CD73 as before.35, 36, 37
Effect of BMP-4 on the viability of SCAPs
The effect of BMP-4 on SCAP viability was investigated as follows: SCAPs were seeded into 24-well culture plates (1 × 104 or 1 × 105 cells/well) for 24 h to achieve non-confluent and near-confluent cultures. Then, the culture medium was aspirated and replenished by fresh DMEM containing 10 % FBS with various concentrations of BMP-4 (0, 10, 25, 50, 100, 200 ng/ml) for five days. We collected the cultured medium to measure various marker proteins using ELISA. Finally, cells were rinsed and then incubated in a medium comprising MTT (0.5 mg/ml) for 2 h. Viable cells converted T into formazan, which was dissolved in dimethyl sulfoxide and quantified at an optical density of OD540 using a Dynatech Microwell plate reader (Dynatech Labs. Inc., Chantilly, VA, USA).35,38, 39, 40
Effect of BMP-4 on Osx, N-cadherin, CTGF and SPARC mRNA expression in SCAPs
To investigate the effect of BMP-4 on the expression of various regulatory molecules related to differentiation and mineralization, such as Osx, N-cadherin, CTGF, and SPARC, confluent SCAPs were used. SCAPs (1.5 × 106 cells/10-cm dishes or 6-well plates) were exposed to BMP-4 at concentrations ranging from 0 to 200 ng/ml for 24 h. The Macherey–Nagel NucleoSpin RNA II isolation kits were used for RNA isolation. The isolated RNA was subjected to RNA quantification and reverse transcription. The produced cDNA was then subjected to real-time polymerase chain reaction (PCR) amplification and quantification.41,42 The PCR reaction mixtures contained a SYBR master mix, specific primer pairs, cDNA, and diethylpyrocarbonate water. The PCR conditions were as follows: Stage 1, 95 °C for 30 s (1 cycle); Stage 2, 95 °C for 10 s and 60 °C for 30 s for 40 cycles. The following specific primers were used: Osx (GCCAGAAGCTGTGAAACCTC and GCTGCAAGCTCTCCATAACC),43 N-cadherin (GATGTTGAGGTA CAGAATCGT and GGTCGGTCTGGATGGCGA);44 CTGF (TTCCAGAG CAGCTGCAAGTA and TGGAGATTTTGGGAGTACGG),45 SPARC (AAGATCCATGAGAATGAGAAG and AAAAGCGGGTGG TGCAATG),46 β-actin (AAGAGAGGCATCCTCACCCT and TACATGGCTGG GGTGTTGAA). To quantify PCR results, the delta/delta cycle threshold values (ΔCt = mean ΔCt [treated] - mean delta (Δ) Ct [control]) were used to calculate the alterations in gene expression. Changes in the study groups relative to the control (solvent) group were measured via the 2-ΔΔCtmethod and used for data presentation. In all PCR experiments, the β-actin mRNA expression was used as the internal control.
In some experiments, SCAPs were pretreated for 1 h with LDN193189 or SB431542 prior to co-incubation with BMP-4 for 24 h. Following this, RNA was isolated to verify the signaling pathways mediated by BMP-4-induced events.
Effect of BMP-4 on the protein expression of Osx, N-cadherin, CTGF, and SPARC in SCAPs
Western blotting
SCAPs (1.5 × 106 cells/10-cm dishes or 6-well culture plates) were treated with BMP-4 for 24 h. Western blot was then performed to analyze the expression of various matrix and differentiation markers (Osx, N-cadherin, CTGF, SPARC).41,47 Cell lysates were prepared, and protein concentrations were quantified using Bio-Rad protein assay kits. Equal amounts of protein were subjected to 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein bands were transferred to the polyvinylidene difluoride (PVDF) membrane, blocked for 30 min, and then incubated for 2 h with anti-human GAPDH, Osx, N-cadherin, CTGF, and SPARC antibodies. After washing three times with Tris-buffered saline with 0.1 % Tween-20 (10 mM Tris, pH 7.5; 0.1 % Tween-20, 100 mM NaCl), the membranes were incubated with secondary antibodies and then rinsed. The protein band images were visualized on Fuji X-ray films by Amersham-enhanced chemiluminescence reagents. In some experiments, SCAPs were pretreated with SB431542 or LDN193189 for 1 h before the addition of BMP-4. Cells were then co-incubated for 24 h before protein isolation to verify the mediated signaling pathways for BMP-4-induced events.
Immunofluorescent staining
To visualize the effect of BMP-4 on protein expression in SCAPs, cells (1 × 105 cells) were inoculated into a 24-well plate with coverslips, and incubated in a control solvent or numerous concentrations of BMP-4 for 24 h. Immunofluorescent staining was conducted as described previously,37 by using various antibodies including Osx, N-cadherin, CTGF, and SPARC etc., and isotype control. Cell samples were then subjected to 1-h staining in secondary antibodies conjugated with tetramethylrhodamine (red fluorescence) or fluorescein isothiocyanate (green fluorescence) for 30 min, and counterstained with 1:1000 (v/v) of 4’,6-diamidino-2-phenylindole for nucleus staining. The cellular immunofluorescent staining pictures were photographed with an Olympus IX71 microscope assisted by the DP Controller/Manager software (Olympus Corporation, Tokyo, Japan).
Effect of BMP-4 on PAI-1, uPA, and uPAR mRNA expression in SCAPs
SCAPs (1.5 × 106 cells/10 cm dishes) were inoculated and treated with different concentrations of BMP-4. We isolated the total RNA for reverse transcription and real-time PCR analysis.36,37 The specific primer nucleotide sequences were as follows: uPA (GCCCTCCTCTCCTCCAGAAGAA and GTAGACGATGTAGTCCTCCTTC); uPAR (ATGGATGCTCCTCTGAAGAG and CACAGTCT GGCAGTCATTAG); and PAI-1 (ATGGGATTCAAGATTGATGA and TCAGTATAGTTGAACTTG TT).36,37,48
Effect of BMP-4 on the protein expression and production of uPA, uPAR, and PAI-1 in SCAPs
A total of 1.5 × 106 SCAPs were seeded into 10-cm culture dishes. After cell adhesion for 24 h, cells were treated with various concentrations of BMP-4 (0–200 ng/ml). Western blotting was performed as mentioned above,41,47 but the PVDF membranes were blotted first with anti-human uPA, PAI-1, uPAR, and GAPDH primary antibodies for 2 h. Membranes were thereby hybridized in horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h, and images were developed and photographed by a LAS-4000 Image Reader (Fujifilm, Tokyo, Japan).
For measurement of uPA, suPAR, and PAI-1 production, SCAPs were prepared and treated as described above. In addition, a culture medium was collected to quantify uPA, suPAR, and PAI-1 concentrations using ELISA.
Effect of BMP-4 on various signal transduction pathway molecules in SCAPs
SCAPs (1.5 × 106 cells) were treated with different concentrations of BMP-4 for 24 h, and changes in protein expression of different signal transduction molecules (p-Smad2, p-Smad3, and p-Smad1/5/8) were evaluated using western blotting, as described before.36
Inhibition of signal transduction on BMP-4-induced events in SCAPs
To evaluate whether BMP-4-induced events on SCAPs were mediated by Smad2/3 or Smad1/58 signaling pathways, SCAPs were pretreated with LDN193189 or SB431542 for 1 h before the addition of the solvent (control) or BMP-4 (100 or 200 ng/ml). Culture medium was collected for uPA, suPAR, and PAI-1 analysis. The cell layer was collected to isolate RNA and proteins for real-time PCR or Western blotting analysis of Osx, N-cadherin, CTGF, SPARC or PAI-1, uPAR expression, as described above.
Statistical analysis
More than three independent experiments were executed. Quantified data were examined by paired Student's t-test. A P-value <0.05 was considered a statistically significant difference between the two groups.
Results
Effect of BMP-4 on the viability of SCAPs
BMP-4 had no marked influence on SCAP cell viability at non-confluent conditions as indicated by MTT results (P > 0.05) (Fig. 1A). BMP-4 also exhibited little stimulatory or inhibitory effect on the viability of SCAPs at a confluent state (Fig. 1B). Accordingly BMP-4 showed no marked effect on cell viability of SCAPs with and without SB431542 or LDN193189 (Supplement Fig. 1A and B) and BMP-4 showed little effect on cell viability even in serum-free conditions (Supplement Fig. 1C and D). No obvious differences in cell morphology of SCAPs was noted between control (solvent-treated) and BMP-4 (200 ng/ml)-treated cells (Fig. 1C and D).
Figure 1.
Effect of BMP-4 on the viability of SCAPs. (A) not confluent SCAPs (1 × 104 cells/well) were exposed to BMP-4 for 5 days. (B) Near confluent SCAPs (1 × 105 cells/well) were exposed to BMP-4 for 5 days. Cell viability was estimated by MTT assay. Results were expressed as % of control (as 100 %). ∗denotes statistically significant difference when compared with solvent control group. (C) Morphology of SCAPs in cultured medium for 5 days, (D) Morphology of SCAPs after exposure to BMP-4 (200 ng/ml) for 5 days. One representative picture was shown. Effect of BMP-4 on the mRNA expression of various differentiation markers as analyzed by realtime PCR. (E) Osterix expression, (F) N-cadherin expression, (G) CTGF expression, (H) SPARC expression in SCAPs. Results were expressed as fold of control (as 1). ∗denotes statistically significant difference when compared with control. BMP-4: bone morphogenetic protein-4; SCAP: stem cells from apical papilla; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; CTGF: connective tissue growth factor; SPARC: secreted protein acidic and rich in cysteine.
Effect of BMP-4 on Osx, N-cadherin, SPARC and CTGF mRNA expression of SCAPs
We investigated whether BMP-4 stimulates the osteoblastic and odontoblastic differentiation of SCAPs. Our results showed that BMP-4 at concentrations over 25 ng/ml induced Osx mRNA expression of SCAPs (Fig. 1E). Additionally, BMP-4 at concentrations over 10 ng/ml promoted the mRNA expression of N-cadherin (Fig. 1F), as indicated by the result of real-time PCR. BMP-4 also stimulated CTGF mRNA expression at concentrations greater than 25 ng/ml (Fig. 1G). Furthermore, BMP-4 at concentrations over 10 ng/ml stimulated SPARC mRNA expression of SCAPs (Fig. 1H).
Effect of BMP-4 on protein expression of Osx, N-cadherin, CTGF, and SPARC in SCAPs
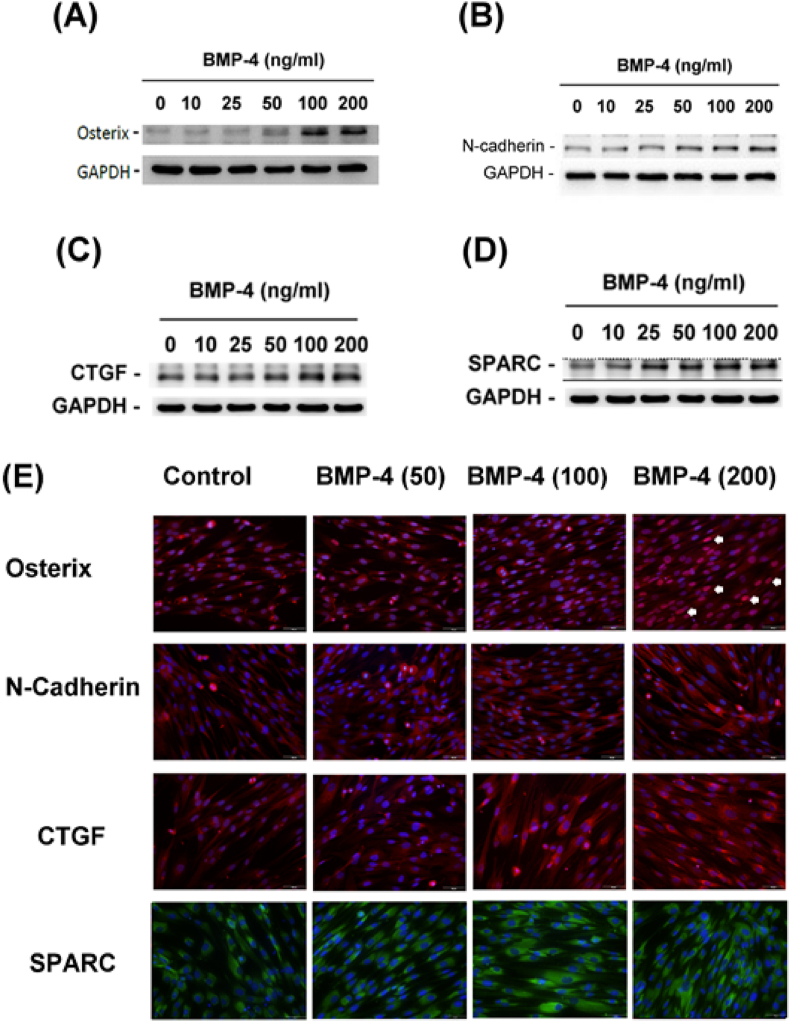
We also found that BMP-4 had a stimulatory effect at concentrations over 50 ng/ml on Osx and N-cadherin protein expression in SCAPs, as revealed by western blotting results (Fig. 2A and B). The results also found the stimulatory effect of BMP-4 on CTGF and SPARC protein expression in SCAPs (Fig. 2C and D). Similarly, immunofluorescent staining results indicated that BMP-4 increased Osx expression (red fluorescence) in the nucleus (arrowheads) (Fig. 2E). The protein expression of N-cadherin, as well as the CTGF (red fluorescence) and SPARC (green fluorescence) protein expression in the cytosol, also increased in SCAPs after exposure to BMP-4 (Fig. 2E).
Figure 2.
Effect of BMP-4 on the protein expression of various differentiation markers as analyzed by western blotting or immunofluorescent staining. (A) Osterix protein expression, (B) N-cadherin protein expression, (C) CTGF protein expression, and (D) SPARC protein expression as analyzed by western blotting. Immunofluoresent staining for analysis of the protein expression of (E) Osterix, N-cadherin, CTGF, and SPARC, respectively, by solvent control, and 50, 100 and 200 ng/ml BMP-4. One representative western blotting and immunofluorescent staining (400x, original magnification) picture was shown. BMP-4: bone morphogenetic protein-4; connective tissue growth factor; SPARC: secreted protein acidic and rich in cysteine.
Effect of BMP-4 on expression and production of PAI-1, uPA, and uPAR in SCAPs
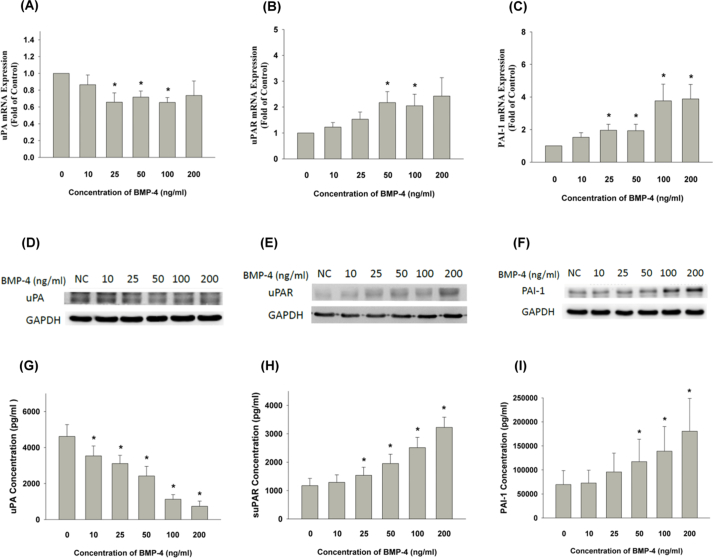
Exposure to BMP-4 decreased cellular uPA mRNA expression of SCAPs (Fig. 3A). However, BMP-4 stimulated uPAR and PAI-1 mRNA expression of SCAPs at concentrations greater than 50 ng/ml and 25 ng/ml, respectively (Fig. 3B and C). Accordingly, BMP-4 also inhibited uPA protein expression (Fig. 3D), but the stimulatory effect of BMP-4 on uPAR and PAI-1 protein expression was noted in SCAPs (Fig. 3E and F). Similarly, BMP-4 at concentrations over 10 ng/ml decreased the uPA production of SCAPs during the five days of exposure (Fig. 3G). BMP-4 also separately stimulated the production of both suPAR and PAI-1 in SCAPs at concentrations above both 25 ng/ml and 50 ng/ml (Fig. 3H and I).
Figure 3.
Effect of BMP-4 on uPA, uPAR, and PAI-1 mRNA expression, protein expression and production in SCAPs. (A) Exposure of SCAPs to BMP-4 decreased cellular uPA mRNA expression, (B) BMP-4 stimulated uPAR mRNA expression of SCAPs, (C) BMP-4 induced PAI-1 mRNA expression, ∗denotes statistically significant difference when compared with control (P < 0.05) as analyzed by real-time PCR. (D) BMP-4 also inhibited uPA protein expression, (E) BMP-4 stimulated uPAR protein expression in SCAPs, (F) BMP-4 stimulated PAI-1 protein expression in SCAPs. One representative Western blot picture was shown. (G) Effect of BMP-4 on uPA production of SCAPs, (H) Effect of BMP-4 on suPAR production of SCAPs, (I) Effect of BMP-4 on PAI-1 production of SCAPs as analyzed by enzyme-linked immunosorbant assay. Results were expressed as Mean ± SE (pg/ml). ∗indicates statistically significant difference when compared with control (P < 0.05). BMP-4: bone morphogenetic protein-4; uPA: urokinase plasminogen activator; uPAR: urokinase plasminogen activator receptor; PAI-1: plasminogen activator inhibitor-1; SCAP: stem cells from apical papilla; suPAR: soluble urokinase plasminogen activator receptor.
Effect of BMP-4 on signaling of Smad2/3, and Smad1/5/8 in SCAPs
BMP-4 stimulated Smad1/5/8 phosphorylation and activation at concentrations higher than 50 ng/ml (Fig. 4A). Similarly, BMP-4 also induced Smad2 and Smad3 phosphorylation of SCAPs (Fig. 4B). Both LDN193189 (1 & 5 μM) and SB431542 effectively suppressed the BMP-4-induced phosphorylation of Smad1/5/8 (Supplement Fig. 2A and B). Furthermore, LDN193189 also attenuated the BMP-4-induced p-Smad2 and p-Smad3 protein expression in SCAPs (Supplement Fig. 2C and D).
Figure 4.
The involvement of Smad2/3 and smad1/5/8 in BMP-4 signaling. (A) Effect of BMP-4 on Smad1/5/8, p-Smad1/5/8 and GAPDH protein expression of SCAPs,. (B) Effect of BMP-4 on Smad2/3, p-Smad2, p-Smad 3 and GAPDH protein expression of SCAPs. One representative Western blot picture were shown. BMP-4: bone morphogenetic protein-4; SCAP: stem cells from apical papilla; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Effect of LDN and SB431542 on BMP-4-induced events in SCAPs
Moreover, LDN193189 (a Smad1/5/8 inhibitor) effectively attenuated the BMP-4-induced Osx mRNA expression of SCAPs (Fig. 5A). SB431542 (a Smad2/3 inhibitor) also prevented the Osx mRNA expression of SCAPs (Fig. 5B). The BMP-4-induced Osx protein expression was also decreased by co-treatment with LDN193189 and SB431542 (Fig. 5C and D).
Figure 5.
Effect of LDN193189 and SB431542 on BMP-4-induced Osx, N-cadherin, CTGF and SPARC expression in SCAPs. (A) Effect of LDN193189 on BMP-4-induced Osx mRNA expression, (B) Effect of SB431542 on BMP-4-induced Osx mRNA expression, (C) Effect of LDN193189 on BMP-4-induced Osx protein expression, (D) Effect of SB431542 on BMP-4-induced Osx protein expression. (E) Effect of LDN193189 on BMP-4-induced N-cadherin mRNA expression, (F) Effect of SB431542 on BMP-4-induced N-cadherin mRNA expression, (G) Effect of LDN193189 on BMP-4-induced CTGF mRNA expression, (H) Effect of SB431542 on BMP-4-induced CTGF mRNA expression, (I) Effect of LDN193189 on BMP-4-induced CTGF protein expression, (J) Effect of SB431542 on BMP-4-induced CTGF protein expression, (K) Effect of LDN193189 on BMP-4-induced SPARC mRNA expression, (L) Effect of SB431542 on BMP-4-induced SPARC mRNA expression. For western blotting, one representative result was shown. For real-time PCR, mRNA expression results were expressed as fold of control. ∗denotes statistically significant difference when compared with control. #denotes statistically significant difference when compared with BMP-4-treated group. BMP-4: bone morphogenetic protein-4; osx: osterix; connective tissue growth factor; SPARC: secreted protein acidic and rich in cysteine; SCAP: stem cells from apical papilla.
LDN and SB431542 also suppressed the BMP-4-induced N-cadherin mRNA expression of SCAPs (Fig. 5E and F). LDN193189 & SB431542 further prevented the BMP-4-induced CTGF mRNA expression of SCAPs (Fig. 5G and H). LDN193189 and SB431542 also attenuated the BMP-4-induced CTGF protein expression of SCAPs (Fig. 5I and J). Accordingly, LDN193189 and SB431542 also prevented the BMP-4-induced SPARC mRNA expression of SCAPs (Fig. 5K and L).
Effect of LDN193189 and SB431542 on BMP-4-induced mRNA, protein expression, and production of plasminogen activation-associated molecules
LDN193189 partly reversed the BMP-4-induced decrease of uPA production in SCAPs (Fig. 6A). In contrast, LDN193189 prevented the BMP-4-induced production of suPAR and PAI-1 in SCAPs (Fig. 6B and C). SB431542 by itself stimulated the uPA production of SCAPs. It also attenuated the BMP-4-induced decline of uPA production (Fig. 6D). Similar to LDN193189, SB431542 also suppressed the BMP-4-induced suPAR and PAI-1 production in SCAPs (Fig. 6E and F). Western blotting results also showed that LDN193189 inhibited the BMP-4-induced uPAR and PAI-1 protein expression (Fig. 6G). Accordingly, SB431542 further attenuated the BMP-4-induced uPAR and PAI-1 protein expression in SCAPs (Fig. 6H).
Figure 6.
Effect of LDN193189 and SB431542 on BMP-4-induced uPA, PAI-1, and uPAR expression and production in SCAPs. (A) Effect of LDN193189 on BMP-4-induced decline of uPA production, (B) Effect of LDN193189 on BMP-4-induced suPAR production, (C) Effect of LDN193189 on BMP-4-induced PAI-1 production, (D) Effect of SB431542 on BMP-4-induced decline of uPA production, (E) Effect of SB431542 on BMP-4-induced suPAR production, (F) Effect of SB431542 on BMP-4-induced PAI-1 production. Results were expressed as pg/ml (Mean ± SE). ∗denotes statistically significant difference when compared with control. #denotes statistically significant difference when compared with BMP-4-treated group. (G) Effect of LDN on BMP-4-induced uPAR and PAI-1 protein expression of SCAPs as analyzed by western blotting. (H) Effect of SB431542 on BMP-4-induced uPAR and PAI-1 protein expression of SCAPs. One representative western blotting result was shown. BMP-4: bone morphogenetic protein-4; uPA: urokinase plasminogen activator; uPAR: urokinase plasminogen activator receptor; PAI-1: plasminogen activator inhibitor-1; SCAP: stem cells from apical papilla; suPAR: soluble urokinase plasminogen activator receptor.
Discussion
Clinical observations and animal studies strongly suggest that stem cells residing in the apical papilla play a strong role in root formation. Inducing the proliferation and ingrowth of SCAPs into the root canal and pulp chamber through the apical foramen, with subsequent extracellular matrix protein deposition and differentiation of SCAPs, is considered to be a key factor for pulp regeneration, apexogenesis, and clinical success.4,49 Growth factors like PDGF, bFGF, TGF-β, and BMPs in the blood clot, whether released from dentin (by acidogenic bacteria, acid etching or Ca(OH)2 treatment, and others) or added exogenously, may potentially influence the biological activities of SCAPs, promote the differentiation of underlying stem cells, increase dentinogenesis, osteogenesis and cementogenesis, and improve the clinical success of apexogenesis.
While BMP-4 might induce cellular differentiation, it showed little influence on the growth of SCAPs in the present study. Accordingly, transfection of vascular endothelial growth factor (VEGF) increased cell proliferation, whereas transfection of both VEGF and BMP-2 decreased the cell proliferation of SCAPs.50 Transfection of the BMP-2 expression vector into SCAPs showed no marked effect on cell proliferation relative to SCAPs transfected with the control vector.51 However, BMP4 even promotes the self-renewal of some embryonic and somatic stem cells, as demonstrated by Cheng et al. (2022).52 BMP-4 also stimulated the growth (viability) of HDPCs (near confluent, 10 % FBS) at 3 and 5 days of exposure, but not at 1, 2, and 7 days. BMP-4 also induced the differentiation of HDPCs.53 The effect of BMP-4 on proliferation and differentiation may be affected by cell density, confluent status, cell type (SCAPs, muscle stem cells, dental pulp cells or tumor cells), BMP-4 and serum concentration, exposure time, and more. This point can be addressed in future studies.
In this study, we discovered that BMP-4 may induce the expression of CTGF and variable osteogenic and odontogenic differentiation markers, such as N-cadherin, Osx, and SPARC in SCAPs. CTGF is a cysteine-rich extracellular matrix protein involved in the control of various cellular functions and biological processes, such as chondrogenesis, osteogenesis, and angiogenesis, which are crucial for skeletal repair and regeneration.54 CTGF expression is higher in odontoblast-like cells near dental caries and is involved in the reparative dentinogenesis of dental pulp via stimulation of mineralization.55 Exogenous BMP-1 was found to be internalized in dental pulp cells to stimulate CTGF expression.56 We noticed increased CTGF expression in SCAPs by BMP-4, suggesting that BMP-4 and CTGF contributed to revascularization, repair, and reparative dentinogenesis.
However, cadherins are known as important cell–cell adhesion molecules for stem cell differentiation. Moreover, cadherins may function as both ligands and receptors. Cadherin-mediated signaling plays important roles during cellular proliferation, development, differentiation, apoptosis and pathogenesis.57 There are a wide variety of cadherins. Among these, N-cadherin plays a role during tooth development in humans. It is essential for odontoblast differentiation and function, both developmentally and pathologically.58 Re-expression of N-cadherin has been shown to occur in cultured primary pulp cells, which differentiate into odontoblast-like cells. In this study, the enhancement of N-cadherin expression may suggest that BMP-4 assists odontoblastic and osteogenic differentiation and benefits the dental repair and regeneration activities of SCAPs.
Osx is an essential transcription factor for osteoblast and odontoblast differentiation.59 In Runt-related transcription factor 2 (Runx2)-presented mesenchymal cells, Osx expression stimulates cellular differentiation into osteoblasts and subsequently induces bone formation.60,61 Osx generally operates downstream of Runx2, which is also vital for osteogenesis and odontogenesis. Osx overexpression in bone marrow-derived stem cells accelerates osseointegration after implantation,62 SPARC, as a non-collagenous protein rich in mineralized tissues, may regulate extracellular matrix assembly and cross-linking. It is involved in osteoblast and odontoblast differentiation of mineralized tissues, such as periodontal ligament, dental pulp and bone.63,64 Cannabidiol stimulates the osteogenic differentiation of SCAPs with the induction of SPARC.65 The induction of Osx, CTGF, N-cadherin, and SPARC expression in SCAPs suggests a stimulatory effect of BMP-4 on osteogenesis and odontogenesis. The results indicate that BMP-4 may enhance the osteogenic and odontoblastic differentiation of SCAPs.
Collagen is the most profound extracellular protein and an essential component of the dentinal matrix. Plasmin, a protease activated from plasminogen, is involved in collagen remodeling. In addition, PAI-1 was found to accelerate odontoblastic differentiation of SCAPs,66 and provoke cementoblast differentiation of human periodontal ligament stem cells.67 PAI-1 has also been observed to increase the expression of Runx2, Osx, and Smad4 during odontogenesis, with functions essential for extracellular matrix turnover and bone remodeling.66 In the present research, BMP-4 decreased uPA expression and production in SCAPs. In contrast, BMP-4 increased uPAR and PAI-1 expression and production in SCAPs. Moreover, recombinant PAI-1 was recently found to accelerate the odontoblast differentiation of SCAPs,66 and uPAR was found to induce the migration and differentiation of mesenchymal cells.68 These results indicate the possible influence of BMP-4 on migration and matrix accumulation, possibly contributing to osteogenic and odontogenic differentiation of SCAPs.
Recently, we discovered that SCAPs express ALK1, ALK3, ALK5, betaglycan, TGF-β-RII, and endoglin mRNA.35 It is well known that the TGF-β superfamily of intracellular signaling advances through non-canonical Smad-independent and canonical Smad-dependent pathways. The Smad1, Smad5 and Smad8 (also known as Smad9) are the main signaling molecules for the Smad-dependent pathway of BMPs. Intriguingly we found BMP-4 induced canonical Smad1/5/8 and also Smad2/3 signaling activation. Moreover, LDN193189 inhibited the BMP-4-induced p-Smad2/3 expression, and SB431542 attenuated the BMP-4-induced p-Smad1/5/8 expression, implicating the obvious cross-talk between both signaling mechanisms. Similarly, BMP-4 is also shown to provoke the activation and phosphorylation of both Smad2/3 and Smad1/5/8 in human granulosa cells.69 These results indicate that both Smad2/3 and Smad1/5/8 pathways are important for BMP-4-induced events.
We consistently found that SB431542 (the ALK5/Smad2/3 inhibitor) pre-treatment and co-incubation prevented the BMP-4-induced Osx, N-cadherin, CTGF, SPARC, PAI-1 and uPAR expression or production. LDN193189, a Smad1/5/8 inhibitor, also attenuated the BMP-4-induced Osx, N-cadherin, CTGF, SPARC, uPAR and PAI-1 expression and secretion in SCAPs. These results suggest that both signaling pathways are crucial for BMP-4-induced activities in SCAPs. SB431542 can inhibit TGF-β signaling via ALK4, ALK5, ALK7, which contain similar kinase domains. However, it showed no marked effects on BMP signaling via other BMP-binding ALKs, such as ALK2, 3, 6.70 CTGF is shown to antagonize BMP-4 and enhance TGF-β signaling.71 BMP-4 and TGF-β are also shown to exert antagonistic effects in pulmonary artery smooth muscle cells in Smad-dependent or independent manners.72 Accordingly, BMP-4 increased both Smad1/5/8 and Smad2/3 signaling in granulosa cells.69 More studies are necessary to further delineate the cross-talk of Smad1/5/8 and Smad2/3 in BMP-4 signaling.
In conclusion, these results indicate that BMP-4 might enhance the osteogenic and odontogenic differentiation of SCAPs, and contribute to revascularization, repair, and reparative dentinogenesis. For clinical pulpal regeneration and apexogenesis, following the control of pulpal and root canal infections, inducing blood clot formation within the root canal can provide a scaffold for the migration and proliferation of SCAPs into the root canal space. BMP-4 present in the blood clot, released from dentin or added exogenously during the revascularization procedures in combination with other scaffolds such as collagen sponge and others, may stimulate both ALK3/6-Smad1/5/8 and ALK5-Smad2/3 signaling. This activation influences matrix accumulation and migration, and the differentiation of odontoblast and osteoblast by inducing the expression and production of Osx, N-cadherin, CTGF, SPARC, PAI-1 and uPAR (Fig. 7). These processes are important for the success of clinical revascularization procedures, contributing to the calcification of root canal walls and new root formation (apexogenesis).
Figure 7.
Proposed mechanism of BMP-4-induced changes of SCAP and their regulation by Smad signaling. Apical papilla is present in the apical region of necrotic dental pulp with infected root canals. After control of pulpal/root canal infection, induction of blood clot formation into the root canal may serve as a scaffold for migration/proliferation of SCAP into the root canal space. BMP-4 in the blood clot, released from dentin or added exogenously, may stimulate both Smad1/5/8 and Smad2/3 signaling, thereby affect the matrix accumulation, migration or odontoblast/osteoblast differentiation via induction of Osterix, N-cadherin, CTGF, SPARC, PAI-1 and uPAR expression/production in SCAP. BMP-4: bone morphogenetic protein-4; SCAP: stem cells from apical papilla; connective tissue growth factor; SPARC: secreted protein acidic and rich in cysteine; uPAR: urokinase plasminogen activator receptor; PAI-1: plasminogen activator inhibitor-1.
Declaration of competing interest
The authors declare no conflict of interest for this submission.
Acknowledgments
The authors would like to thank Ms Ying-Yin Chen, Mr Shuan-Yu Du, Ms Yu-Ting Chen and Yu-Ting Hsiao for their technical assistance and doing some of the experiments. This study was supported by Chang Gung Memorial Hospital (CMRPF1G0101, CMRPF1G0102, CMRPF1F0071, CMRPF1H0061, CMRPF1H0062, CMRPF1H0063, CMRPF3E0022, CMRPF3E0023, NMRPF3E0041, NMRPF3E0042, NMRPF3E0043, NMRPF3H0061, NMRPF3H0062, NMRPF3H0071, NMRPF3H0072, NMRPF3H0073, CMRPF1K0071, CMRPF1K0072, NMRPF3L0031, NMRPF3L0032, NMRPF3L0041, NMRPF3L0042, ZMRPF3L0111, ZMRPF3L0121, ZMRPF3M0051, ZMRPF3M0061, CMRPF1M0081), the National Sciences and Technology Council, Taiwan, ROC (MOST104-2314- B-255-010-MY3, MOST106-2314-B-002-033-MY2, MOST106-2314- B-002-034-MY2, MOST107-2314-B-255-009-MY3, MOST107-2314-B-255-008-MY2, MOST108-2314-B-002-043-MY3, MOST110-2314-B255-002-MY3. MOST110-2314-B-255-003-MY3; MOST111-2314-B002-109-MY3, MOST111-2314-B002-107-MY3), National Taiwan University Hospital (NTUH101-S1862, NTUH102-S2180, NTUH103- S2368, NTUH104-S2658, NTUH106-S3467, NTUH106-UN-001, NTUH107-003875, NTUH108-004156, NTUH110-S4815) & Kaohsiung Medical University (KMU-Q111004, KMU-110KK040, KMU-DK(A)-110002, KMUH111-1T09).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2024.11.002.
Contributor Information
Jiiang-Huei Jeng, Email: jhjeng@kmu.edu.tw, jhjeng@ntu.edu.tw.
Hsiao-Hua Chang, Email: hhchangpedo@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Flanagan T.A. What can cause the pulps of immature, permanent teeth with open apices to become necrotic and what treatment options are available for these teeth. Aust Endod J. 2014;40:95–100. doi: 10.1111/aej.12087. [DOI] [PubMed] [Google Scholar]
- 2.Iwaya S.I., Ikawa M., Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 3.Chueh L.H., Huang G.T. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205–1213. doi: 10.1016/j.joen.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Li L., Pan Y., Mei L., Li J. Clinical and radiographic outcomes in immature permanent necrotic evaginated teeth treated with regenerative endodontic procedures. J Endod. 2017;43:246–251. doi: 10.1016/j.joen.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Pecci-Lloret M.P., Nandin-Muttoni G., Pecci-Lloret M.R., Guerrero-Girones J., Rodriguez-Lozano F.J. Scaffolds for pulp revitalization: a systematic review of randomized clinical trials. Ann Anat. 2022;243 doi: 10.1016/j.aanat.2022.151936. [DOI] [PubMed] [Google Scholar]
- 6.Friedlander L.T., Cullinan M.P., Love R.M. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–962. doi: 10.1111/j.1365-2591.2009.01622.x. [DOI] [PubMed] [Google Scholar]
- 7.Leite M.L., Soares D.G., Anovazzi G., et al. Bioactivity effects of extracellular matrix proteins on apical papilla cells. J Appl Oral Sci. 2021;29 doi: 10.1590/1678-7757-2021-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q., Gao Y., He J. Stem cells from the apical papilla (SCAPs): past, present, prospects, and challenges. Biomedicines. 2023;11:2047. doi: 10.3390/biomedicines11072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paduano F., Marrelli M., White L.J., Shakesheff K.M., Tatullo M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanov A.A., Kuznetsova A.V., Popova O.P., Danilova T.I., Latyshev A.V., Yanushevich O.O. Influence of extracellular matrix components on the differentiation of periodontal ligament stem cells in collagen I hydrogel. Cells. 2023;12:2335. doi: 10.3390/cells12192335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonoyama W., Liu Y., Fang D., et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1 doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang G.T., Sonoyama W., Liu Y., Liu H., Wang S., Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonoyama W., Liu Y., Yamaza T., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J.W., Yuan G.H., Chen Z. Pulp regeneration: current approaches and future challenges. Front Physiol. 2016;7:58. doi: 10.3389/fphys.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima M., Reddi A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 16.Sieber C., Kopf J., Hiepen C., Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Graf D., Malik Z., Hayano S., Mishina Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016;27:129–139. doi: 10.1016/j.cytogfr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashiro T., Tummers M., Thesleff I. Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res. 2003;82:172–176. doi: 10.1177/154405910308200305. [DOI] [PubMed] [Google Scholar]
- 20.Hosoya A., Kim J.Y., Cho S.W., Jung H.S. BMP4 signaling regulates formation of Hertwig's epithelial root sheath during tooth root development. Cell Tissue Res. 2008;333:503–509. doi: 10.1007/s00441-008-0655-z. [DOI] [PubMed] [Google Scholar]
- 21.Seki D., Takeshita N., Oyanagi T., et al. Differentiation of odontoblast-like cells from mouse induced pluripotent stem cells by Pax9 and Bmp4 transfection. Stem Cells Transl Med. 2015;4:993–997. doi: 10.5966/sctm.2014-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z., Wang J., Dong R., et al. Depletion of MEIS2 inhibits osteogenic differentiation potential of human dental stem cells. Int J Clin Exp Med. 2015;8:7220–7230. [PMC free article] [PubMed] [Google Scholar]
- 23.Qu B., Liu O., Fang X., et al. Distal-less homeobox 2 promotes the osteogenic differentiation potential of stem cells from apical papilla. Cell Tissue Res. 2014;357:133–143. doi: 10.1007/s00441-014-1833-9. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M.S., Akhtar N., Jamil H.M., Banik R.S., Asaduzzaman S.M. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3 doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M., Goldman G., MacDougall M., Chen S. BMP signaling pathway in dentin development and diseases. Cells. 2022;11:2216. doi: 10.3390/cells11142216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel J., Meuller T.D. Specification of BMP signaling. Cells. 2019;8:1579. doi: 10.3390/cells8121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X., Chang H.M., Yi Y., Leung P.C.R., Sun Y. Bone morphogenetic protein 2 upregulates SERPINE2 expression through noncanonical SMAD2/3 and p38 MAPK signaling pathways in human granulosa-lutein cells. Faseb J. 2021;35 doi: 10.1096/fj.202100670RR. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H.J., Klausen C., Li Y., Zhu H., Wang Y.L., Leung P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling. Cell Death Dis. 2018;9:174. doi: 10.1038/s41419-017-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G.T., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakopoulou A., About I. Stem cells of dental origin: current research trends and key milestones towards clinical application. Stem Cell Int. 2016;2016 doi: 10.1155/2016/4209891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim B.C., Jun S.M., Kim S.Y., et al. Engineering three dimensional micro nerve tissue using postnatal stem cells from human dental apical papilla. Biotechnol Bioeng. 2017;114:903–914. doi: 10.1002/bit.26205. [DOI] [PubMed] [Google Scholar]
- 32.Park C.S., Hong O.K., Kim M.K., et al. Serum bone morphogenetic protein-4 contributes to discriminating coronary artery disease severity. Medicine. 2015;94 doi: 10.1097/MD.0000000000001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Um I.W., Ku J.K., Lee B.K., Yun P.Y., Lee J.K., Nam J.H. Postulated release profile of recombinant human bone morphogenetic protein-2 (rhBMP-2) from demineralized dentin matrix. J Korean Assoc Oral Maxillofac Surg. 2019;45:123–128. doi: 10.5125/jkaoms.2019.45.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grawish M.E., Grawish L.M., Grawish H.M., et al. Demineralized dentin matrix for dental and alveolar bone tissues regeneration: an innovative scope review. Tissue Eng Regen Med. 2022;19:687–701. doi: 10.1007/s13770-022-00438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang H.H., Chang M.C., Wu I.H., et al. Role of ALK5/Smad2/3 and MEK1/ERK signaling in transforming growth factor beta 1-modulated growth, collagen turnover, and differentiation of stem cells from apical papilla of human tooth. J Endod. 2015;41:1272–1280. doi: 10.1016/j.joen.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.C., Chang H.H., Hsieh W.C., et al. Effects of transforming growth factor-β1 on plasminogen activation in stem cells from the apical papilla: role of activating receptor-like kinase 5/Smad 2 and mitogen-activated protein kinase kinase (MEK)/extracelllular signal-regulated kinase (ERK) signalling. Int Endod J. 2020;53:647–659. doi: 10.1111/iej.13266. [DOI] [PubMed] [Google Scholar]
- 37.Chang M.C., Chen N.Y., Chen J.H., et al. bFGF stimulated plasminogen activation factors, but inhibited alkaline phosphatase and SPARC in stem cells from apical papilla: involvement of MEK/ERK, TAK1 and p38 signaling. J Adv Res. 2022;40:95–107. doi: 10.1016/j.jare.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeng J.H., Hsieh C.C., Lan W.H., et al. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol Toxicol. 1998;14:383–389. doi: 10.1023/a:1007591426267. [DOI] [PubMed] [Google Scholar]
- 39.Chang M.C., Chen J.H., Lee H.N., et al. Inducing cathepsin L expression/production, lysosomal activation, and autophagy of human dental pulp cells by dentin bonding agents, camphorquinone and BisGMA and the related mechanisms. Biomater Adv. 2023;145 doi: 10.1016/j.bioadv.2022.213253. [DOI] [PubMed] [Google Scholar]
- 40.Chang M.C., Chen L.I., Chan C.P., et al. The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials. 2010;31:8164–8171. doi: 10.1016/j.biomaterials.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 41.Chang M.C., Wang T.M., Chien H.H., et al. Effect of butyrate, a bacterial by-product, on the viability and ICAM-1 expression/production of human vascular endothelial cells: role in infectious pulpal/periapical diseases. Int Endod J. 2022;55:38–53. doi: 10.1111/iej.13614. [DOI] [PubMed] [Google Scholar]
- 42.Chang M.C., Zhong B.H., Lee H.N., et al. Melatonin exerts anti-fibrinolytic effects by regulating IL-1β-induced changes in uPA, uPAR, and PAI-1 expression/production in human dental pulp cells. J Food Drug Anal. 2022;30 doi: 10.38212/2224-6614.3415. article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morsczeck C. Gene expression of runx2, Osterix, c-fos, DLX-3, DLX5, and MSX-2 in dental follicle cells during osteogenic differentiation in vitro. Calcif Tissue Int. 2006;78:98–102. doi: 10.1007/s00223-005-0146-0. [DOI] [PubMed] [Google Scholar]
- 44.Hotz B., Arndt M., Dullat S., Bhargava S., Buhr H.J., Hotz H.G. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 45.Kantarci A., Black S.A., Murawel P., et al. Epithelial and connective tissue cell CTGF/CCN2 expression in gingival fibrosis. J Pathol. 2006;210:59–66. doi: 10.1002/path.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X., Liu F.C., Wang Y.Y., Gao J. Secreted protein acidic and rich in cysteine promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells and acquisition of cancer stem cell phenytypes. J Gastroenterol Hepatol. 2019;34:1860–1868. doi: 10.1111/jgh.14692. [DOI] [PubMed] [Google Scholar]
- 47.Chang M.C., Wu H.L., Lee J.J., et al. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. [DOI] [PubMed] [Google Scholar]
- 48.De Petro G., Tavian D., Copeta A., Portolani N., Giulini S.M., Barlati S. Expression of urokinase-type plasminogen activator (u-PA), u-PA receptor, and tissue-type PA messenger RNAs in human hepatocellular carcinoma. Cancer Res. 1998;58:2234–2239. [PubMed] [Google Scholar]
- 49.He L., Zhong J., Gong Q., et al. Regenerative endodontics by cell homing. Dent Clin. 2017;61:143–159. doi: 10.1016/j.cden.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W., Zhang X., Ling J., Wei X., Jian Y. Osteo-/odontogenic differentiation of BMP-2 and VEGF gene co-transfected human stem cells from apical papilla. Mol Med Rep. 2016;13:3747–3754. doi: 10.3892/mmr.2016.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W., Zhang X., Ling J., et al. Proliferation and odontogenic differentiation of BMP-2 gene transfected stem cells from human tooth apical papilla: an in vitro study. Int J Mol Med. 2014;34:1004–1012. doi: 10.3892/ijmm.2014.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng H., Gao X., Huard M., et al. Bone morphogenetic protein 4 rescues the bone regenerative potential of old muscle-derived stem cells via regulation of cell cycle inhibitors. Stem Cell Res Ther. 2022;13:385. doi: 10.1186/s13287-022-03047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun N., Jiang T., Wu C., Sun H., Zhou Q., Lu L. Expression and influence of BMP-4 in human dental pulp cells cultured in vitro. Exp Ther Med. 2018;16:5112–5116. doi: 10.3892/etm.2018.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnott J.A., Lambi A.G., Mundy C., et al. The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr. 2011;21:43–69. doi: 10.1615/critreveukargeneexpr.v21.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muromcahi K., Kamio N., Matsumoto T., Matsushima K. Role of CTGF/CCN2 in reparative dentinogenesis in human dental pulp. J Oral Sci. 2012;54:47–54. doi: 10.2334/josnusd.54.47. [DOI] [PubMed] [Google Scholar]
- 56.Muromachi K., Kamio N., Matsuki-Fukushima M., et al. CCN2/CTGF expression via cellular uptake of BMP-1 is associated with reparative dentinogenesis. Oral Dis. 2015;21:778–784. doi: 10.1111/odi.12347. [DOI] [PubMed] [Google Scholar]
- 57.Yulis M., Kusters D.H.M., Nusrat A. Cadherins: cellular adhesive molecules serving as signalling mediators. J Physiol. 2018;596:3883–3898. doi: 10.1113/JP275328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heymann R., About I., Lendahl U., Franquin J.C., Obrink B., Mitsiadis T.A. E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am J Pathol. 2002;160:2123–2133. doi: 10.1016/S0002-9440(10)61161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S., Gluhak-Heinrich J., Wang Y.H., et al. Runx2, osx, and dspp in tooth development. J Dent Res. 2009;88:904–909. doi: 10.1177/0022034509342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu H., Doll B., McNelis T., Hollinger J.O. Osteoblast differentiation in vitro and in vivo promoted by osterix. J Biomed Mater Res A. 2007;83:770–778. doi: 10.1002/jbm.a.31356. [DOI] [PubMed] [Google Scholar]
- 61.Nakashima K., Zhou X., Kunkel G., et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 62.Xu B., Zhang J., Brewer E., et al. Osterix enhances BMSC-associated osseointegration of implants. J Dent Res. 2009;88:1003–1007. doi: 10.1177/0022034509346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosset E.M., Bradshaw A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016;52–54:78–87. doi: 10.1016/j.matbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trombetta J.M., Bradshaw A.D. SPARC/osteonectin functions to maintain homeostasis of the collagenous extracellular matrix in the periodontal ligament. J Histochem Cytochem. 2010;58:871–879. doi: 10.1369/jhc.2010.956144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrescu N.B., Jurj A., Soritau O., et al. Cannabidiol and vitamin D3 impact on osteogenic differentiation of human dental mesenchymal stem cells. Medicina. 2020;56:607. doi: 10.3390/medicina56110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin B., Choung P.H. Recombinant human plasminogen activator inhibitor-1 accelerates odontoblastic differentiation of human stem cells from apical papilla. Tissue Eng Part A. 2016;22:721–732. doi: 10.1089/ten.tea.2015.0273. [DOI] [PubMed] [Google Scholar]
- 67.Jin H., Choung H.W., Lim K.T., et al. Recombinant human plasminogen activator inhibitor-1 promotes cementogenic differentiation of human periodontal ligament stem cells. Tissue Eng Part A. 2015;21:2817–2828. doi: 10.1089/ten.tea.2014.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallabhaneni K.C., Tkachuk S., Kiyan Y., et al. Urokinase receptor mediates mobilization, migration and differentiation of mesenchymal stem cells. Cardiovasc Res. 2011;90:113–121. doi: 10.1093/cvr/cvq362. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H., Tian S., Klausen C., Zhu H., Liu R., Leung P.C.K. Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol Cell Endocrinol. 2016;428:17–27. doi: 10.1016/j.mce.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Inman G.J., Nicolas F.J., Callahan J.F., et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 71.Abreu J.G., Ketpura N.I., Reversade B., De Robertis E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Upton P.D., Davies R.J., Tajsic T., Morrell N.W. Transforming growth factor-1 represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol. 2013;49:1135–1145. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.