Abstract
Bovine viral diarrhea virus (BVDV) persistently infected (PI) calves represent significant sources of infection to susceptible cattle. The objectives of this study were to determine if PI calves transmitted infection to vaccinated and unvaccinated calves, to determine if BVDV vaccine strains could be differentiated from the PI field strains by subtyping molecular techniques, and if there were different rates of recovery from peripheral blood leukocytes (PBL) versus serums for acutely infected calves. Calves PI with BVDV1b were placed in pens with nonvaccinated and vaccinated calves for 35 d. Peripheral blood leukocytes, serums, and nasal swabs were collected for viral isolation and serology. In addition, transmission of Bovine herpes virus 1 (BHV-1), Parainfluenza-3 virus (PI-3V), and Bovine respiratory syncytial virus (BRSV) was monitored during the 35 d observation period.
Bovine viral diarrhea virus subtype 1b was transmitted to both vaccinated and nonvaccinated calves, including BVDV1b seronegative and seropositive calves, after exposure to PI calves. There was evidence of transmission by viral isolation from PBL, nasal swabs, or both, and seroconversions to BVDV1b. For the unvaccinated calves, 83.2% seroconverted to BVDV1b. The high level of transmission by PI calves is illustrated by seroconversion rates of nonvaccinated calves in individual pens: 70% to 100% seroconversion to the BVDV1b. Bovine viral diarrhea virus was isolated from 45 out of 202 calves in this study. These included BVDV1b in ranch and order buyer (OB) calves, plus BVDV strains identified as vaccinal strains that were in modified live virus (MLV) vaccines given to half the OB calves 3 d prior to the study. The BVDV1b isolates in exposed calves were detected between collection days 7 and 21 after exposure to PI calves. Bovine viral diarrhea virus was recovered more frequently from PBL than serum in acutely infected calves. Bovine viral diarrhea virus was also isolated from the lungs of 2 of 7 calves that were dying with pulmonary lesions. Two of the calves dying with pneumonic lesions in the study had been BVDV1b viremic prior to death. Bovine viral diarrhea virus 1b was isolated from both calves that received the killed or MLV vaccines. There were cytopathic (CP) strains isolated from MLV vaccinated calves during the same time frame as the BVDV1b isolations. These viruses were typed by polymerase chain reaction (PCR) and genetic sequencing, and most CP were confirmed as vaccinal origin. A BVDV2 NCP strain was found in only 1 OB calf, on multiple collections, and the calf seroconverted to BVDV2. This virus was not identical to the BVDV2 CP 296 vaccine strain. The use of subtyping is required to differentiate vaccinal strains from the field strains. This study detected 2 different vaccine strains, the BVDV1b in PI calves and infected contact calves, and a heterologous BVDV2 subtype brought in as an acutely infected calf. The MLV vaccination, with BVDV1a and BVDV2 components, administered 3 d prior to exposure to PI calves did not protect 100% against BVDV1b viremias or nasal shedding. There were other agents associated with the bovine respiratory disease signs and lesions in this study including Mannheimia haemolytica, Mycoplasma spp., PI-3V, BRSV, and BHV-1.
Résumé
Les veaux infectés de façon persistante (PI) par le virus de la diarrhée virale bovine (BVDV) représentent une source d’infection significative pour les bovins susceptibles. Les objectifs de cette étude étaient de déterminer si les veaux PI pouvaient transmettre l’infection à des veaux vaccinés ou non, de déterminer si les souches vaccinales de BVDV pouvaient être différencier des isolats cliniques de veaux PI par des méthodes de typage moléculaire, et s’il y avaient des taux de recouvrement de virus différents à partir des leucocytes du sang périphérique (PBL) versus le sérum de veaux infectés de manière aiguë. Des veaux PI avec le BVDV de type 1b ont été placés dans des enclos avec des veaux vaccinés et non-vaccinés pendant 35 jours. Des PBL, du sérum et des écouvillons nasaux ont été prélevés pour isolement viral et sérologie. De plus, la transmission du virus herpès bovin de type 1 (BHV-1), du virus parainfluenza-3 (PI-3V) et du virus respiratoire syncytial bovin (BRSV) a été surveillée durant les 35 j de la période d’observation.
Suite à l’exposition aux veaux PI, le BVDV de type 1b été transmis autant aux veaux vaccinés que non-vaccinés, incluant des veaux séropositifs et séronégatifs pour BVDV1b. La transmission a été mise en évidence par isolement viral à partir des PBL, des écouvillons nasaux, ou des deux, et par séroconversion au BVDV1b. Une séroconversion envers BVDV1b a été notée chez 83,2 % des veaux non-vaccinés. Le degré élevé de transmissibilité de BVDV1b à partir de veaux PI est démontré par les taux de séroconversion des veaux non-vaccinés gardés dans des enclos individuels (allant de 70 à 100 %). Des isolats de BVDV ont été obtenus de 45 des 202 veaux dans cette étude. Ce nombre incluait des isolats provenant de veaux de ranchs et d’acheteurs (OB), ainsi que des isolats identifiés comme souches vaccinales que l’on trouve dans les vaccins vivants modifiés (MLV) administrés à la moitié des veaux OB 3 jours avant l’étude. Les isolats de BVDV1b provenant des veaux exposés ont été détectés entre les jours de collecte 7 à 21 suite à l’exposition aux veaux PI. Le BVDV a été isolé plus fréquemment à partir des PBL que du sérum chez les veaux infectés de manière aiguë. Le BVDV a également été isolé des poumons de 2 des 7 veaux qui sont morts avec des lésions pulmonaires. Deux des veaux qui sont morts avec des lésions de pneumonie dans cette étude étaient virémiques pour BVDV1b avant leur mort. Le BVDV1b a été isolé des deux veaux qui avaient été vaccinés avec un vaccin tué ou MLV. Des isolats cytopathogènes (CP) ont été obtenus de veaux vaccinés avec le vaccin MLV durant la même période que l’isolement de BVDV1b. Ces virus ont été typés par réaction d’amplification en chaîne par la polymérase (PCR) et séquençage génique, et la plupart des virus CP ont été confirmés comme d’origine vaccinale. Une souche BVDV2 NCP a été isolée à partir d’un seul veau OB lors de plusieurs prélèvements d’échantillons, et une séroconversion a été notée chez cet animal. Ce virus n’était pas identique à la souche vaccinale BVDV2 CP 296. L’utilisation du typage génique est requise afin de différencier les souches vaccinales des souches de terrain. Dans cette étude deux souches vaccinales différentes ont été trouvées, la souche BVDV1b chez des veaux PI et des veaux infectés suite à un contact, et un sous-type de BVDV2 trouvé chez un veau infecté. La vaccination à l’aide de MLV, avec BVDV1a et BVDV2, 3 jours avant l’exposition à des veaux PI n’a pas conféré une protection de 100 % contre une virémie ou l’excrétion nasale de BVDV1b. D’autres agents associés à des problèmes et lésions respiratoires ont été trouvés dans cette étude : Mannheimia haemolytica, Mycoplasma spp., PI-3V, BRSV et BHV-1.
(Traduit par Docteur Serge Messier)
Introduction
Bovine viral diarrhea viruses (BVDV) cause infection and disease in cattle and involve 1 or more organ systems (1,2). Bovine viral diarrhea virus has been isolated from several clinical forms of disease and from necropsy samples, including cattle with signs and/or lesions of bovine respiratory disease (BRD) (2).
Bovine viral diarrhea viruses are classified by biotype and genotype (1). The biotypes, cytopathic (CP) and noncytopathic (NCP) are based on the presence or absence of visible cytopathic effects (CPE) in infected cell cultures. Bovine viral diarrhea virus genotypes (1 and 2) are detected by polymerase chain reaction (PCR) for nucleotide differences and antigenic differences (3,4). Type I BVDV strains can be further subdivided into BVDV1a and BVDV1b by differential PCR and nucleic acid sequencing (5,6). Bovine viral diarrhea virus genotypes have been associated with particular disease forms. In one study, BVDV NCP biotypes were isolated more frequently than CP biotypes and BVDV1 NCP biotypes were isolated more frequently than BVDV2 genotypes from cattle with history of BRD (2). Bovine viral diarrhea virus 1 genotypes were isolated more frequently than BVDV2 genotypes from necropsy cases of calves with fibrinous pneumonia (2). In another study, BVDV1b subtype was more frequently isolated than BVDV1a (68.3% versus 31.7%), with almost equal distribution of BVDV1a and BVDV1b from cattle with BRD history (7). However, BVDV1b was more frequently isolated than BVDV1a from calves that died with pneumonia (7).
Bovine viral diarrhea viruses have been associated with BRD in calves of mixed source, auction market derived, commingled, transported, and those under observation for BRD signs and lesions (8,9). These BVDV isolations and seroconversions have occurred along with Parainfluenza-3 virus (PI-3V), Bovine respiratory syncytial virus (BRSV), Bovine adenovirus (BAV-7), and Bovine herpes virus-1 (BHV-1). The primary causes of bacterial pneumonias in BRD, Mannheimia haemolytica and Pasteurella multocida, were also important contributors to BRD in other studies (8,9). Bovine viral diarrhea virus 1b was the predominant BVDV subtype in these studies, although BVDV1a and BVDV2 were also present (8,9).
Persistently infected (PI) cattle are a major source of exposure for susceptible cattle. The PI calves are the result of fetal infections with NCP strains occurring between 42 to 125 d of gestation (10). The PI calves shed virus in all secretions, especially in the nasal secretions, throughout their lives. To illustrate efficiency of transmission, in a prior study, a pen with 1 PI calf that was positive for BVDV1b caused 13 out of 19 (68.4%) calves exposed to seroconvert to BVDV1b (9).
The purpose of this study was 6-fold: 1) to determine the rate of BVDV1b infection in susceptible calves exposed to PI BVDV1b calves; 2) to determine if BVDV infections occur in ranch calves previously vaccinated with a killed BVDV vaccine; 3) to determine if modified live virus (MLV) vaccination with a BVDV vaccine 3 d prior to exposure would reduce BVDV infection; 4) to determine differences in BVDV vaccine strains versus PI field strains by subtyping molecular techniques; 5) to determine if BVDV was detected more frequently from peripheral blood leukocytes (PBL) than serum in acutely infected calves; and 6) to determine the rate of transmission of other viruses, such as BHV-1, PI-3V, and BRSV.
Materials and methods
Cattle
The cattle used in this study represented 2 sources. There were 100 calves purchased by an order buyer (OB) in Arkansas from multiple sale barns and delivered to the OB pens in Hope, Arkansas on October 19, 2001. There they received initial treatments and vaccinations, and samples were collected including nasal swabs for viral isolation, blood stored in ethylene diamine tetraacetic acid (EDTA) for isolating the virus from peripheral blood leukocytes (PBL), and clotted blood samples for viral serologic testing. The calves were tagged individually with consecutive numbers, with the 50 even numbered calves receiving a MLV vaccine containing BHV-1, BVDV1a, BVDV2a, PI-3V, and BRSV (Titanium 5; Agrilabs, St. Joseph, Missouri, USA). The remaining 50 calves served as nonvaccinates.
The October 19, 2001 collection and processing represented day −3 of the study. The calves were then shipped via semi-trailer truck to research feedyards in Clayton, New Mexico. Upon arrival, they mixed with ranch calves and BVDV1b PI calves on October 22, 2001 (day 0 of the study).
One hundred and two ranch calves were from a northeastern New Mexico ranch. The calves were born in between March and April 2001 and were initially involved in a study to determine the immune response to a killed vaccine containing BHV-1, BVDV1a, BVDV2a, PI-3V, and BRSV (Triangle 4 + type II BVD; Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and to study maternal antibody decay (Proceedings of 45th Annual Meeting of AAVLD, October 18–22, 2001, St. Louis, Missouri, USA). The calves were tagged individually with consecutive numbers with the 51 even numbered calves receiving the killed vaccine and 51 odd numbered calves remaining unvaccinated. Calves were vaccinated on June 29, 2001 at branding and then again on October 1, 2001. On both dates samples were collected from the calves for viral isolation and serology. The ranch calves were then weaned and delivered to the Clayton, New Mexico research facilities where they were mixed with the OB calves from Arkansas and the PI BVDV calves on October 22, 2001.
The PI calves (n = 10) contained BVDV1b NCP. These calves were born between March and April 2001 and were delivered to the Clayton, New Mexico facility and mixed with the OB and ranch calves on day 0, October 22, 2001. These were naturally occurring PI calves from a Kansas ranch.
On day 0, the calves were assigned to each of 10 pens. There were calves in pens with ranch vaccinates, ranch unvaccinated, OB vaccinates, and OB unvaccinated. There were equal numbers of vaccinated and unvaccinated ranch calves (n = 5 each) and vaccinated and nonvaccinated OB calves (n = 5 each) in each pen (n = 8), with the exception of 2 pens containing 11 ranch calves and 10 OB calves. One PI BVDV1b calf was placed in each pen for the duration of the study (n = 10). The calves were in the pens that were 40 × 100 feet in with a 34 ft long bunk space.
The calves were processed and placed in the pens on day 0 with samples collected for viral isolation from nasal swabs and blood stored in ethylene diamine tetraacetic acid (EDTA) treated tubes for PBL and serology analysis. Similar samples were collected on days 7, 14, 21, and 35 (completion date of study). Nasal swabs were also collected for viral isolation on day 3. The vaccinated OB calves were vaccinated on day 14 with a monovalent BRSV MLV vaccine (Titanium BRSV; Agrilabs). The isolation and transmission of M. haemolytica and P. multocida, the primary causes of bacterial pneumonias in BRD; vaccination against M. haemolytica and P. multocida; and clinical signs of sick cattle are subjects of other investigations.
Lung samples were collected at necropsy from each calf during this study. These samples were tested for viruses and bacteria (8,9). Samples were also collected for histopathological study.
Serologic tests
A virus neutralization test (VNT) in Madin-Darby bovine kidney (MDBK) cells in 96-well microtiter plates was used to quantitate virus-neutralizing antibodies to BVDV types 1a, 1b, and 2a, PI3V, and BRSV. The viruses used in this test were CP BVDV type 1a (Singer strain), CP BVDV1b (TGAC 8HB), CP BVDV type 2a (125-C strain), PI3V (SF-4 strain), and a BRSV vaccine strain (9). The 1:4 final dilution was the lowest dilution tested. A plaque reduction assay in MDBK cells in 24-well plates was used to detect virus-neutralizing antibodies to the Colorado strain of BHV-1 (9). The 1:10 final dilution was the lowest dilution tested. In this study, 0 or negative titers represented < 1:4 for BVDV types 1a, 1b, and 2, PI3V, and BRSV, and < 1:10 for BHV-1. Titers of ≤ 4 for BRS were considered negative. Titers expressed represent reciprocals of the endpoint titers.
Microbiologic studies
The virus isolations were performed at the Oklahoma Animal Disease Diagnostic Laboratory (OADDL). A monolayer enzymelinked immunosorbent assay (ELISA) was used to detect BVDV (9,11). To isolate viruses, nasal swabs, PBLs, and lung samples were inoculated onto bovine turbinate (BT) monolayers in 24-well plates, as previously described (9,11). Cytopathic agents other than BVDV were tested for by using direct fluorescent antibody tests to detect BRSV, PI3V, and BHV-1. The BVDV isolates were typed with the use of differential PCR and sequencing of the 5’ untranslated region (5’-UTR), as previously described (5,6,9). Bacterial isolation from lung tissues was also performed by the OADDL (9).
Pathological studies
Lung samples collected at necropsy were fixed in 10% buffered formalin and processed for histopathology. Sections were stained with hematoxylin and eosin, and examined by one of the authors (A.W.C.) who was blinded to the source, who then rendered a morphologic diagnosis.
Statistical analysis
Due to the problems arising from having a small number of samples, the percentages of isolations and of seroconversions were compared using a two-sided Fisher’s Exact Tests and PROC FREQ (SAS, version 8.2; SAS Institute, Cary, North Carolina, USA). All tests were performed at a level of significance of P < 0.05.
Results
Serologic findings and virology status upon arrival
The 102 ranch calves were negative for viruses in PBL and nasal secretions on June 29, 2001; October 1, 2001; and October 22, 2001 (day 0). No viruses were detected in the nasal secretions in the 100 OB calves on day −3 or day 0 prior to the commingling. On day −3, the OB calves were 99% seronegative to BHV-1, 75% to BVDV1a, 75% to BVDV1b, 85% to BVDV2, 48% to PI-3V, and 68% to BRSV. Only 5/100 calves had BRSV antibody titers < 1:4. This number was similar to other studies, as 63 calves had titers of 4, which may be nonspecific inhibitors. Titers of ≤ 4 were considered negative for BRSV antibodies.
The ranch calves at entry were 11/102 (10.8%) seronegative to BVDV1b; 8 calves were unvaccinated seronegative and 3 calves were vaccinated seronegative. The geometric mean for the vaccinated calves was 17.6, (range, 0 to 256 titers) and was 10.7 for the unvaccinated calves (range, 0 to 512 titers). One hundred out of 102 (98.0%) ranch calves had BVDV1a antibodies and 84/102 (82.4%) had BVDV2 antibodies, with 15 seronegative unvaccinated and 3 seronegative vaccinates. There were only 2 ranch calves (1 each to vaccinates and unvaccinated) that were seronegative to BRSV. There were 40/102 ranch calves that were seropositive to BHV-1; 28 vaccinates and 12 unvaccinated.
Calves that died: lung lesions and isolates
The lesions in the lungs of the 7 calves that were dying during the study were consistent with those of pneumonia caused by M. haemolytica — primarily fibrinopurulent pneumonia (Table I). Mycoplasma spp. were isolated from 4 cases; PI-3V from 1 case and BVDV NCP strains from 2 cases, calves numbers 34 and 85. At the time of treatment for BRD, BVDV NCP strains were isolated from PBL from 2 calves (numbers 34 and 83) that died (Table I).
Table I.
Pulmonary lesions, bacteria, and viruses isolated from calves dying during the study
| Animal number | Day of death | Type of pneumonia | Bacteria/Mycoplasma isolated | Virus isolated |
|---|---|---|---|---|
| 25 | 29 | Chronic, moderate, multifocal fibrinopurulent pleuropneumonia with thrombosis and necrosis and bronchiolar necrosis |
Mycoplasma spp.
No bacteria |
PI-3V |
| 34 | 15 | Acute severe, fibrinopurulent pleuropneumonia with extensive hemorrhage and lobular necrosis | No bacteria
Mycoplasma spp. |
BVDV
NCP a |
| 83 | 21 | Subacute, chronic, fibrinopurulent pleuropneumonia with bronchiolar dilatation and epithelial hyperplasia |
Mannheimia haemolytica Mycoplasma spp. |
Noneb |
| 85 | 10 | Acute, severe, fibrinopurulent pleuropneumonia with multifocal hemorrhage and necrosis |
M. haemolytica Mycoplasma spp |
BVDV
NCP |
| 241 | 3 | Acute, severe, fibrinopurulent pleuropneumonia |
M. haemolytica No mycoplasma |
None |
| 275 | 3 | Acute, severe, fibrinopurulent pleuropneumonia with multifocal hemorrhage and lobular necrosis |
M. haemolytica No mycoplasma |
None |
| 285 | 3 | Acute, moderate fibrinopurulent pleuropneumonia |
M. haemolytica No mycoplasma |
None |
Bovine viral diarrhea virus (BVDV)1b noncytopathic (NCP) isolated from peripheral blood leukocytes (PBL) on day 14
Bovine viral diarrhea virus 1b NCP isolated from PBL on days 14 and 21
Viral isolates from cattle
There were 45 cattle from which BVDV was isolated, either from PBL or nasal swabs (Table II). Bovine viral diarrhea virus was isolated from both vaccinates and unvaccinated calves. Bovine viral diarrhea virus isolates were grown again in cell culture, reconfirmed as BVDV, and subtyped (Table II). There were 40 calves that had isolates that were amplified and subtyped (Table II). The CP vaccine strains, BVDV1a C24V and BVDV2 296, were isolated from PBL on the day 10 collection post vaccination (day −3 + day 7 collection after study began). Each of the 10 pens containing a PI calf had calves that became infected with BVDV1b NCP. The isolation of BVDV1b strains in exposed calves occurred between days 7 and 21 after exposure to the PI calves (Table II). There were 32 calves that had BVDV1b in PBL or nasal swabs identical to the PI strain. Eleven ranch calves became infected with BVDV1b: 8/51 unvaccinated and 3/51 vaccinated. Twenty-one OB calves also became infected with BVDV1b isolates: 8/50 vaccinated and 13/50 unvaccinated. Bovine viral diarrhea virus 2 NCP strain was isolated from 1 OB calf on both PBL and nasal swabs on multiple collections from days 3 to 9. This calf developed higher numbers of antibodies to BVDV2 (0 at day −3 to 8192 at day 35) than to BVDV1a (0 to 128) or BVDV1b (0 to 512). All other calves in that pen (2) had higher titers to BVDV1b than to BVDV2. This BVDV2 was genetically different from the BVDV2 vaccine strain. No other calf became infected with this subtype. It is most likely that this was an acute infection not detected by virus isolation on day −3 or day 0, that the calf had been exposed prior to purchase and assembly at the OB.
Table II.
Bovine viral diarrhea virus (BVDV) isolations from calves
| Pen number | Animal number | Origin | Collection day | Sample origin | Vaccination status | BVDV subtype |
|---|---|---|---|---|---|---|
| 2 | 13 | R | 14 | PBL | N | BVDV1b NCP a |
| 53 | R | 14 | PBL | N | BVDV1b NCP | |
| 57 | R | 14 | PBL | N | BVDV1b NCP | |
| 58 | R | 21 | PBL | V | BVDV1b NCP | |
| 225 | OB | 14 | PBL | N | BVDV1b NCP | |
| 294 | OB | 3 | NS | V | BVDV2 NCP b | |
| 7 | NS | BVDV2 NCP b | ||||
| 7 | PBL | BVDV2 NCP b | ||||
| 9 | PBL | BVDV2 NCP b | ||||
| 4 | 46 | R | 10 | PBL | V | BVDV1b NCP |
| 286 | OB | 14 | PBL | V | BVDV1b NCP | |
| 6 | 34 | R | 13 | PBL | V | BVDV 1b |
| 14 | PBL | V | BVDV NCP | |||
| 201 | OB | 11 | PBL | N | BVDV1b NCP | |
| 213 | OB | 14 | PBL | N | BVDV1b NCP | |
| 237 | OB | 7 | PBL | N | BVDV1b NCP | |
| 242 | OB | 7 | PBL | V | BVDV2 CP c | |
| 8 | 214 | OB | 7 | PBL | V | BVDV1a CP d |
| 222 | OB | 7 | PBL | V | BVDV1b NCP | |
| 238 | OB | 14 | PBL | V | BVDV NCP | |
| 253 | OB | 7 | PBL | N | BVDV1b NCP | |
| 258 | OB | 7 | PBL | V | BVDV1a CP d | |
| 289 | OB | 7 | PBL | N | BVDV NCP | |
| 10 | 41 | R | 14 | PBL | N | BVDV1b NCP |
| 206 | OB | 11 | NS | V | BVDV1b NCP | |
| 221 | OB | 7 | PBL | N | BVDV1b NCP | |
| 273 | OB | 7 | PBL | N | BVDV1b NCP | |
| 290 | OB | 7 | PBL | V | BVDV1b NCP | |
| 293 | OB | 14 | PBL | N | BVDV1b NCP | |
| 298 | OB | 7 | PBL | V | BVDV 2c CP | |
| 14 | PBL | V | BVDV 2c CP | |||
| 15 | 15 | R | 14 | PBL | N | BVDV1b NCP |
| 220 | OB | 7 | PBL | V | BVDV1b NCP | |
| 236 | OB | 7 | PBL | V | BVDV1b NCP | |
| 17 | 83 | R | 14 | PBL | N | BVDV1b NCP |
| 17 | PBL | N | BVDV NCP | |||
| 101 | R | 14 | PBL | N | BVDV1b NCP | |
| 211 | OB | 7 | PBL | N | BVDV1b NCP | |
| 243 | OB | 7 | PBL | N | BVDV1b NCP | |
| OB | 14 | PBL | N | BVDV1b NCP | ||
| 252 | OB | 7 | PBL | V | BVDV CP | |
| 283 | OB | 14 | PBL | N | BVDV NCP | |
| 19 | 63 | R | 7 | PBL | N | BVDV1b NCP |
| 276 | OB | 7 | PBL | V | BVDV CP | |
| 291 | OB | 14 | PBL | N | BVDV1b NCP | |
| 21 | 247 | OB | 14 | PBL | N | BVDV1b NCP |
| 284 | OB | 7 | PBL | V | BVDV1b NCP | |
| 292 | OB | 7 | PBL | V | BVDV2 CP c | |
| 299 | OB | 7 | PBL | N | BVDV1b NCP | |
| 23 | 224 | OB | 7 | PBL | V | BVDV1b NCP |
| 232 | OB | 7 | PBL | V | BVDV2 CP c | |
| 11 | PBL | V | BVDV CP | |||
| 264 | OB | 7 | PBL | V | BVDV1a CP d |
Bovine viral diarrhea virus 1b noncytopathic (NCP), identified to persistently infected (PI) strain
Bovine viral diarrhea virus 2 NCP, different from BVDV2 vaccinal cythopathic (CP) strain
Bovine viral diarrhea virus 2 CP, vaccine strain, 296
Bovine viral diarrhea virus 1a CP,C24V vaccine strain
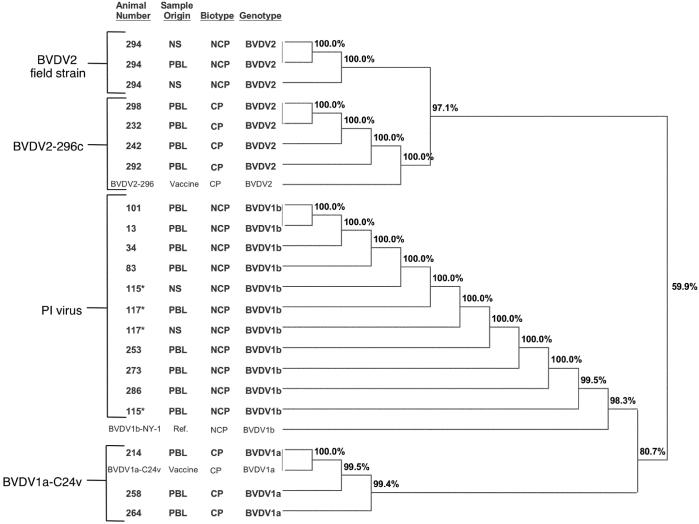
The phylogenetic analysis for representative BVDV subtypes is shown in Figure 1. There were no CP strains isolated from unvaccinated calves. It is presumed that the CP strains that were not amplified and subtyped were of vaccine origin. There were 7 BVDV isolates from the OB calves subsequent to vaccination with a MLV vaccine containing BVDV1a (C24V) and BVDV2 (296), both CP strains. These isolates were from collections 10 to 17 d post vaccination (day −3 + day 14 of study). Both the CP strains, C24V and 296, were identified by subtyping. Thus, there were 7 calves identified with confirmed vaccine strains.
Figure 1.
Dendogram representing the relatedness of nucleotide sequences of 5’-UTR from reference strains of Bovine viral diarrhea virus (BVDV) including vaccinal strains, representative persistently infected (PI) isolates, and isolates from calves exposed to PI calves.
The PI-3V isolates (21 calves) were found on day 21 in nasal swabs with viral isolates from both vaccinated and unvaccinated calves. The isolates were all from calves that had been treated earlier (19/21), and only 2 PI-3V isolates were from calves that remained healthy throughout the study. In addition, the only BHV-1 isolated was on day 7 from a nasal swab from 1 PI calf.
Seroconversions to BVDV1b, PI-3V, and BRSV in the surviving calves
There were 98/102 ranch calves that survived throughout the study (through day 35) and 97/100 OB calves that survived. Sera were tested from the earliest collection for OB calves, day −3 (time at which MLV vaccine was given to half the OB calves), day 0, and day 35. For the basis of seroconversions for the OB calves, the vaccination date (day −3) was used along with day 35. For the ranch calves, day 0 and day 35 antibody titers were compared. Seroconversion was defined as 4-fold or greater increase in antibody titers for BVDV1a, BVDV1b, BVDV2a, PI-3V, and BRSV; and 2-fold or greater increase for BHV-1. There was evidence of active infection during this study based on seroconversions by the unvaccinated calves representing both the ranch and OB calves: 79/95 (83.2%) to BVDV1b, 54/95 (56.8%) for PI-3V, 36/95 (37.9%) for BRSV, and 1/95 (1.1% for BHV-1).
There were 83/98 (84.7%) surviving ranch calves that seroconverted to BVDV1b and 87/97 (89.7%) surviving OB calves, for a total of 170/195 (87.2%) (Tables III and IV). There was a higher seroconversion rate (P < 0.05) for vaccinated ranch calves, 47/50 (94.0%), compared to unvaccinated ranch calves, 36/48 (75.0%). There was no difference in the BVDV1b seroconversion rate between OB vaccinates, 44/50 (88.0%), and OB unvaccinated, 43/47 (91.5%) (P > 0.05). There was a high rate of BVDV1b seroconversions in the pens, ranging from 80% to 95% (Table IV). There was a high rate of seroconversions to BVDV1b among the unvaccinated calves in each pen. The seroconversion rates ranged from 7/10 (70%) for pen 23 to 10/10 (100%) for pen 2. There was no difference in the range of BVDV1b antibody titers in OB calves after vaccination (0 to 32 up to 1024) or unvaccinated (0 to 32 up to 1024) after exposure to BVDV1b PI calves.
Table III.
Bovine viral diarrhea virus (BVDV)1b seroconversions for ranch and order buyer (OB) cattle
| Source | Vaccination | Seroconversion |
|---|---|---|
| Ranch | Yes | 47/50 (94.0%) |
| None | 36/48 (75.0%) | |
| OB | Yes | 44/50 (88.0%) |
| None | 43/47 (91.5%) |
Table IV.
Bovine viral diarrhea virus (BVDV)1b seroconversions by pen
| Ranch
|
OB
|
||||
|---|---|---|---|---|---|
| Pen number | VAX | NonVAX | VAX | NonVAX | Total |
| 2 | 4/5 | 5/5 | 5/5 | 5/5 | 19/20 |
| 4 | 4/4 | 3/4 | 4/5 | 4/5 | 15/18 |
| 6 | 4/5 | 5/5 | 4/5 | 4/4 | 17/19 |
| 8 | 5/5 | 3/4 | 5/5 | 3/4 | 16/18 |
| 10 | 4/5 | 4/5 | 3/5 | 5/5 | 16/20 |
| 15 | 5/5 | 4/5 | 5/5 | 4/4 | 18/19 |
| 17 | 6/6 | 3/4 | 5/5 | 5/5 | 19/20 |
| 19 | 5/5 | 4/6 | 5/5 | 4/5 | 18/21 |
| 21 | 5/5 | 3/5 | 4/5 | 4/5 | 16/20 |
| 23 | 5/5 | 2/5 | 4/5 | 5/5 | 16/20 |
| 47/50 (94.0%) | 36/48 (75.0%) | 44/50 (88.0%) 43/47 (91.5%) | |||
| Total ranch calves: 83/98 (84.7%) | Total OB calves: 87/97 (89.7%) | ||||
Total ranch + OBB: 170/195 (87.2%)
OB — order buyer; VAX — vaccinated calves; NonVAX — unvaccinated calves
Seroconversions also occurred in the study to BRSV and PI-3V. There were BRSV seroconversions in ranch calves, 39/98 (39.8%), and 65/97 (67.0%) OB calves, 104/195 (53.3%) total. For the ranch calves the BRSV vaccinates had higher seroconversion rates than unvaccinated (P < 0.05), 58% versus 20.8%. There were more OB BRSV vaccinates seroconverting, 78%, compared to OB unvaccinated, 55.3% (P < 0.05). There were PI-3V seroconversions in ranch calves, 54/98 (55.1%), and 62/97 (63.9%) OB calves, 116/195 (59.5%) total. No ranch calves seroconverted to BHV-1 in this study, 0/98. This was not unexpected as the vaccination of ranch calves had occurred approximately 3.5 months and 3 wk prior to the study. There were 42/50 OB vaccinated calves that seroconverted to BHV-1, but only 1/48 unvaccinated OB calf seroconverted. This calf had BHV-1 antibodies at day –3 with increasing antibodies on days 0 and 35 suggesting that the calf was undergoing an acute infection at time of purchase and initial assembly at day –3. There were 2 PI calves that seroconverted to BHV-1 during the study. They were in different pens and not with the OB calf above.
Effect of BVDV1b antibody titer and exposure to PI calves at entry in ranch calves
The association between BVDV1b antibody titer at entry (day 0) for ranch calves and protection against viremia is illustrated in Table V. Antibody titers to BVDV1b of 64 and below did not prevent viremias in the 11 ranch calves.
Table V.
Bovine viral diarrhea virus (BVDV)1b antibody titer in ranch calves at day 0 exposure to persistently infected (PI) BVDV1b calves and time of initial virus identification from peripheral blood leukocytes (PBL)
| Calf number | Antibody titer | Vaccine status | Day of viral isolation |
|---|---|---|---|
| 13 | 0 | N | 14 |
| 15 | 8 | N | 14 |
| 34 | 16 | V | 13 |
| 41 | 4 | N | 14 |
| 46 | 0 | V | 10 |
| 53 | 8 | N | 14 |
| 57 | 0 | N | 14 |
| 58 | 64 | V | 21 |
| 63 | 4 | N | 7 |
| 83 | 8 | N | 14 |
| 101 | 4 | N | 14 |
N — unvaccinated; V — vaccinated
Bovine viral diarrhea virus 1b antibody titers at time of exposure (day 0) to PI calves did not prevent infection (anamnestic response), as measured by increased antibody titers (4-fold or greater). All ranch calves with titers of 8 or below seroconverted after BVDV1b exposure. Seroconversion rates in calves with various antibody titers were: 16/17 for titer of 16, 15/21 for titer of 32, 4/10 for titer of 64, 2/2 for titer of 128, and 1/2 for titer of 256.
Collection samples for determining BVDV acute infections: serum versus PBL
To determine if BVDV could be isolated from sera simultaneous to isolation from PBL positive samples, companion sera for all calves with PBL positives were tested for BVDV by cell culture inoculation. Also, serum from the PBL positive cattle, either 1 wk prior to or 1 wk after the PBL positive collection, were also tested. There were 42 cattle with PBL positive samples, and BVDV was isolated from 16/42 (38.1%) sera concurrently. Bovine viral diarrhea virus was not isolated from sera collected before or after the PBL positive collection. The use of PBL for diagnosis of acute infections yielded over twice as many BVDV positives. Throughout this study BVDV was isolated from PBL, nasal swabs, and sera from the PI calves.
Discussion
The results from this study clearly demonstrated that PI BVDV infected calves readily transmitted BVDV infection to control calves as based on: 1) seroconversions indicating active infections, and 2) viral isolations from infected contact calves. An 83.2% seroconversion rate to BVDV1b was observed in unvaccinated calves after PI calf exposure, and BVDV was isolated from 32/202 (15.8%) exposed calves. Similar results have been reported in other studies (9,12). In one study, a PI cow and her PI calf were placed in a pen with susceptible heifers for 24 h, and 22/35 (62.9%) heifers seroconverted to BVDV indicating infection (12). In a field study, a pen of cattle with 1 PI calf present had a seroconversion rate of 13/19 (68.4%) (9). Transmission to vaccinated and unvaccinated heifers by PI cattle was recently demonstrated (13). Eleven BVDV vaccinated pregnant heifers and 7 unvaccinated pregnant heifers were in a pen with 3 PI heifers. All unvaccinated heifers became infected, with the virus being isolated from leukocytes and sera from all 7 and from nasal swabs in 5/7 heifers. There were 4/11 vaccinates with virus in leukocytes and 2/11 with positive nasal swabs. This latter study (13) supports the current study, wherein ranch calves that previously received a killed BVDV vaccine often became infected with BVDV1b after exposure to PI calves. However, vaccines used included BVDV1a and BVDV2, and the calves were exposed to PI BVDV1b. Therefore, it could be argued that the BVDV subtype in vaccines is critical for protection against BVDV exposure from PI cattle.
However, several ranch calves in this study with BVDV1b antibodies at the time of exposure that still became infected with BVDV1b from PI exposure. Bovine viral diarrhea virus 1b titers up to 64 did not prevent BVDV1b from being isolated after exposure. In fact, BVDV1b antibody titers up to 256 did not uniformly prevent 4-fold or greater rises in BVDV1b antibody titers. Similar results were observed when calves were fed colostrum containing BVDV antibody and challenged with BVDV2 890 strain (14). Therefore, preexisting antibodies to the BVDV strain shed by PI cattle may not be enough to protect cattle against this source of infection.
This study also demonstrated that the PBL would be the preferred sample to isolate BVDV in acutely infected animals. There were no instances where the virus was in the serum and not in the PBL, and in the majority of acutely infected cattle in this study, the PBL were positive and the serum negative by viral isolation in cell culture. Future studies should be conducted to assay sera and PBL for BVDV using PCR to detect viral nucleic acid after viral isolation in cell culture is negative.
Vaccination with a MLV vaccine containing BVDV1a and BVDV2a 3 d prior to exposure to PI calves with BVDV1b did not prevent infection in the vaccinated calves. Even though the vaccinated calves responded with antibodies against BVDV, the onset of immunity preventing infection to a heterologous BVDV strain (1b, in this case) requires more than 3 d. Future studies should examine administration of vaccine 2 to 4 wk, or more, prior to exposure to PI calves.
This study also supports the need to clearly and definitively identify the BVDV detected by viral isolation in cell culture by use of PCR and genomic sequencing. Cell culture studies detect biotypes, CP versus NCP. Isolates detected by cell culture from sick cattle that received MLV vaccines 2 to 3 wk prior may be erroneously incriminated as the causative pathogen. A prior study detected vaccine strains of BVDV in calves’ PBL as early as 3 to 7 d after MLV vaccination with the virus being cleared by day 14 (15). Reliance on biotype CP status to identify the vaccine strain may also be incorrect.
In conclusion, PI BVDV1b calves are very efficient sources of infection for susceptible calves in the field and can be used experimentally to simulate natural exposure. Vaccines to prevent BVDV infection must stimulate immunity to antigenically diverse BVDV strains and must be given sufficiently prior to exposure to stimulate appropriate immunity. In addition, molecular techniques should be used to differentiate field strains rom MLV vaccine strains as well as new or reemerging BVDV strains.
Footnotes
Dr. Duff’s current address is Department of Animal Sciences, University of Arizona, Tucson, Arizona, 85721, USA.
References
- 1.Baker JC. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim. 1995;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- 2.Fulton RW, Saliki JT, Confer AW, et al. Bovine viral diarrhea virus cytopathic and noncytopathic biotypes and type 1 and 2 genotypes in diagnostic laboratory accessions: clinical and necropsy samples from cattle. J Vet Diagn Invest. 2000;12:33–38. doi: 10.1177/104063870001200106. [DOI] [PubMed] [Google Scholar]
- 3.Pellerin CJ, van den Hurk J, Lecomte J, Tussen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994;203:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- 4.Ridpath JF, Bolin SR, Dubovi EJ. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994;205:66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- 5.Ridpath JF, Bolin SR. Differentiation of types 1a, 1b, and 2 bovine viral diarrhoea virus (BVDV) by PCR. Molec Cell Probes. 1998;12:101–106. doi: 10.1006/mcpr.1998.0158. [DOI] [PubMed] [Google Scholar]
- 6.Ridpath JF, Neill JD, Frey M, et al. Phylogenetic, antigenic and clinical characterization of type 2 BVDV from North America. Vet Microbiol. 2000;77:145–155. doi: 10.1016/s0378-1135(00)00271-6. [DOI] [PubMed] [Google Scholar]
- 7.Fulton RW, Ridpath JF, Confer AW, et al. Bovine viral diarrhea virus antigenic diversity: impact on disease and vaccination programs. Biologicals. 2003;31:89–95. doi: 10.1016/s1045-1056(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 8.Fulton RW, Purdy CW, Confer AW, et al. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can J Vet Res. 2000;64:151–159. [PMC free article] [PubMed] [Google Scholar]
- 9.Fulton RW, Ridpath JF, Saliki JT, et al. Bovine viral diarrhea virus (BVDV1b): predominant BVDV subtype in calves with respiratory disease. Can J Vet Res. 2002;66:181–190. [PMC free article] [PubMed] [Google Scholar]
- 10.McClurkin AW, Littledike ET, Cutlip RC, et al. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med. 1984;48:156–161. [PMC free article] [PubMed] [Google Scholar]
- 11.Saliki JT, Fulton RW, Hull SR, et al. Micotiter virus isolation and enzyme immunoassays for detection of bovine viral diarrhea virus in cattle serum. J Clin Microbiol. 1997;35:803–807. doi: 10.1128/jcm.35.4.803-807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan-MR, Kirkland PD, Richards SG, et al. Increased reproductive losses in cattle infected with bovine pestivirus around the time of insemination. Vet Rec. 1993;133:39–43. doi: 10.1136/vr.133.2.39. [DOI] [PubMed] [Google Scholar]
- 13.Patel JR, Shilletto RW, Williams J, Alexander DCS. Prevention of transplacental infection of bovine foetus by bovine viral diarrhoea virus through vaccination. Arch Virol. 2002;147:2453–2463. doi: 10.1007/s00705-002-0878-3. [DOI] [PubMed] [Google Scholar]
- 14.Bolin SR, Ridpath JF. Assessment of protection from systemic infection or disease afforded by low to intermediate titers of passively acquired neutralizing antibody against bovine viral diarrhea virus in calves. Am J Vet Res. 1995;56:755–759. [PubMed] [Google Scholar]
- 15.Fulton RW, Saliki JT, Burge LJ, Payton ME. Humoral immune response and assessment of vaccine virus shedding in calves receiving modified live virus vaccines containing bovine herpesvirus-1 and bovine viral diarrhea 1a. J Vet Med B. 2003;50:31–37. doi: 10.1046/j.1439-0450.2003.00608.x. [DOI] [PubMed] [Google Scholar]