Abstract
Nano-biochar considers a versatile and valuable sorbent to enhance plant productivity by improving soil environment and emerged as a novel solution for environmental remediation and sustainable agriculture in modern era. In this study, roles of foliar applied nanobiochar colloidal solution (NBS) on salt stressed tomato plants were investigated. For this purpose, NBS was applied (0%, 1% 3% and 5%) on two groups of plants (control 0 mM and salt stress 60 mM). Tween-20 was used as a surfactant to prolong NBS effective stay on plant leaf surface. The results showed that 3% NBS application effectively improved the plant height, plant biomass, fruit count and fruit weight under non-stressed and stressed plants. In addition, 3% NBS application further increased the plant pigments such as chlorophyll by 72% and 53%, carotenoids by 64% and 40%, leaf relative water content by 4.1 fold and 1.07 fold under both conditions, respectively. NBS application stabilized the plasma membrane via reducing electrolyte leakage by 30% as well as reduced the lipid peroxidation rates by 46% and 29% under non-stressed and stressed plants, respectively. 3% NBS application also significantly enhanced the plants primary and secondary metabolites, as well as activities of antioxidant enzymes compared to control plants. Overall, NBS foliar application significantly improved all growth and yield indices, pigments, primary and secondary metabolites, leaf water content, antioxidant enzyme activities as well as reduced electrolyte leakage and lipid peroxidation rates in tomato to combat stress conditions. In future, studies on nano biochar interactions with soil microbiota, surface modifications, long-term environmental impacts, reduced methane gas emissions, and biocompatibility could provide insights into optimizing its use in sustainable agriculture.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87399-5.
Keywords: Nanobiochar, Biochemical indices, Growth, Yield, Metabolites, Antioxidant enzymes
Subject terms: Physiology, Plant sciences
Introduction
Salinity is a significant environmental stressor and diminishes the plant’s yield. It disturbs irrigated soils and reduce one-third of food production. Saline soil has an impact on ecosystems, land use, and agriculture productivity1. Salinity caused physiological mechanisms change which are responsible for growth and developmental regulations as it interferes with both nitrogen and carbon metabolism in plants2. Growth and physiological traits, including stem length, leaf area, relative water content, chlorophyll concentration, and yield output, decreased when exposed to salinity3. High salinity has been linked to the generation and formation of reactive oxygen species (ROS) in plant cells4. On the other hand, overproduction of reactive oxygen species damage membrane lipids causing membrane injury5.
Additionally, a higher amount of Na+ and Cl− ions negatively impact protein synthesis, plant lipid metabolism, and enzyme functioning. Furthermore, the presence of such ions in excess amount can promote reactive oxygen species (ROS) production that impose very fatal oxidative stress on plants6. Salt exclusion through channel proteins and upregulation of ROS scavenging system are among the basic mechanisms for structural protection of cellular organelles 7.
In past, farmers have been utilized both organic and artificial fertilizers to improve production and maintain soil productivity8. The usage of chemical fertilizers harms the environment and crops by causing water pollution, greenhouse gas emissions, and N leaching9. A substance like charcoal called biochar is being used more frequently in agriculture with the goal of enhancing agricultural yield, reducing greenhouse gas emissions, and improving soil fertility10. Biochar has a high capacity for adsorbing salt, which could lower plant Na+ absorption and mitigate the negative effects of soil salinity11. .
Additionally, it has been said that biochar can lower exchangeable acidity12. Biochar (BC) contains significant amounts of inorganic carbonates and minerals like calcium and magnesium that are good for plant growth. The properties of soil and carbon sequestration may be improved by biochar13. The beneficial effects of biochar may be ascribed to the fact that it increases the amount of carbon in the soil, which enhances soil quality, leads to an increase in relative water content, and increases plant height, leaf number, and leaf area per plant14.
In recent era, nanobiochar (NB) is synthesized from ordinary biochar with different techniques. Generally, mechanical grinding is used for nanosize particle generation. Despite mechanical methods, direct NB manufacturing by flash heating resulted in graphitic nanosheets. Depending on the feedstock sources and pyrolysis temperature, the weight of the biochar nanoparticles (NPs) ranges from 1.6 to 2.6% of the total generated biochar particles15. In order to overcome the limitations of biochar, NB has been developed, increasing its physical, chemical, and structural properties. For instance, its greater surface area brought about by its nanosize and the abundance of functional groups with enhanced mechanical and thermal stability, as recently reported by Noreen and Abd-Elsalam, (2021)16. NB can be used to boost the growth of plants like wheat and rice by alleviating salt stress17. The best perspective has been provided by nanotechnology in agriculture for plant nutrition 18. Previous literature has been highlighted the potential of nano-sized biochar to enhance the growth, biomass and mineral composition of raddish and carrot plants19,20. Further, foliar application of nano-biochar colloidal suspension ameliorated the negative effects of drought stress in tomato21 and salinity stress in quinoa and wheat22. Recent reports also displayed that nano-biochar application enhanced the growth of wheat seedlings by activating the hydrolyzing and nitrogen metabolic enzymes under salt stress23,24. However, the effects of foliar applied nano-biochar colloidal solution in alleviation of salt stress through improved metabolites and antioxidant enzyme activities in vegetables remains unexplored yet.
The tomato is regarded as one of the more well-liked fruits or vegetables, with a wide distribution over the world and a significant economic significance25. Tomatoes are highly sensitive to environmental factors such as temperature, light, and changes in irrigation throughout the growth phase of the plant26. Tomato can be grown in areas with some soil salinization due to its moderate resistance towards soil salts27. However, the nutritional profiles of tomatoes which include vitamins, carotenoids, and phenolic compounds, are mostly effected by salt stress28.
In recent years, the use of nanotechnology in agriculture, a rapidly developing industry, has made significant progress29, due to its capacity to offer viable and timely solutions essential for sustainable agriculture30. The best perspective on plant nutrition has been provided by nanotechnology applied as nanoparticles (NPs). The most popular way for giving plants the vital nutrients they need is soil application. In this instance, plant roots take up the provided nutrients. However, when sprayed as foliar sprays in the proper amounts, taller plants can absorb mineral nutrients more efficiently. While in most of the cases foliar fertilization have been seen as a visible, affordable technique to add nutrients to the plants for more effective fertilization31. Under challenging circumstances like biotic and abiotic stresses32and normal conditions as well the application of nanoparticles in the agriculture sector could be considered as one of the effective methods to increase agricultural output.
Materials and methods
Experiment detail
An experiment with the pots was designed at the wire-house of The University of Lahore field area in spring 2020–2021 to assess the effects of foliar applied nano-biochar (supplied by Shaanxi Dainong Huitai Biological Health Agricultural Technology Co., Ltd China). Characterization and elemental composition of this nano-biochar solution has been published earlier33. All laboratory analysis was carried out in General Botany Laboratory, Institute of Molecular Biology and Bio-technology, UOL Lahore. Tomato seedlings were supplied by The Vegetables Research Institute, AARI, Faisalabad, Pakistan. Briefly, five tomato seedlings with two to three leaves were transplanted into clay pots with loam-organic manure soil (3:1) weighing around 20 g per pot. Four replicates of each treatment were used for study the each parameter. The experiment was arranged in a completely randomized design (CRD).
Application of salt and NBS solution
After one week of transplantation, once the field capacity condition was achieved, tomato plants were irrigated by salt solution adjusted to the electrical conductivity of 6 dS/m by adding NaCl (equivalent to 60 mM). A screening experiment was conducted to identify an optimal salt stress level for tomato plants. 60 mM NaCl was chosen because it produced a notable stress response.
The control plants were not irrigated with salt solution. Following two weeks of salt stress treatment, both the control and salt groups received foliar applications of Nano-biochar solution (NBS) in four different concentrations: 0%, 1%, 3%, and 5%. Three applications of nano-biochar sprays were applied with an interval of five days. In order to guarantee effective spray penetration into leaves, 0.5% tween-20 was added to the nano-biochar solution.
Sample collection
Plant samples were collected after 20 days of initial foliar application of NBS for biochemical analysis and preserved at -20ºC in refrigerator. While fresh plant samples were uprooted at maturity (65 days after planting) to investigate the plant fresh and dry biomass and some physiological attributes. Yield indices were recorded at the end of the experiment.
Pigments analysis
Pigment analysis was performed as reported by Arnon, (1949)34to determine the contents of chlorophyll a, b, total chlorophyll, and carotenoids in the fresh leaves of tomato in both control as well as salt treated groups by determining the absorbance at 663, 645, and 480 nm wavelengths using the UV/VIS spectrophotometer (HALO SB-10). The chlorophyll a and b contents, total chlorophyll contents, and carotenoid contents were calculated using formulas as mentioned by Yoshida et al. (1980)35and results were expressed as (mg/g FW).
Estimation of membrane stability indices
The relative water content (RWC) was determined by using method of Barr and Weatherley, (1962)36. For this purpose, fully expanded topmost leaves were collected from each treatment groups. Then quickly weigh out the leaves to avoid moisture loss. After that, leaves were dipped in deionized water for 4 h. In next, leaves were taken out from water and placed on blotting paper to remove extra surface water and again weight out to determine turgid weight. In next step, leaves were placed in oven at 55 °C for 24 h and again weigh out to find the dry weight. The relative water content was recorded using following formula:
Electrolyte leakage% was estimated using method as reported by Lutts, (1996)37. The proportion of electrolyte leakage (EL) in response to stress damage was calculated. In this method, fully expanded fresh leaves were collected and cut in to 5–10 leaf discs (about 1–1.5 cm diameter) without midrib or major veins and placed 6 leaf discs in each test tubes. 10 ml distilled water was added in each tube. Disc samples were incubated at room temperature for 24 h in shaker. In next day, electrical conductivity of test solutions was read which designated as EC1. Sample solutions were than autoclaved at 120°C for 20 min and EC2 was recorded afterwards.
EC % was calculated by using following formula:
To determine the lipid peroxidation rates in terms of the content of malondialdehyde (MDA) production, the method proposed by Heath and Packer, (1968)38was adopted. For this purpose, 0.1 g leaf tissue from each test sample was homogenized in 0.5 ml of 0.1% TCA. Homogenate was centrifuged for 10 mints and supernatant was collected. After that, 0.5 ml of supernatant was mixed with 1.5 ml of 0.5% TBA solution which was diluted in 20% TCA. This mixture was incubated in water bath for 25 mints at 95 °C. The reaction was ended up by incubating the samples on ice bath. Development of Light pink color showed the presence of MDA contents. Absorbance was measured at 532 and 600 nm on spectrophotometer. MDA concentration was calculated by using the Lambert-Beer law with an extinction coefficient of εΜ = 155 mM− 1cm− 1.
Determination of primary metabolites
Leaf samples were extracted in phosphate buffer solution (0.2 M) for biochemical examination after 3 weeks of NBS treatments. Total free amino acids were estimated by Hamilton and Van Slyke (1943) 39and OD measured at 570 nm. Total soluble sugars were determined by using the method of Riazi et al. (1985)40 and measurements were taken at 620 nm using spectrophotometer.
Determination of secondary metabolites
The method described by Pekal and Pyrzynska, (2014)41 was used to calculate the total flavonoid contents at 465 nm OD. Further the total phenolic content of leaves was assessed using the Folin-Denis reagent as reported by Julkunen-Titto (1985)42and OD was measured at 765 nm.
Antioxidant enzyme activities
Catalase and peroxidase enzyme activities were tested using the Chance and Maehly (1955)43method. The enzyme activities of catalase (CAT) and peroxidase (POD) were measured as enzyme units (EU) per gram or ml of fresh tissue weight (EU/g FW) and per milligram of protein (EU/mg protein). Superoxide dismutase activity was measured using the Dhindsa et al. (1981)44 approach. The superoxide dismutase activity was determined as enzyme unit (EU) on per g fresh basis and per mg protein.
Statistical analysis
The data from 4 repeats were subjected to two-way analysis of variance (ANOVA) and analyzed by computer based software STATISTIX 8.1. Significant differences among treatments were analyzed by the Least Significant Difference (LSD) test. The least significant difference was used to compare means at p ≤ 0.05.
Results
NBS foliar application promoted growth indices under salt stress
To investigate the effects of NBS colloidal solution on tomato growth, yield and biomass, various parameters were studied as shown in Table 1. It was noted that salinity reduced the root and shoot lengths by 21.6% and 16.6%, respectively, as compared to control plants. Biomass of a plant comprises of root and shoot dry as well as fresh weights. In current study, plant fresh biomass decreased by 53.9% and plant dry biomass by 64.5% in only saline stressed plants as compared to control plants. However, the foliar application of NBS solution accelerated the growth characteristics under both stressed and non-stressed. Foliar application of 3% NBS solution increased the shoot length by 44 and 53%, and root length by 35 and 36% under non-stressed and stressed plants, respectively compared to their respective controls. While 1% NBS application increased the fresh biomass by 26 and 67% and plant dry biomass by 67 and 80.3% under non-stressed and stressed plants, respectively when compared to their respective control samples.
Table 1.
The effect of foliar application of NBS on growth and biomass of tomato under salt stress.
| Condition | Foliar (%) |
Shoot length (%) | Root length (%) | Shoot fresh weight (g) | Shoot dry weight (g) | Root fresh weight (g) | Root dry weight (g) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 16.6c | 9.6bc | 20.9c | 5.0cd | 3.7b | 1.4bcd |
| 1 | 21.0b | 11.2b | 25.4b | 8.7a | 5.8a | 1.8abc | |
| 3 | 24.6a | 13.0a | 25.0a | 8.9a | 5.6a | 1.8ab | |
| 5 | 17.3c | 10.8bc | 19.9c | 7.3b | 3.9b | 1.3cd | |
| Salinity (60 mM) | 0 | 13.0d | 8.0d | 12.42d | 4.0d | 2.4d | 1.1d |
| 1 | 18.0c | 10.0bc | 20.47c | 7.7ab | 4.4b | 1.5abc | |
| 3 | 20.0b | 10.9bc | 19.0b | 7.2b | 4.1bc | 1.5abcd | |
| 5 | 10.0e | 9.4cd | 16.3e | 5.6c | 3.0c | 1.2cd | |
| ANOVA (F Value) | |||||||
| Salinity (S) | *** | *** | *** | *** | *** | *** | |
| Foliar (F) | *** | *** | *** | *** | *** | *** | |
| Salinity*foliar | ** | ns | ** | ns | ns | ns | |
Each value is a mean of three replicates; different alphabetic letters indicate significant differences (P ≤ 0.05) among treatments, * and *** indicate significant at p ≤ 0.05 and p ≤ 0.001 respectively; ns indicate non-significant difference.
The foliar application of NBS solution also accelerated yield characteristics of tomato under both stressed and non-stressed conditions (Table 2).
Table 2.
The effect of foliar application of NBS on yield attributes of tomato under salt stress.
| Condition | Foliar (%) | Number of leaves (n) | Number of flowers (n) | Number of fruits(n) | Fruit weight per plant (g) |
|---|---|---|---|---|---|
| Control | 0 | 14.3bc | 9.6cde | 4.0bc | 11.2c |
| 1 | 17.0a | 15.0a | 5.0ab | 15.1b | |
| 3 | 13.0cd | 11.3bc | 6.0a | 17.6a | |
| 5 | 11.6de | 8.0e | 4.0bc | 13.6b | |
| Salinity | 0 | 11.3de | 9.0be | 3.0c | 6.6d |
| 1 | 15.3ab | 13.3ab | 4.0bc | 10.1c | |
| 3 | 12.6cde | 10.6cd | 5.0ab | 13.5b | |
| 5 | 10.6e | 7.6e | 3.0 c | 9.4c | |
| ANOVA (F_Value) | |||||
| Salinity (S) | *** | *** | *** | *** | |
| Foliar (F) | *** | ** | *** | *** | |
| Salinity*foliar | * | ns | ns | ns | |
Each value is a mean of three replicates; different alphabetic letters indicate significant differences (P ≤ 0.05) among treatments, * and *** indicate significant at p ≤ 0.05 and p ≤ 0.001 respectively; ns indicate non-significant difference.
It is noted that only salt treated plants exhibited reduction in yield attributes compared to control plants like the number of leaves reduced by 20.9%, flowers by 6.25%, fruits by 25% and fruit weight per plant by 41% as compared to plants which are not supplemented with salt. However, 1% NBS application increased the number of leaves by 19.2% and 35.3%, number of flowers by 56% and 47.7% under non-stressed and stressed plants, respectively compared to their respective control. While 3% NBS application exerted stimulatory effect on number of fruits as fruit no increased by 50% and 25%, and fruit weight per plant was increased by 57% and 1.04 fold, under non-stressed and stressed plants, respectively compared to respective control plants. Figure 1a, b showed the effects of different concentrations of NBS on plant morphology, fruit size and number under non-stressed and stressed conditions. These results suggest that NBS at 1% and 3% concentration exerted significant positive effects on plant growth, biomass and fruit count.
Fig. 1.
Effect of different concentrations of NBS on (a) plant morphology, fruit size and number without stress (b) with stress NBS; nanobiochar solution, S; salinity (60 mM).
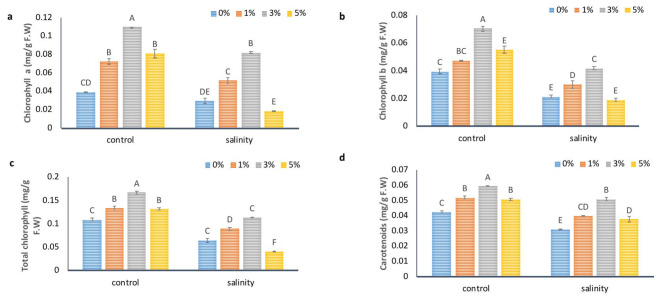
Foliar application of NBS improved photosynthetic pigments
In order to further investigate the association of NBS with photosynthesis, photosynthetic pigments were analyzed. Data in Fig. 2 showed the response of foliar application of NBS solution on plant photosynthetic pigments under non-stressed and stressed conditions. As salinity had a considerable negative effect on tomato leaf pigments. In salt stress, chlorophyll a was reduced by 25%, chlorophyll b by 46%, total chlorophyll by 40%, and carotenoids content by 26.73% compared to control plants (Fig. 2a, b, c, d). But exogenous application of 3% NBS enhanced the plant pigments such as chlorophyll a by 1.76 fold and 1.82 fold, chlorophyll b by 79 and 95%, total chlorophyll by 53 and72%, and carotenoids contents by 40and 64% under non-stressed and stressed plants, respectively compared to their respective control plants. These results clearly suggest that 3% NBS application significantly improved the photosynthetic machinery which ultimately improved the growth and yield attributes of tomato plants.
Fig. 2.
The effect of different concentrations of foliar application of NBS on leaf pigments (a) chlorophyll a, (b) chlorophyll b, (c) total chlorophyll and (d) carotenoid contents in leaves of tomato under salinity. Mean values are the average of three replicates. The upper case alphabetic letters indicate significant differences (P ≤ 0.05) among treatments using Tukey’s test.
Foliar NBS improved the plasma membrane stability in salt stressed plants
As salinity stress is well known for membrane system destructions, ionic toxicity as well as increase in membrane permeability which leads towards high relative conductivity in plants. To evaluate the foliar applied NBS efficiency on plant plasma membrane stability under stressed conditions, various parameters such as leaf RWC%, EL% as well as MDA contents were investigated (Fig. 3a, b, c). The leaf water status of the tomato plant was significantly influenced by salt stress. Salt stress reduced the leaf RWC (%) by 54% as compared to control plants. But 1% NBS application raised the water content by 69% and 1.78 fold while 3% NBS increased the RWC(%) by 1.46 fold and 2.55 fold under non-stressed and stressed plants, respectively when compared to their respective control groups. Further, salt stress increased the EL(%) by 32% compared to control plants. Moreover, foliar applied 3% NBS significantly reduced the EL (%) by 28% under non-stressed and stressed plants, respectively compared to their respective control samples.
Fig. 3.
The effect of different concentrations of foliar application of NBS on membrane stability parameters (a) relative water content (b) Electrolyte leakage % (c) Malondialdehyde contents in leaves of tomato under salinity. Mean values are the average of three replicates. The upper case alphabetic letters indicate significant differences (P ≤ 0.05) among treatments using Tukey’s test.
Since MDA is a byproduct of membrane lipid peroxidation and is regarded as a reliable indicator of oxidative stress, higher MDA contents indicate greater levels of oxidative stress. In this study, salinity stress increased the MDA contents by 39% as compared to the control. But foliar application of 5% NBS greatly reduced the MDA contents by 46% and 29% under non-stressed and stressed conditions. MDA contents decreased gradually with increasing concentration of NBS under both conditions. These results suggests, that NBS application stabilized the plasma membrane via lowering lipid peroxidation rate, reduced leakage of electrolytes as well as improvement in leaf water status.
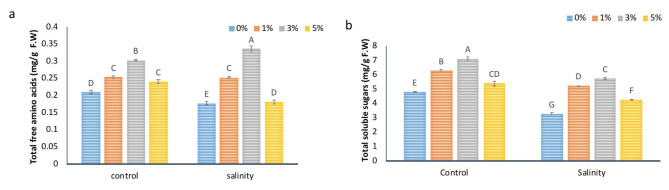
NBS application altered the levels of plant metabolites
To further evaluate the influence of NBS on plant metabolism under stressed and non-stressed conditions various metabolites contents were calculated such as total free amino acids, total soluble sugars, total phenolics contents and flavonoids contents. Data in Fig. 4a, b showed the effect of NBS foliar application on plant primary metabolites such as free amino acids and soluble sugars. It is noted that the total amount of soluble sugars and amin acids slightly decreased in salt stressed plants, but significant improvement was noticed when treated with NBS. In salt stress, the contents of total soluble sugars and total free amino acids were reduced by 31% and 45%, respectively, as compared to control plants The foliar application of NBS, on the other hand, showed a rising tendency for both soluble sugars and total free amino acids. At 3% NBS, total soluble sugars increased by 48% and 75% under non-stressed and stressed plants, respectively. Further, total free amino acids contents were checked. Salt stress decreased the total free amino acids by 15% however, 3% NBS application raised levels of free amino acids by 42% and 44% under non-stressed and stressed plants, respectively as compared to their respective control groups.
Fig. 4.
The effect of different concentrations of foliar application of NBS on primary metabolites in leaves of tomato under salinity. Mean values are the average of three replicates. The upper case alphabetic letters indicate significant differences (P ≤ 0.05) among treatments using Tukey’s test.
NBS application also altered the contents of plant secondary metabolites as shown in Fig. 5a, b. Salt stress decreased the total phenolics and flavonoids contents by 44% and 43% as compared to control plants, respectively. But 3% NBS application improved the phenolics contents by 71% and 2.76 fold as well as flavonoids contents by 1.29 fold and 3.5 fold under non-stressed and stressed plants, respectively compared to their respective control samples. NBS application rapidly increased the levels of secondary metabolites especially under salt stress conditions. These results strongly suggested that NBS application worked very well at all basic cellular levels of plant growth, as healthy plant metabolism and rapid production of primary and secondary metabolites lead towards stress tolerance in plants.
Fig. 5.
The effect of different concentrations of foliar application of NBS on secondary metabolites (a) phenolics (b) flavonoids in leaves of tomato under salinity. Mean values are the average of three replicates. The upper case alphabetic letters indicate significant differences (P ≤ 0.05) among treatments using Tukey’s test.
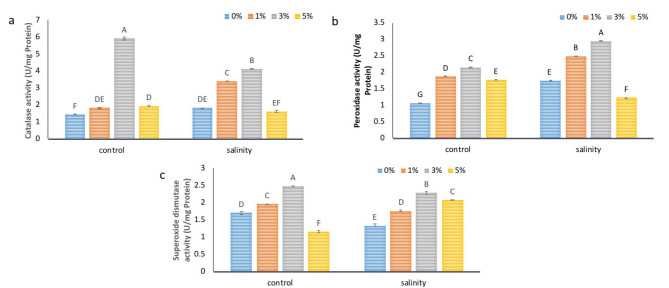
NBS application improved the antioxidant defense system in tomato
Different antioxidant enzyme activities were checked to further evaluate the roles of NBS application in plant defense, especially ROS scavenging capability (Fig. 6a, b,c). Data showed that salt stress increased the CAT and POD enzyme activities by 27% and 63% while SOD activities decreased by 21% as compared to control plants, respectively. Meanwhile, 3% NBS application increased the CAT activity by 3.11 fold and 1.26 fold, POD activity by 1.01 fold and 68%, and SOD activity was increased by 45% and 70% under non-stressed and stressed plants, respectively compared to their respective controls. 1% NBS and 5% NBS application also improved the antioxidant enzyme activities as compared to non-NBS treated plants under both conditions which clearly showed the positive role of NBS in activation of antioxidant defense system in plant defense against stress conditions.
Fig. 6.
The effect of different concentrations of foliar application of NBS on antioxidant enzyme activities (a) catalase (b) peroxidase (c) superoxide dismutase in leaves of tomato under salinity. Mean values are the average of three replicates. The upper case alphabetic letters indicate significant differences (P ≤ 0.05) among treatments using Tukey’s test.
Discussion
Application of NBS may benefit crop production by altering the ability of soil to hold water, fostering biotic interactions as the foliar application of NBS is another sources of macro and micronutrients supplementation to the plants. Due to its capacity to offer prospective and quick solutions essential for sustainable agriculture, the application of nanotechnology in agriculture is a subject that is rapidly evolving, has gained significant ground recently29,30. In general, nanoparticles are prospective materials for the site-specific delivery of nucleotides, proteins, and chemicals to accomplish important objectives including enhancing crop growth and yield as well as resistance to stressful signals45. Reported data suggests that adding nanoparticles to plants can considerably reduce the negative impacts of certain severe circumstances, such as salt stress, and hence control plant adaptations32,46.
As nano-carbon is the major constituent of this NBS solution, and it adsorb nitrogen from ammonia as well as release hydrogen ions which enhance water and mineral absorption of plants. Due to this phenomenon, nutrients uptake such as N, P and K can be increased which has been proven by field experiment in which interactive application of N and nano-carbon boosted the quality and output of rice45,47. In terms of reducing salt stress, NBS have thus far provided encouraging results23,24. In this study, we summaries recent ground-breaking progresses in the development of salt stress tolerance in tomato plants using NBS.
Abiotic stresses including salinity stress showed negative effects reduced the growth and yield parameters of plant. Findings of the current study demonstrated that the growth metrics including the height of the plant, the length of the shoots and roots, the fresh and dry weights of the shoots and roots, the quantity of leaves, flowers, and fruits, and the weight of the fruits per plant were decreased under the salt stressed condition. While an increase in these parameters have been observed by the foliar application of NBS at the levels of 1%, 3%, and 5% under both stressed and non-stressed conditions. Although, 3% NBS showed maximum increase in plant height, root length, and shoot length. A decrease in the plant FW, root length, biomass, number of leaves, yield, stem length and root length of tomato plant has been reported in response to salinity45,46. And the amendments of soil with biochar increased a number of characteristics in tomato plant, including plant height, number of leaves, fresh and dried weights of both above- and below-ground plant sections, and leaf relative water content (LRWC)48,49.
Our results are in accordance to the findings of above described researchers. In another study, fertigation and foliar application of three nano-fertilizers revealed higher number of leaves and flowers by the application of 1% NBS while 3% NBS exhibited an increase in fruit weight per plant and fruit number per plant in tomato plants grown in saline environment50. Furthermore, carbon nanotubes also altered the lipid content, flexibility, and permeability of the root plasma membranes which resulted in greater aquaporin transduction under salt-stressed conditions48.
The current study revealed that reduction in photosynthetic pigments in tomato under salt stress were significantly improved by the foliar application of NBS, although an increase in pigments was also observed in non-stressed plants under the influence of NBS application. These results are in accordance to the findings of51, they worked on olive and reported an increase in total chlorophyll, chlorophyll a and b, and total soluble sugars by the application of BNP. An increase in carotene in fruits of tomato plants was observed under the effect of CuNPs52. These findings strongly suggest that carbon based nano-biochar has ability to dense the stomata and longer roots which in turns enhance water absorption and mineral uptake in plants. It has been proven by a latest report in which multiwalled carbon nano-tubes increased the chlorophyll contents and photosynthetic activity in rice53,54.
Previously, Nano materials application have both positive and negative effects on different plant species depends on nature, concentration and type of plants. Therefore, the effects of NBS on membrane stability indices were investigated. Results of this study showed that a decrease in electrolyte leakage was observed by the foliar treatment at 5% NBS, while, relative water content increased at 3% NBS in both the stressed and non-stressed tomato plants. These results are in accordance to the reports of Sassine50et al., (2020), who worked on tomato plants and experienced more cellular leakage under the effect of nanofertilizer in saline condition.
Similar outcomes were exhibited under the impact of carbon nanotubes (MWCNTs) on broccoli grown in saline regimes where the rate of photosynthesis and water uptake increased51. It might be due to fact that, NBS has ability to produce more pores in cell wall and plasma membrane which can enhance water transport as well as transduction of aquaporins to alleviate harmful effects of salt stress.
Application of NBS exhibited an increase in amino acids and total soluble sugars in stressed and non-stressed tomato plants, although maximum value was observed under the effect 3%NBS. The increased protein content may result from the up-regulation of N metabolism-related enzymes and better photosystems I and II photosynthetic efficiency55. Similarly, Shafiq et al., (2019)56 found that pretreatment of wheat seedlings with polyhydroxy fullerene NPs decreased salt stress by elevating antioxidant activities, amino acids, chlorophyll content, soluble sugars, and K+ and P contents. As stress increased the ROS production in plants, therefore to cope with its deleterious effects, phenolic compounds synthesis, activation of PAL enzymes (phenylalanine ammonium lyase) and flavonoids are major processes which are promoted by NB application57. According to the current findings, the phenolic and flavonoids were increased in stressed tomato plants than the non-stressed plants. Although, the maximum flavonoid content in leaves were observed at 1% NBS, while, the total phenolic were maximum in plants when treated with 3% NBS, foliarly. Similar to this, potassium treatment has been shown to improve the quality of figs by significantly increasing the antioxidant activity and phenolic components in fig fruit58. Khan (2016)59 in addition to total phenols demonstrated that these NPs boost SOD and POX activity in tomato grown under salt stress59,60 In compliment to these results, Guerrero et al., (2017)61 reported that CuNPs adsorbed on CsPVA raised the overall phenol and flavonoid content in jalapeo peppers.
Antioxidant enzyme activity enhanced under stress condition62, similar findings were observed in the current study that a considerably higher POD and CAT activity, while a slight decrease in SOD activity was recorded in stressed tomato plants as compared to the non-stressed plants. The activity of above said antioxidants increased in 3% of a NBS treated tomato plants under saline environment. These results are in agreement to the findings of Hernandez et al., (2018)52 as they have observed an increasing expression of SOD and jasmonic acid (JA) genes led to reduced ionic and oxidative stress when CuNPs were tested on tomato plants under salt stress.
In Helianthus annuus, the foliar supply of FeNPs boosted the activities of polyphenol oxidase, CAT, and POD when grown under saline environment61. Moreover, application Fe3O4 and ZnONPs on the leaves of Moringa reduced the deleterious effects of salt stress by increasing the activity of certain enzymes63. It can be corelated at transcriptomics level as DRE/CRT is a well knows drought responsive Cis-element in DREB transcription factor family which showed upregulation under abiotic stresses and application of nano-particles64. Intriguingly, it was noted that NBS at low concentrations such as 1% and 3% significantly enhanced the plant secondary metabolites and antioxidant enzyme activities. It might be due to fact that C nanoparticles in low concentrations protected the tomato plants from salt stress, maintained the levels of ROS and other neutralizing factors and stimulates the antioxidant defense system65. Foliar application of NBS showed significant effect on malonaldehyde(MDA) content under salinity as 5% NBS reduced the level of MDA in leaves of stressed and non-stressed tomato plants. Similar results were found by the application of TiO2 nanoparticles (rutile phase), they improved oxidative stress tolerance by modifying a number of processes, including the formation of superoxide radicals, hydrogen peroxide, MDA content, and also induced antioxidant enzymes activities i.e. superoxide dismutase, catalase, ascorbate peroxidase, and guaiacol peroxidase on the photochemical reaction of chloroplasts of Spinacia oleracea plants64. Lipid peroxidation, membrane degradation are harmful effects of ROS66. The production of ROS should be controlled, not only to prevent the injurious effects of ROS but also to ensure proper execution of their signaling functions67.
A promising method for improving soil health and remediating contaminated soil is nanobiochar. It is necessary to carefully evaluate its possible effects on the environment, especially the mobility and deposition of nanoparticles in soil. Even though it can increase microbial activity and immobilize heavy metals. But, its transportation and effects on soil microbial communities needed careful management techniques and continued research. According to Chen et al. (2017)68, biochar nanoparticles can migrate pollutants throughout the soil profile, which may harm groundwater. Because of its special qualities and uses, nano black carbon can be applied as a soil amendment, suspending agent, and nano fertilizer in a variety of environmental platforms69. Compared to regular biochar, nanobiochar has a stronger ability to migrate, improving soil texture, moisture, and flow rate but getting weaker with depth70. According to Mahmoud et al. (2024)71, nanobiochar provides an environmentally friendly platform for crop improvement while also greatly enhancing soil health and maize production72. Compared to pure biochar, nanobiochar-based composites are far more successful at improving soil for heavy metal remediation73. However, there are a number of advantages to using nanobiochar for agricultural productivity and soil remediation. The long-term effects must be thoroughly investigated in order to create safe and efficient soil application techniques.
Conclusion
Our study findings indicated that foliar application of NBS at low concentrations such as 1% and 3% positively contributed towards the development of various growth and yield attributes in tomato by stabilizing the plasma membrane, improved water uptake and photosynthetic pigments, by production of total free amino acids, sugars, total phenolics and flavonoids both under stressed and non-stressed environments. Higher production of primary and secondary metabolites further supported the ROS scavenging systems by activating antioxidant enzymes. NBS foliar applied plants also exhibited less lipid peroxidation rates compared to non-NBS treated plants under stress conditions. Thus we believe that Despite the potential benefits of nanomaterials, their effects on plants require thorough evaluation as nano particles in higher concentrations may exert negative effects on all levels of ecosystem. Currently, several researchers are focusing on the interaction of carbon based nano materials with plant systems; however, this field is still in the nascent stage, and further research is required to understand the molecular mechanisms that influence plant growth and toxicity. Despite the potential benefits of nanomaterials, their effects on plants require thorough evaluation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R393) of King Saud University, Riyadh, Saudi Arabia.
Abbreviations
- MDA
Malonaldehyde
- JA
Jasmonic acid
- SOD
Superoxide dismutase
- NBS
Nanobiochar colloidal solution
- ROS
Reactive oxygen species
- BC
Biochar
- NB
Nanobiochar
- NPs
Nanoparticles
- SA
Salicylic acid
- RWC
Relative water content
- EU
Enzyme unit
Author contributions
[JS], [ZN], [NA], idea formulation and design the experiment. [MYA], [CC] added valuable suggestions during experiment and manuscript improvement. [AAS], [ZN], contributed various inputs during the manuscript preparation and supervision. [MI], [NA], performed data analysis and design the manuscript. Validation, funding contributed by [SS]. [MI] involved in drafting article, revision, arranged the figures, tables and manuscript description. [MKG] validation, reviewing, funding, drafting. All the authors read and approved the finalized manuscript for publication.
Funding
Researchers supporting the project (RSP2025R393) at King Saud University, Riyadh, Saudi Arabia.
Data availability
All data is presented in this manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors give permission to the publisher to publish this research work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zaib-un-Nisa, Email: zaib.nisa@imbb.uol.edu.pk.
Anis Ali Shah, Email: anisalibot@gmail.com.
References
- 1.Machado, R. M. A. & Serralheiro, R. P. Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae3, 30 (2017). [Google Scholar]
- 2.Elkelish, A. A., Soliman, M. H., Alhaithloul, H. A. & El-Esawi, M. A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem.137, 144–153 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Hafez, Y. et al. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy10, 630 (2020). [Google Scholar]
- 4.Chawla, S., Jain, S. & Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L). J. Plant. Biochem. Biotechnol.22, 27–34 (2013). [Google Scholar]
- 5.Hasanuzzaman, M. et al. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol. Biochem.126, 173–186 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Roy, S. J., Negrão, S. & Tester Salt resistant crop plants. Curr. Opi N Biotech.26, 115–124 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Suo, J., Zhao, Q., David, L., Chen, S. & Dai, S. Salinity response in chloroplasts: insights from gene characterization. Int. J. Mol. Sci.18, 1011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chukwu, L. I., Ano, A. O. & Asawalam, D. O. Effects of poultry manure and NPK fertilizer on soil properties and nutrient uptake of maize (Zea mays L.) plants growth in an Ultisol. in Proceedings of the 36th Annual conference of the Soil Science Society of Nigeria (SSSN) on 7th–11th March (2012).
- 9.Liu, Z. et al. Emergy-based indicators of the environmental impacts and driving forces of non-point source pollution from crop production in China. Ecol. Indic.121, 107023 (2021). [Google Scholar]
- 10.Abiven, S., Schmidt, M. W. I. & Lehmann, J. Biochar by design. Nat. Geosci.7, 326–327 (2014). [Google Scholar]
- 11.Zhang, Z., Wang, Q., Wang, H., Nie, S. & Liang, Z. Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (GRSP). Sci. Total Environ.581–582, 657–665 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Chang, R., Sohi, S. P., Jing, F., Liu, Y. & Chen, J. A comparative study on biochar properties and cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut.254, 113123 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Antala, M., Sytar, O., Rastogi, A. & Brestic, M. Potential of karrikins as novel plant growth regulators in agriculture. Plants9, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei, W. et al. Biochar effects on crop yields and nitrogen loss depending on fertilization. Sci. Total Environ.702, 134423 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Wang, D., Zhang, W., Hao, X. & Zhou, D. Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environ. Sci. Technol.47, 821–828 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Noreen, S. & Abd-Elsalam, K. A. Biochar-based nanocomposites: A sustainable tool in wastewater bioremediation. in Aquananotechnology 185–200Elsevier, (2021).
- 17.Akhter, A., Hage-Ahmed, K., Soja, G. & Steinkellner, S. Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycopersici. Front. Plant Sci.6, 529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, R. & Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ.514, 131–139 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Khaliq, H. et al. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. J. Plant. Growth Regul.42, 715–3729 (2023). [Google Scholar]
- 20.Mehr-un-Nisa, S. F. et al. Physiological effects of some engineered nanomaterials on radish (Raphanus sativus L.) intercropped with pea (Pisum sativum L). Environ. Sci. Pollut Res.30, 78353–78366 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Mubashir, A. et al. Effect of foliar application of nano-nutrients solution on growth and biochemical attributes of tomato (Solanum lycopersicum) under drought stress. Front. Plant Sci.13, 1066790 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tourajzadeh, O., Piri, H., Naserin, A. & mahdi Cahri, M. Effect of nano biochar addition and deficit irrigation on growth, physiology and water productivity of quinoa plants under salinity conditions. Environ. Exp. Bot.217, 105564 (2024). [Google Scholar]
- 23.Yousaf, W. et al. Supplementation of nano-biochar improved growth and physiological attributes in wheat seedlings exposed to salt stress through enhanced activity of hydrolysing and nitrogen metabolic enzymes and regulation of crucial metabolites. South. Afr. J. Bot.167, 500–508 (2024). [Google Scholar]
- 24.Mehmood, H. M. et al. Synergistic effects of soil and foliar nano-biochar on growth, nitrogen metabolism and mineral uptake in wheat varieties. J. King Saud University-Science. 36(9), 103392 (2024). [Google Scholar]
- 25.Savić, S. et al. Comparative effects of regulated deficit irrigation (RDI) and partial root-zone drying (PRD) on growth and cell wall peroxidase activity in tomato fruits. Sci. Hortic.117, 15–20 (2008). [Google Scholar]
- 26.Murshed, R., Lopez-Lauri, F. & Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol. Mol. Biology Plants19, 363–378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggio, A., De Pascale, S., Fagnano, M. & Barbieri, G. Saline agriculture in Mediterranean environments. Italian J. Agron.6, 7 (2011). [Google Scholar]
- 28.Li, Z. et al. A tomato ERF transcription factor, SlERF84, confers enhanced tolerance to drought and salt stress but negatively regulates immunity against Pseudomonas syringae Pv. Tomato DC3000. Plant Physiol. Biochem.132, 683–695 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Zulfiqar, F. & Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem.160, 257–268 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Hofmann, T. et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food1, 416–425 (2020). [Google Scholar]
- 31.Girma, K. et al. Determination of optimum rate and growth stage for Foliar-Applied phosphorus in corn. Commun. Soil. Sci. Plant. Anal.38, 1137–1154 (2007). [Google Scholar]
- 32.Khan, I. et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant. Physiol. Biochem.156, 221–232 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Khaliq, B., Sarwar, H., Akrem, A., Azam, M. & Ali, N. Isolation of napin from Brassica nigra seeds and coagulation activity to turbid pond water. Water Supply22, 6050–6058 (2022). [Google Scholar]
- 34.Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.24, 1 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida, F., Shimizu, S. & Kohno, H. Mineral nutrition, of cultured chlorophyllous cells of tobacco VII. Effects of the NH4/NO3 ratio and of cl in the media on the growth and ionic balance of cells. Plant Cell Physiol.21(6), 1095–1107 (1980). [Google Scholar]
- 36.Barr, H. D. & Weatherley, P. E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J. Biol. Sci.15, 413–428 (1962). [Google Scholar]
- 37.Lutts, S., Kinet, J. M. & Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativaL.) Cultivars differing in salinity resistance. Ann. Bot.78, 389–398 (1996). [Google Scholar]
- 38.Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.125, 189–198 (1968). [DOI] [PubMed] [Google Scholar]
- 39.Hamilton, P. B. & Van Slyke, D. D. The gasometric determination of free amino acids in blood Filtrates by the Ninhydrin-Carbon Dioxide Method. J. Biol. Chem.150, 231–250 (1943). [Google Scholar]
- 40.Riazi, A., Matsuda, K. & Arslan, A. Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J. Exp. Bot.36, 1716–1725 (1985). [Google Scholar]
- 41.Pękal, A. & Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods7, 1776–1782 (2014). [Google Scholar]
- 42.Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem.33(2), 213–217 (1985). [Google Scholar]
- 43.Chance, B. & Maehly, A. C. [136] assay of catalases and peroxidases. 764–775. 10.1016/S0076-6879(55)02300-8 (1955).
- 44.Dhindsa, R. S., Plumb-Dhindsa, P. A. M. E. L. A. & Thorpe, T. A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot.32, 93–101 (1981). [Google Scholar]
- 45.Rastogi, A. et al. Application of silicon nanoparticles in agriculture. 3 Biotech.9, 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad, I. & S Akhtar, M. Use of nanoparticles in alleviating salt stress. Salt Stress Microbes Plant. Interactions: Causes Solut.199-215. (Springer, 2019). [Google Scholar]
- 47.Wu, M-Y. Effects of incorporation of nano-carbon into slow-released fertilizer on rice yield and nitrogen loss in surface water of paddy soil. In: 2013 Third International Conference on Intelligent System Design and Engineering Applications. Geneva: IEEE; pp. 676–81. (2013).
- 48.Hossain, M. K., Strezov, V., Chan, K. Y. & Nelson, P. F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere78, 1167–1171 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Liu, Z. et al. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena (Amst). 123, 45–51 (2014). [Google Scholar]
- 50.Sassine, Y. N. et al. Mitigation of salt stress on tomato crop by using foliar spraying or fertigation of various products. J. Plant. Nutr.43, 2493–2507 (2020). [Google Scholar]
- 51.Hegazi, E. S., El-Motaium, R. A., Yehia, T. A. & Hashim, M. E. Effect of foliar boron application on boron, chlorophyll, phenol, sugars and hormones concentration of olive (Olea europaea L.) buds, leaves, and fruits. J. Plant. Nutr.41, 749–765 (2018). [Google Scholar]
- 52.Hernández-Hernández, H. et al. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules23, 178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Ballesta, M. C., Chelbi, N., Lopez-Zaplana, A. & Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant. Physiol. Biochem.146, 23–30 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Joshi, A. et al. Plant nanobionic effect of multi-walled carbon nanotubes on growth, anatomy, yield and grain composition of rice. BioNanoScience (2020).
- 55.Huang, M., Yin, X., Chen, J. & Cao, F. Biochar application mitigates the efect of heat stress on rice (Oryza sativa L.) by regulating the root-zone environment. Front. Plant. Sci.12, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shafiq, F., Iqbal, M., Ali, M. & Ashraf, M. A. Seed pre-treatment with polyhydroxy fullerene nanoparticles confer salt tolerance in wheat through upregulation of H 2 O 2 neutralizing enzymes and phosphorus uptake. J. Soil. Sci. Plant. Nutr.19, 734–742 (2019). [Google Scholar]
- 57.Martínez-Ballesta, M., Zapata, L., Chalbi, N. & Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol.14, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaaliche, B., Ladhari, A., Zarrelli, A. & Mimoun, M. B. Impact of foliar potassium fertilization on biochemical composition and antioxidant activity of fig (Ficus carica L). Sci. Hortic.253, 111–119 (2019). [Google Scholar]
- 59.Khan, M. N. Nano-titanium dioxide (nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill). J. Plant. Sci.11, 1–11 (2016). [Google Scholar]
- 60.Martinez, V. et al. Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules23, 535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinedo-Guerrero, Z. H. et al. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules22, 926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torabian, S., Farhangi-Abriz, S. & Zahedi, M. Efficacy of FeSO 4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Indian J. Plant. Physiol.23, 305–315 (2018). [Google Scholar]
- 63.Soliman, A. S., El-feky, S. A. & Darwish, E. Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J. Hortic. For.7, 36–47 (2015). [Google Scholar]
- 64.Lei, Z. et al. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res.121, 69–79 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Akbudak, M. A., Filiz, E. & Kontbay, K. DREB2 (dehydration-responsive element-binding protein 2) type transcription factor in sorghum (Sorghum bicolor): Genome-wide identification, characterization and expression profiles under cadmium and salt stresses. 3 Biotech 8, 426. (2018). [DOI] [PMC free article] [PubMed]
- 66.El Ghazali, G. E. Suaeda Vermiculata Forssk. Ex JF Gmel.: structural characteristics and adaptations to salinity and drought: a review. Int. J. Sci.9, 28–33 (2020). [Google Scholar]
- 67.Hasanuzzaman, M. & Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res.143, 1758–1776 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Chen, M. et al. Transport and retention of biochar nanoparticles in a paddy soil under environmentally-relevant solution chemistry conditions. Environ. Pollut.230, 540–549 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Lian, F. & Xing, B. From Bulk to Nano: Formation, Features, and Functions of Nano-Black Carbon in Biogeochemical Processes (Environmental science & technology, 2024). [DOI] [PubMed]
- 70.Li, P. et al. Migration rules and mechanisms of Nano-Biochar in Soil columns under various transport conditions. Nanomaterials14. (2024). [DOI] [PMC free article] [PubMed]
- 71.Mahmoud, E. et al. Enhancing Maize Yield and Soil Health through the Residual Impact of Nanomaterials in Contaminated Soils to Sustain Food. Nanomaterials, 14. (2024). [DOI] [PMC free article] [PubMed]
- 72.Zörb, C., Geilfus, C. M. & Dietz, K. J. Salinity and crop yield. Plant. Biol.21, 31–38 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Mandal, S. et al. Progress and future prospects in biochar composites: application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol.51, 219–271 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is presented in this manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.