Abstract
This study aimed to investigate the role of myosteatosis, sarcopenia, and perioperative serum biomarkers as independent predictors of major complications within 180 days following radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC). We retrospectively analyzed of 127 MIBC patients who underwent RC between 2013 and 2023 at a single institution. Preoperative body composition was assessed using CT scans at the L3 vertebral level to measure psoas muscle density (PMD), skeletal muscle density (SMD), axial muscle density (AMD), and muscle indices. Novel inflammatory and nutritional markers, including serum chloride levels within 24 h post-surgery, were also evaluated. Major complications were defined as Clavien-Dindo grade ≥ 3. Multivariate analysis was performed to identify independent predictors of postoperative complications. Among the cohort, 30.7% of patients experienced major complications within 90 days, and 36.2% within 180 days. Sepsis was the most common major complication (19 of 241 complications, 7.9%). Ten patients died during the observation period. Myosteatosis (p = 0.002) and postoperative serum chloride levels (p < 0.001) were significant independent predictors of 180-day major complications. Patients with low PMD had an adjusted odds ratio (OR) of 3.959 for developing major complications, while increased serum chloride levels were associated with a reduced risk of complications (OR = 0.985). Multivariate analysis reveals associations between myosteatosis, aging, and anemia. Myosteatosis and perioperative serum chloride levels are significant predictors of major complications after RC for MIBC. Incorporating body composition analysis and early serum chloride monitoring into perioperative care may improve risk stratification and patient outcomes following RC.
Keywords: Myosteatosis, Skeletal muscle, Cystectomy, Serum chlorides, Postoperative complications
Subject terms: Bladder, Risk factors, Cancer imaging, Bladder cancer
Introduction
Bladder cancer is the 10th most commonly diagnosed cancer globally and the 13th leading cause of cancer-related mortality1. Muscle-invasive bladder cancer (MIBC) presents significant challenges due to its aggressive nature, with about 25% of patients either presenting with or progressing to this advanced stage2. Radical cystectomy (RC) remains the standard treatment for patients with MIBC, involving complete bladder removal and creation of either an external urinary diversion or a continent urinary reservoir. Despite its curative potential, RC is associated with substantial postoperative complications, with morbidity and mortality rates ranging between 40 and 75%, particularly in older individuals3–5.
Several studies have highlighted the importance of identifying risk factors to tailor treatment strategies for these patients. Sarcopenia, characterized by reduced muscle mass has been shown to predict poor cancer prognosis and survival6,7. However, emerging evidence suggests that muscle quality, particularly myosteatosis (fat infiltration into skeletal muscle), may be an even stronger predictor of adverse surgical outcomes. Myosteatosis has been associated with poorer survival in several cancers, though its role in bladder cancer remains underexplored8–10.
In addition to body composition, novel serum biomarkers representing inflammatory and nutritional status—such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI), and prognostic nutritional index (PNI) — have gained attention as potential predictors of postoperative complications11–15. Among these, serum chloride levels have emerged as significant perioperative markers, with studies suggesting that both hypo- and hyperchloremia can affect surgical outcomes16–20. Combining these serum markers with imaging biomarkers like sarcopenia and myosteatosis could offer a more comprehensive preoperative risk stratification approach for MIBC patients.
Given that preoperative CT scans and serum biomarkers analyses are routinely used for MIBC patients, incorporating these novel predictors into preoperative evaluation can enhance risk assessment. This study aims to assess the predictive value of preoperative sarcopenia, myosteatosis, along with novel serum biomarkers in forecasting major complications using standardized reporting methods. Understanding these relationships could lead to improved patient counseling and personalized prehabilitation strategies, ultimately reducing postoperative complications.
Materials and methods
Study design and patients
This retrospective, single-center cohort study was conducted at Siriraj Hospital, Mahidol University, Bangkok, Thailand, over a 10-year period (January 2013 to June 2023). The study included 127 patients with MIBC who underwent RC. Patients receiving neo-adjuvant cisplatin-based chemotherapy or immuno-oncology (IO) treatments were also included. Exclusions criteria comprised patients lacking a preoperative abdominal CT scan within 60 days of surgery, those diagnosed with non-urothelial cancers, patients receiving adjuvant chemotherapy, IO, or radiation within 180 days postoperatively, and those lost to follow-up before the 180-day postoperative period. Routine follow-ups were conducted at 1, 3, and 6 months post-surgery. This study was approved by the Siriraj institutional Review Board. (SIRB 828/2565(IRB1)). Due to the retrospective nature of the study, the Siriraj institutional Review Board waived the need of obtaining informed consent. All methods in this study were conducted in compliance with the relevant guidelines and regulations outlined by the SIRB.
Data collection
Demographic and clinical data were retrieved from medical records, including gender, age at surgery, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, age-adjusted Charlson comorbidity index (ACC index), neoadjuvant treatment (NAT) status, surgical approach (open, laparoscopic or robotic), and type of urinary diversion (cutaneous ureterostomy, ileal conduit, or neobladder). Postoperative complications were categorized using the Clavien-Dindo classification (CDC), with major complications defined as grade 3 or higher. Length of hospital stay (LOS) and intensive care unit (ICU) stay duration were also recorded. Serum chloride levels were measured upon admission and within 24 h post-surgery.
Image analysis of body composition
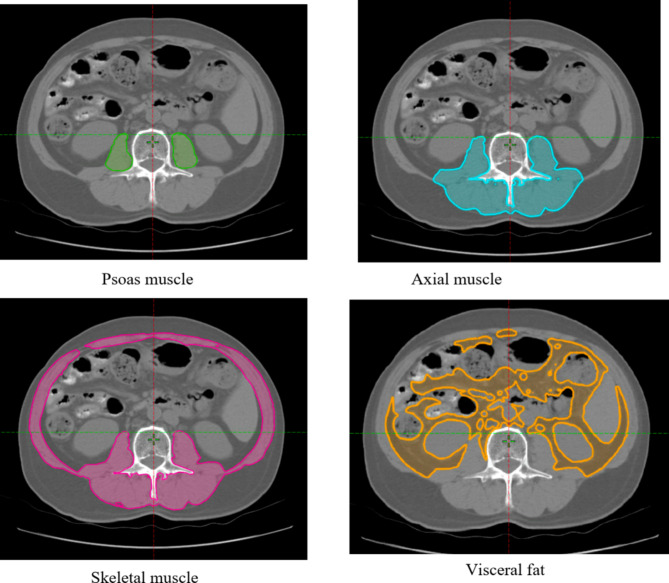
Preoperative non-contrast abdominal CT images were analyzed to assess body composition parameters. Two trained urologists, blinded to patient outcomes, conducted the image analyses using the ARIA Oncology Information System (Varian medical system, Inc., CA, USA), under the supervision of a certified radiologist. The regions of interest included the psoas, axial, skeletal muscles, and visceral fat. These areas were manually outlined on axial CT images at the L3 vertebral level. The software automatically calculated muscle area (in cm2) and muscle density (in Hounsfield units, HU). Sarcopenia was assessed by normalizing the muscle area to patient height (cm2/m2). Myosteatosis was evaluated by measuring the mean muscle density (HU) at the L3 level. (Fig. 1). The outcomes from the two urologists were averaged, and this value was used for further analysis.
Fig. 1.
Measurement of muscle area and density on axial non-contrast computed tomography at the L3 level.
Novel inflammatory and nutritional serum biomarkers
Baseline blood tests collected on the day of admission were used to calculate novel inflammatory and nutritional markers.
Neutrophil-to-lymphocyte ratio (NLR).
Platelet-to-lymphocyte ratio (PLR).
Systemic inflammatory response index (SIRI) (calculated as the product of neutrophils and monocytes, divided by the lymphocyte count).
Prognostic nutritional index (PNI) [(10 × serum albumin {g/dL}) + (0.005 × lymphocytes/µL)].
These biomarkers were analyzed as dichotomous variables based on previously reported thresholds for bladder cancer12,13.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 29 (IBM Corp, Armonk, NY, USA). Demographic data were presented as mean ± standard deviation for normally distributed variable or median with range for non-normally distributed variables. Inter-rater reliability for body composition measurements was assessed using intraclass correlation coefficients (ICC). Group comparisons were performed using independent t-tests or Mann–Whitney U tests for continuous variables and chi-square test or Fisher’s exact tests, for categorical variables. Receiver operating characteristic (ROC) curves were constructed for psoas muscle density (PMD), axial muscle density (AMD), and skeletal muscle density (SMD), and their area under the ROC curve (AUC) were calculated. A backward stepwise logistic regression model was employed to identify independent predictors of postoperative complications. Variables with p < 0.2 in univariate analysis were included in the multivariate models. Statistical significance was set at p < 0.05 for all analyses.
Results
Patient demographics are summarized in Table 1. The median age of the patients was 66.87 years (range: 41–92), and 103 patients (81.1%) were male. Among the 127 patients, 51 (40.2%) received NAT, specifically 18 with dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC), 17 with gemcitabine/cisplatin, 9 with pembrolizumab, and 7 with gemcitabine/carboplatin. Most patients (81.1%, 103 patients) underwent open RC, and 82.5% (106 patients) had an ileal conduit urinary diversion. Pathologic findings showed pT0-pT2 stages in 63 patients (49.6%) and pT3-T4 stages in 64 patients (50.4%). Pathological lymph node metastasis was found in 35 patients (27.6%). A high comorbidity burden (ACC index > 4) was noted in 55.9% (71 patients). Preoperative anemia and hypoalbuminemia were observed in 65.4% (83 patients and 21.3% (27 patients), respectively. None of the patients exhibited hyperchloremia before surgery, while two (1.6%) developed postoperative hypochloremia, both experiencing major complications.
Table 1.
Demographic characteristics of the study population.
| Characteristics | CDC 0–2 | CDC 3–5 | Total | P value |
|---|---|---|---|---|
| N (% of 81) | N (% of 46) | N (% of 127) | ||
| Gender | ||||
| Male | 63 (77.8) | 40 (87) | 103 (81.1) | 0.765 |
| ACC index > 4 | 43 (53.1) | 28 (60.9) | 71 (55.9) | 0.081 |
| Age* | 66.83 ± 9.02 | 66.96 ± 9.02 | 66.87 ± 8.98 | 0.938 |
| BMI (kg/m2)* | 23.75 ± 4.80 | 23.99 ± 4.16 | 23.84 ± 4.56 | 0.780 |
| BMI | ||||
| < 18.4 kg/m2 | 15 (18.5) | 4 (8.7) | 19 (15) | 0.613 |
| 18.5–29.9 kg/m2 | 61 (75.3) | 38 (82.6) | 99 (78) | |
| ≥ 30 kg/m2 | 5 (6.2) | 4 (8.7) | 9 (7.1) | |
| ASA | ||||
| ASA 1 | 18 (17.1) | 4 (18.2) | 22 (17.3) | 0.236 |
| ASA 2 | 43 (41) | 4 (18.2) | 47 (37) | |
| ASA 3 | 44 (41.9) | 14 (63.6) | 58 (45.7) | |
| Neoadjuvant treatment | 32 (39) | 19 (41.3) | 51 (40.2) | 0.380 |
| Diversion | ||||
| Ileal conduit | 71 (87.7) | 35 (76.1) | 106 (82.5) | 0.198 |
| Neobladder | 6 (7.4) | 4 (8.7) | 10 (7.9) | |
| Ureterostomy | 4 (4.9) | 7 (15.2) | 11 (8.7) | |
| Laboratory results | ||||
| NLR* | 3.50 ± 3.27 | 4.90 ± 9.12 | 4.01 ± 6.08 | 0.162 |
| PLR* | 169.80 ± 109.03 | 169.39 ± 116.38 | 169.65 ± 111.29 | 0.940 |
| SIRI* | 3.03 ± 8.43 | 3.24 ± 5.14 | 3.11 ± 7.39 | 0.099 |
| PNI* | 48.74 ± 7.18 | 47.44 ± 8.12 | 48.27 ± 7.53 | 0.865 |
| CKD stage ≥ 3a | 40 (49.4) | 27 (58.7) | 67 (52.8) | 0.312 |
| Hemoglobin (g/dl)* | 11.55 ± 1.86 | 11.08 ± 1.75 | 11.38 ± 1.83 | 0.077 |
| Anemiab | 49 (60.5) | 34 (73.9) | 83 (65.4) | 0.127 |
| Platelet count (/ul)* | 288,876 ± 127,443 | 267,173 ± 96,346 | 281,015 ± 117,205 | 0.635 |
| Thrombocytopeniac | 3 (3.7) | 5 (10.9) | 8 (6.3) | 0.137 |
| Serum albumin (g/dl)* | 3.82 ± 0.56 | 3.88 ± 0.69 | 3.84 ± 0.61 | 0.253 |
| Hypoalbuminemiad | 16 (19.8) | 11 (23.9) | 27 (21.3) | 0.582 |
| Pre-operative serum chloride | ||||
| < 98 | 11 (13.6) | 7 (15.2) | 18 (14.2) | 0.084 |
| 98–110 | 70 (86.4) | 39 (84.8) | 109 (85.8) | |
| Post-operative serum chloride | ||||
| < 98 | 0 (0) | 2 (4.3) | 2 (1.6) | 0.175 |
| 98–110 | 75 (92.6) | 44 (95.7) | 119 (93.7) | |
| > 110 | 6 (7.4) | 0 (0) | 6 (4.7) | |
CDC Clavien–Dindo classification, ACC index age-adjusted Charlson comorbidity index, BMI body mass index, ASA American Society of Anesthesiologists, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SIRI systemic inflammation response index, PNI prognostic nutritional index, CKD chronic kidney disease.
aCKD stage ≥ 3 is GFR < 60 mL/min/1.73 m2.
bAnemia is serum hemoglobin < 12 g/dL.
cThrombocytopenia is platelet count < 150,000/µL.
dHypoalbuminemia is serum albumin < 3 g/dL.
*Mean ± SD.
During the 180-day observation period, 46 patients (36.2%) experienced major complications, with 39 patients (30.7%) developing complications within the first 90 days. A total of 241 complication events were reported, of which 92 (38.2%) were classified as major. The most frequent major complications were sepsis (19 events, 7.9%), small bowel obstruction (13 events, 5.4%), and hydronephrosis (13 events, 5.4%). The 180-day mortality rate was 7.9% (10 patients). The overall distribution of complication events is presented in Table 2.
Table 2.
Overall postoperative complication events by Clavien–Dindo classification.
| Complications | Grade (Clavien-Dindo) | Management | POD 0-180 N = 241 |
|---|---|---|---|
| Superficial wound infection | Grade 1 | Conservative and antibiotic treatment | 4 (1.7%) |
| Ileus | Grade 1 | Conservative | 8 (3.3%) |
| Anemia | Grade 1 | Conservative | 4 (1.7%) |
| Diarrhea | Grade 1 | Conservative | 2 (0.8%) |
| Acute kidney injury | Grade 1 | Managed by IV fluid | 20 (8.3%) |
| Anemia requiring transfusion | Grade 2 | Blood transfusion | 37 (15.6%) |
| Superficial wound dehiscence | Grade 2 | Antibiotic treatment and saturation under local anesthesia | 2 (0.8%) |
| Delirium | Grade 2 | Antipsychotic | 4 (1.7%) |
| Ileus require treatment | Grade 2 | Replacement of nasogastric tube | 20 (8.3%) |
| Urinary tract infection | Grade 2 | Antibiotic treatment | 24 (10%) |
| Pneumonia | Grade 2 | Antibiotic treatment | 5 (2.1%) |
| Deep vein thrombosis | Grade 2 | Anticoagulants | 5 (2.1%) |
| Arrhythmia | Grade 2 | Conservative, medical treatments | 1 (0.4%) |
| Pulmonary embolism | Grade 2 | Medical treatment | 1 (0.4%) |
| Abdominal collection | Grade 2 | Antibiotic treatment | 5 (2.1%) |
| Volume overload | Grade 2 | Medical treatments | 2 (0.8%) |
| Gouty attack | Grade 2 | Medical treatments | 1 (0.4%) |
| COPD acute exacerbation | Grade 2 | Medical and oxygen treatment | 2 (0.8%) |
| Hypercalcemia | Grade 2 | Medical treatments | 1 (0.4%) |
| Liver abscess | Grade 2 | Antibiotic treatment | 1 (0.4%) |
| Urinary leakage | Grade 2 | Antibiotic treatment | 1 (0.4%) |
| Os stenosis | Grade 3a | PCN insertion | 1 (0.4%) |
| Hydronephrosis | Grade 3a | Ureteral stent / PCN insertion | 13 (5.4%) |
| Lymphocele | Grade 3a | Percutaneous drainage | 2 (0.8%) |
| Urinary leakage | Grade 3b | Anastomosis revision | 2 (0.8%) |
| Small bowel obstruction | Grade 3b | Exploratory laparotomy | 13 (5.4%) |
| Anastomotic bowel leak | Grade 3b | Laparotomy and surgical revision | 1 (0.4%) |
| Stoma ischemia | Grade 3b | Exploratory laparotomy | 4 (1.7%) |
| Wound dehiscence | Grade 3b | Secondary surgical closure | 10 (4.2%) |
| Incisional hernia | Grade 3b | Parastomal hernia repair | 2 (0.8%) |
| Neobladder-cutaneous fistula | Grade 3b | Fistula closure | 1 (0.4%) |
| Small bowel injury | Grade 3b | Exploratory laparotomy | 2 (0.8%) |
| Upper gastrointestinal bleeding | Grade 3b | Esophago-gastroduodenoscopy | 2 (0.8%) |
| Lymphocele | Grade 3b | Open drainage | 1 (0.4%) |
| Myocardial infarction | Grade 4a | Coronary angiography and stent placement | 2 (0.8%) |
| Acute respiratory distress syndrome | Grade 4a | Respiratory support | 1 (0.4%) |
| Acute kidney injury (require hemodialysis) | Grade 4a | Dialysis | 3 (1.2%) |
| Respiratory failure | Grade 4a | Respiratory support | 2 (0.8%) |
| Sepsis | Grade 4b | Multiorgan dysfunction | 19 (7.9%) |
| Death | Grade 5 | 10 (4.2%) |
The reliability and consistency of body composition measurements were assessed using ICC. Excellent agreement was observed across all measurements: PMD (ICC = 0.914), SMD (ICC = 0.939), AMD (ICC = 0.935), psoas muscle index (PMI) (ICC = 0.980), skeletal muscle index (SMI) (ICC = 0.965) and axial muscle index (AMI) (ICC = 0.987). These results reflect high inter-rater reliability for the image analysis.
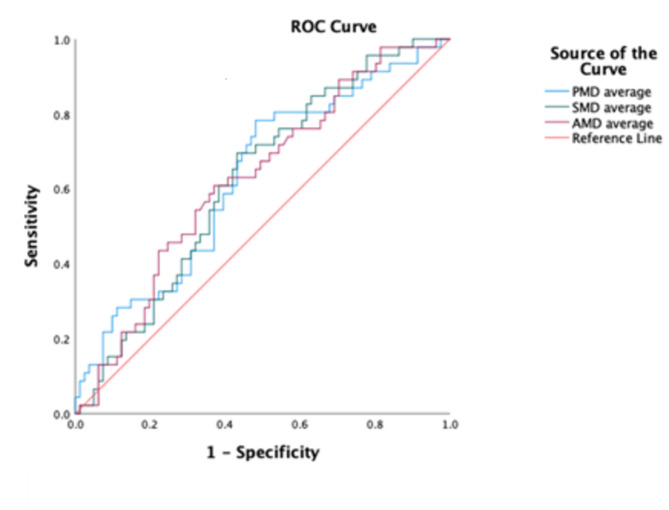
Table 3 compares imaging parameters between patients who developed 180-day postoperative complications (CDC grades 3–5) and those who did not (CDC grades 0–2). A significant association was observed between low PMD, low SMD, low AMD, and high axial muscle index (AMI) with major complications at 180 days postoperatively. Patients with major complications had a median PMD of 40.86 HU, median SMD of 29.7 HU, median AMD of 35.47 HU, and median AMI of 15.23 cm²/m². By contrast, patients without major complications had a median PMD of 43.09 HU (p = 0.016), median SMD of 33.29 HU (p = 0.021), median AMD of 39.16 HU (p = 0.019), and median AMI of 14.35 cm²/m² (p = 0.043). This prompted an evaluation of the predictive value of PMD, SMD, AMD, and AMI for major complications using ROC curves. PMD had an AUC of 0.629, with an optimal cutoff of 43.5 HU (78.3% sensitivity and 51.85% specificity). Similar analyses revealed an AUC of 0.692 for SMD (cutoff: 32.5 HU, 69.6% sensitivity, 56.79% specificity), an AUC of 0.618 for AMD (cutoff: 37.2 HU, 60.9% sensitivity, 61.73% specificity), and an AUC of 0.579 for AMI (cutoff: 15 cm²/m², 52.2% sensitivity, 60.5% specificity). (See Fig. 2: ROC curve demonstrating the myosteatosis cutoff point.)
Table 3.
Comparison of imaging parameters between patients with and without 180-day major complications (CDC 0–2 vs. CDC 3–5).
| CDC 0–2 | CDC 3–5 | Total | P value | |
|---|---|---|---|---|
| (N = 81) | (N = 46) | (N = 127) | ||
| PMD (HU) | 43.09 | 40.86 | 42.91 | 0.016 |
| (36.70–47.12) | (35.45–52.85) | (35.45–52.85) | ||
| SMD (HU) | 33.29 | 29.7 | 31.87 | 0.021 |
| (25.88–37.72) | (23.76–34.40) | (23.76–37.72) | ||
| AMD (HU) | 39.16 | 35.47 | 38 | 0.019 |
| (32.66–44.11) | (29.29–41.02) | (29.29–44.11) | ||
| VFD (HU) | − 80.77 | − 77.48 | − 81.34 | 0.725 |
| (− 90.09 to − 68.45) | (− 91.81 to − 70.72) | (− 91.81 to − 68.45) | ||
| PMI (cm2/m2) | 2.49 | 2.68 | 2.57 | 0.099 |
| (0.49–8.46) | (0.94–5.18) | (0.49–8.46) | ||
| SMI (cm2/m2) | 21.65 | 23.96 | 22.05 | 0.228 |
| (9.33–44.79) | (12.30–39.31) | (9.33–44.79) | ||
| AMI (cm2/m2) | 14.35 | 15.23 | 14.64 | 0.043 |
| (5.29–27.59) | (7.44–25.35) | (5.29–27.59) | ||
| VFI (cm2/m2) | 20.88 | 19.71 | 20.23 | 0.588 |
| (1.56–93.28) | (1.22–60.57) | (1.22–93.28) |
CDC Clavien–Dindo classification, PMD psoas muscle density, SMD skeletal muscle density, AMD axial muscle density, VFD visceral fat density, PMI psoas muscle index, SMI skeletal muscle index, AMI axial muscle index, VFI visceral fat index, HU Hounsfield unit.
Fig. 2.
ROC curve demonstrating the myosteatosis cutoff point.
Table 4 presents the multivariate analysis of factors associated with 180-day major postoperative complications. Patients with PMD ≤ 43.5 HU had a significantly higher likelihood of complications (adjusted odds ratio [OR] 3.959, p = 0.002), as did patients with SMD ≤ 32.5 HU (OR 3.004, p = 0.005) and AMD ≤ 37.2 HU (OR 2.644, p = 0.010). Additionally, patients with AMI of ≥ 15 cm²/m² showed a trend toward increased risk (adjusted OR 2.088, p = 0.071). Postoperative serum chloride levels were inversely associated with complications (adjusted OR 0.985, p = 0.017), suggesting that higher chloride levels reduced the risk of complications. Other factors, including gender, age, BMI, preoperative chronic kidney disease (CKD), hypoalbuminemia, and thrombocytopenia, were not significantly associated with complications.
Table 4.
Factors associated with 180-day postoperative complications.
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| PMD ≤ 43.5 HU | 3.877 | 1.699–8.848 | 0.001 | 3.959 | 1.654–9.480 | 0.002 |
| SMD ≤ 32.5 HU | 3.004 | 1.396–6.466 | 0.005 | |||
| AMD ≤ 37.2 HU | 2.644 | 1.256–5.566 | 0.010 | |||
| VFD | 0.997 | 0.980–1.013 | 0.678 | |||
| PMI | 1.155 | 0.866–1.542 | 0.327 | |||
| SMI | 1.040 | 0.984–1.099 | 0.164 | |||
| AMI ≥ 15 cm2/m2 | 1.823 | 0.877–3.788 | 0.108 | 2.088 | 0.938–4.646 | 0.071 |
| VFI | 0.989 | 0.971–1.008 | 0.267 | |||
| Male | 1.905 | 0.697–5.205 | 0.209 | |||
| Age > 80 | 1.190 | 0.318–4.458 | 0.796 | |||
| ACC index > 4 | 1.375 | 0.659–2.868 | 0.396 | |||
| Neoadjuvant treatment | 0.647 | 0.243–1.719 | 0.382 | |||
| Diversion | ||||||
| Conduit | 1.000 | |||||
| Neobladder | 1.352 | 0.358–5.105 | 0.656 | |||
| Ureterostomy | 3.550 | 0.974–12.941 | 0.055 | |||
| BMI | ||||||
| 18.5–29.9 kg/m2 | 2.336 | 0.721–7.564 | 0.157 | |||
| ≥ 30 kg/m2 | 3.000 | 0.539–16.689 | 0.210 | |||
| NLR > 4.1 | 1.100 | 0.467–2.590 | 0.827 | |||
| PLR > 164.7 | 1.128 | 0.525–2.425 | 0.757 | |||
| SIRI > 2.35 | 1.531 | 0.676–3.469 | 0.307 | |||
| PNI < 45 | 1.382 | 0.626–3.052 | 0.423 | |||
| Anemiaa | 1.850 | 0.836–4.096 | 0.129 | |||
| Pre-op Cl level | 0.967 | 0.869–1.077 | 0.546 | |||
| Post-op Cl level | 0.846 | 0.751–0.953 | 0.006 | 0.985 | 0.759–0.973 | 0.017 |
| CKD stage ≥ 3b | 1.457 | 0.701–3.025 | 0.313 | |||
| Hypoalbuminemiac | 0.976 | 0.306–3.108 | 0.967 | |||
| Thrombocytopeniad | 1.500 | 0.175–12.847 | 0.711 | |||
PMD psoas muscle density, SMD skeletal muscle density, AMD axial muscle density, VFD visceral fat density, PMI psoas muscle index, SMI skeletal muscle index, AMI axial muscle index, VFI visceral fat index, HU Hounsfield unit, ACC index age-adjusted Charlson comorbidity index, BMI body mass index, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SIRI systemic inflammation response index, PNI prognostic nutritional index, Pre-op Cl level preoperative chloride level, Post-op Cl level postoperative chloride level, CKD chronic kidney disease.
aAnemia is serum hemoglobin < 12 g/dL.
bCKD stage ≥ 3 is GFR < 60 mL/min/1.73 m22.
cHypoalbuminemia is serum albumin < 3 g/dL.
dThrombocytopenia is platelet count < 150,000/µL.
Table 5 presents the characteristics of patients with and without myosteatosis, defined by PMD ≤ 43.5 HU. Patients with myosteatosis were more likely to have an ACC index > 4 (65.3% vs. 42.3%, p = 0.01) and were significantly older (mean age: 69.32 years vs. 63.35 years, p < 0.001). Notably, all patients over the age of 80 had myosteatosis. Additionally, patients with myosteatosis had significant lower preoperative serum chloride levels (100.75 ± 3.23 vs. 102 ± 3.41, p = 0.011) and a higher incidence of anemia (73.3% vs. 53.8%, p = 0.023). No significant differences in BMI, diabetes mellitus, or coronary artery disease were observed between the two groups. According to multivariate analysis, older age (p = 0.001) and anemia (p = 0.018) were significantly associated with myosteatosis. (See Table 6)
Table 5.
Demographic characteristics by myosteatosis status.
| Characteristics | Without myosteatosis | With myosteatosis | P value |
|---|---|---|---|
| N (% of 52) | N (% of 75) | ||
| Gender (Male) | 45 (86.5) | 58 (77.3) | 0.193 |
| ACC index > 4 | 22 (42.3) | 49 (65.3) | 0.010 |
| Age* | 63.35 ± 9.50 | 69.32 ± 7.77 | < 0.001 |
| Age ≥ 80 | 0 (0) | 10 (13.3) | 0.005 |
| BMI (kg/m2)* | 23.48 ± 4.55 | 24.08 ± 4.59 | 0.461 |
| Diabetes mellitus | 6 (11.5) | 18 (24) | 0.078 |
| Hypertension | 25 (48.1) | 44 (58.7) | 0.239 |
| Dyslipidemia | 15 (28.8) | 33 (44) | 0.083 |
| Cerebrovascular disease | 2 (3.8) | 3 (4) | 0.965 |
| Coronary artery disease | 6 (11.5) | 9 (12) | 0.937 |
| PMI (cm2/m2)* | 2.8 ± 1.39 | 2.75 ± 1.15 | 0.868 |
| SMI (cm2/m2)* | 23.03 ± 7.07 | 23.04 ± 6.35 | 0.810 |
| AMI (cm2/m2)* | 14.46 ± 4.51 | 14.77 ± 4.08 | 0.657 |
| VFI (cm2/m2)* | 26.74 ± 21.87 | 25.62 ± 18.58 | 0.887 |
| Laboratory results | |||
| Pre-operative serum chloride (mmol/L)* | 102 ± 3.41 | 100.75 ± 3.23 | 0.011 |
| Pre-operative serum chloride | |||
| 98–110 | 47 (90.4) | 62 (82.7) | 0.220 |
| < 98 | 1 (1.9) | 1 (1.3) | |
| Hemoglobin* | 11.68 ± 1.83 | 11.17 ± 1.81 | 0.071 |
| Anemiaa | 28 (53.8) | 55 (73.3) | 0.023 |
| Serum albumin (g/dL)* | 3.81 ± 0.63 | 3.86 ± 0.60 | 0.653 |
| Hypoalbuminemiab | 6 (11.5) | 8 (10.7) | 0.877 |
| Platelet count* | 273,653 ± 101,861 | 286,120 ± 127,180 | 0.739 |
| Thrombocytopeniac | 2 (3.8) | 6 (8) | 0.470 |
| CKD stage ≥ 3d | 24 (46.2) | 43 (57.3) | 0.215 |
| NLR* | 3.24 ± 2.59 | 4.54 ± 7.59 | 0.192 |
| PLR* | 155.21 ± 80.85 | 179.67 ± 127.81 | 0.424 |
| SIRI* | 2.51 ± 7.65 | 3.52 ± 7.23 | 0.462 |
| PNI* | 49.31 ± 49.31 | 47.55 ± 8.32 | 0.286 |
ACC index age-adjusted Charlson comorbidity index, BMI body mass index, PMI psoas muscle index, SMI skeletal muscle index, AMI axial muscle index, VFI visceral fat index, CKD chronic kidney disease, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SIRI systemic inflammation response index, PNI prognostic nutritional index.
aAnemia is serum hemoglobin < 12 g/dL.
bHypoalbuminemia is serum albumin < 3 g/dL.
cThrombocytopenia is platelet count < 150,000/µL.
dCKD stage ≥ 3 is GFR < 60 mL/min/1.73 m2.
*Mean ± SD.
Table 6.
Factors associated with myosteatosis.
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| Male | 0.531 | 0.203–1.390 | 0.193 | |||
| ACC index > 4 | 2.570 | 1.242–5.318 | 0.010 | |||
| Age | 1.087 | 1.037–1.138 | < 0.001 | 1.090 | 1.037–1.145 | 0.001 |
| BMI (kg/m2) | 1.030 | 0.952–1.115 | 0.461 | |||
| Diabetes mellitus | 2.241 | 0.889–6.596 | 0.078 | |||
| Hypertension | 1.533 | 0.752–3.125 | 0.239 | |||
| Dyslipidemia | 1.938 | 0.912–4.117 | 0.083 | |||
| Cerebrovascular disease | 0.960 | 0.155–5.956 | 0.965 | |||
| Coronary artery disease | 1.045 | 0.348–3.139 | 0.937 | |||
| PMI (cm2/m2) | 0.969 | 0.730–1.286 | 0.868 | |||
| SMI (cm2/m2) | 1.000 | 0.948–1.055 | 0.810 | |||
| AMI (cm2/m2) | 1.018 | 0.936–1.107 | 0.657 | |||
| VFI (cm2/m2) | 0.997 | 0.980–1.015 | 0.887 | |||
| Laboratory results | ||||||
| Pre-operative serum chloride (mmol/l) | 0.887 | 0.790–0.995 | 0.011 | 0.906 | 0.800-1.077 | 0.123 |
| Pre-operative serum chloride | ||||||
| 98–110 | 1.971 | 0.657–5.914 | 0.220 | |||
| < 98 | 1.000 | |||||
| Hemoglobin (g/dl) | 0.854 | 0.700-1.041 | 0.071 | |||
| Anemiaa | 2.357 | 1.116–4.979 | 0.023 | 2.691 | 1.188–6.098 | 0.018 |
| Serum albumin (g/dl) | 1.147 | 0.641–2.053 | 0.653 | |||
| Hypoalbuminemiab | 0.833 | 0.353–1.965 | 0.877 | |||
| cThrombocytopenia | 2.174 | 0.471–11.220 | 0.470 | |||
| CKD stage ≥ 3d | 1.568 | 0.769–3.194 | 0.215 | |||
| NLR | 1.062 | 0.953–1.183 | 0.192 | |||
| PLR | 1.002 | 0.999–1.006 | 0.424 | |||
| SIRI | 1.022 | 0.965–1.082 | 0.462 | |||
| PNI | 0.968 | 0.922–1.017 | 0.286 | |||
ACC index age-adjusted Charlson comorbidity index, BMI body mass index, PMI psoas muscle index, SMI skeletal muscle index, AMI axial muscle index, VFI visceral fat index, CKD chronic kidney disease, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SIRI systemic inflammation response index, PNI, prognostic nutritional index.
aAnemia is serum hemoglobin < 12 g/dL.
bHypoalbuminemia is serum albumin < 3 g/dL.
cThrombocytopenia is platelet count < 150,000/µL.
dCKD stage ≥ 3 is GFR < 60 mL/min/1.73 m2.
Discussion
This study evaluated body composition parameters, particularly myosteatosis, and perioperative serum chloride levels as predictors of major complications following RC for MIBC. Our results indicate that myosteatosis, defined by low PMD, and serum chloride levels within the first 24 h post-surgery are significant independent predictors of major complications at 180 days postoperatively.
Myosteatosis has been associated with poor postoperative outcomes in various oncologic surgeries, including gastric, colorectal, and pancreatic surgeries9,10,21–23. However, its role in bladder cancer has been less explored. In this study, we measured PMD, SMD, and AMD through preoperative CT scans at the L3 vertebral level. Our findings suggest that PMD is a particularly practical and reliable marker for identifying high-risk patients. While SMD and AMD also demonstrated predictive value, PMD exhibited the strongest association with major postoperative complications. This finding aligns with the study by Yamashita et al., which reported that low psoas density (< 44 HU) was associated with poorer 2-year cancer-specific survival in patients undergoing RC21,22. However, further exploration is needed to determine appropriate cutoff points and the optimal area for measuring fat infiltration, as most previous studies on bladder cancer have used SMD as a representative of myosteatosis21,22,24.
The mechanisms by which myosteatosis impacts postoperative complications remain speculative. It is thought to impair insulin sensitivity and contribute to systemic inflammation, both of which negatively affect wound healing and immune responses25–28. In our cohort, myosteatosis was associated with older age and a higher incidence of anemia, which may contribute to decreased resilience against major surgery. Notably, all patients over the age of 80 exhibited myosteatosis, consistent with previous findings that linked ectopic fat accumulation in muscles with aging29. Anderson et al. demonstrated that fat accumulations in paraspinal muscles is more significant than in the psoas muscle30. Interestingly, the association between anemia and myosteatosis has not been widely reported. A recent study by De La Cruz-Gongora et al. showed that anemia is significantly associated with incident and persistent sarcopenia31. Moreover, low hemoglobin caused by iron deficiency has been linked to reduced muscle mass, as measured by 24-hours urinary creatinine excretion rates32. Further research is needed to fully understand the causes of myosteatosis and potential preventive measures for postoperative complications in patients with existing myosteatosis.
While sarcopenia has been associated with poorer survival outcomes following RC6,7,33–36, none of the skeletal muscle indices, including SMI, PMI, or AMI, were significantly associated with major complications in our study. Interestingly, AMI exhibited a trend toward higher values in patients with major complications, although it did not reach statistical significance. Hillers et al. recently demonstrated that while increased SMI was associated with improved overall survival in patients with localized renal cell carcinoma, it also increased the risk of complications within 90 days post-surgery37. This suggests that sarcopenia’s role in postoperative complications and cancer survival warrants further study. Our findings suggest that factors beyond sarcopenia—such as myosteatosis, comorbidities, and obesity—may play a more substantial role in influencing surgical complications.
The role of postoperative serum chloride levels remains a topic of debate. Hypochloremia is often a marker of underlying metabolic disturbances, such as metabolic alkalosis, which may arise from intraoperative fluid management. These metabolic imbalances have been shown to affect vascular tone, impair renal perfusion, and disrupt acid-base homeostasis, potentially contributing to complications like acute kidney injury, delayed recovery, and systemic instability38,39. Our study found that immediate postoperative chloride levels were associated with major postoperative complications. Veerakulwatana et al. previously demonstrated that hypochloremia within 24 h post-surgery was a risk factor for postoperative complications40. Similarly, Kimura et al. reported that hypochloremia within 48 h was independently associated with increased hospital mortality following thoracic and abdominal surgery in patients requiring postoperative intensive care20. In contrast, Semler et al. reported that using normal saline, compared to balanced crystalloid solutions, increased incidence mortality, persistent renal dysfunction, and the need for renal-replacement therapy in critically ill adults16. While the protective effect of hyperchloremia in the perioperative period following RC requires further exploration, our study emphasizes the importance of careful monitoring of chloride levels during perioperative care. The choice of intravenous fluid management, particularly between normal saline and balanced crystalloid solutions, may influence postoperative risk mitigation.
This study has several limitations. The retrospective design and single-center setting may introduce selection and assessment biases. Moreover, the study cohort consisted entirely of Thai patients, which may limit the generalizability of our findings to other ethnic populations. Additionally, as the creation of cutoff points generates data-driven results, our findings require validation in independent samples to confirm their broader applicability. Furthermore, we acknowledge the limitation of not reporting the amount and type of intraoperative fluids administered, as well as the potential impact of IV fluid composition during the early postoperative period. Given the emerging evidence on the role of balanced crystalloid solutions and serum chloride levels in surgical outcomes, we believe this topic warrants further investigation. We encourage randomized controlled trials to evaluate the influence of fluid management strategies and serum chloride levels on outcomes in this unique surgical cohort.
Conclusion
This study highlights the significance of myosteatosis and immediate postoperative serum chloride levels as independent predictors of major complications within 180 days following RC for MIBC. Incorporating body composition analysis and early perioperative serum chloride monitoring into the evaluation process may improve the identification of high-risk patients and allow for more personalized surgical and postoperative care. While sarcopenia is a well-established predictor of poor surgical outcomes, our findings suggest that myosteatosis may serve as a stronger and more reliable indicator of adverse outcomes in bladder cancer patients undergoing RC.
Acknowledgements
The authors would like to express gratitude to Miss Jitsiri Chaiyatho and Miss Nerisa Thornsri for their valuable contributions to this study.
Author contributions
W.S.: Writing – original draft, Data curation, Formal analysis. A.T.: Conceptualization, Resources, Data curation. T.T.: Resources. S.J.: Resources. V.W.: Resources. K.J.: Resources. K.M.: Resources, Data curation. N.W.: Resources, Data curation. T.H.: Conceptualization, Methodology, Project administration, Writing – review & editing.
Data availability
The data from this study are not deposited in a publicly accessible repository. However, the data will be made available upon request for academic purposes. Please contact T.H. (the corresponding author) for further details.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Raghavan, D. Chemotherapy and cystectomy for invasive transitional cell carcinoma of bladder. Urol. Oncol.21, 468–474. 10.1016/s1078-1439(03)00145-5 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Fonteyne, V. et al. Curative treatment for muscle invasive bladder Cancer in Elderly patients: a systematic review. Eur. Urol.73, 40–50. 10.1016/j.eururo.2017.03.019 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Chappidi, M. R. et al. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol Oncol 34, 256 e251-256 (2016). 10.1016/j.urolonc.2015.12.010 [DOI] [PMC free article] [PubMed]
- 5.Hollenbeck, B. K. et al. Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer116, 50–56. 10.1002/cncr.24701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith, A. B. et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J. Urol.191, 1714–1720. 10.1016/j.juro.2013.12.047 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Psutka, S. P. et al. Sarcopenia as a predictor of complications and survival following radical cystectomy: A. B. and J Urol. ; 191: 1714–1720. J Urol 192, 1582–1583; discussion 1583 (2014). (2014). 10.1016/j.juro.2014.05.104 [DOI] [PubMed]
- 8.Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol.8, 457–465. 10.1038/nrendo.2012.49 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Zhuang, C. L. et al. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: a propensity score-matched analysis from a large-scale cohort. Surgery166, 297–304. 10.1016/j.surg.2019.03.020 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Kroenke, C. H. et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer124, 3008–3015. 10.1002/cncr.31405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz, G. B. et al. Prognostic value of the preoperative platelet-to-leukocyte ratio for oncologic outcomes in patients undergoing radical cystectomy for bladder Cancer. Clin. Genitourin. Cancer. 15, e915–e921. 10.1016/j.clgc.2017.05.009 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Wang, R., Yan, Y., Liu, S. & Yao, X. Comparison of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios in bladder Cancer patients undergoing Radical Cystectomy. Biomed. Res. Int.2019 (3628384). 10.1155/2019/3628384 (2019). [DOI] [PMC free article] [PubMed]
- 13.Cinar, N. B. et al. Reporting perioperative complications of radical cystectomy: the influence of using standard methodology based on ICARUS and EAU quality criteria. World J. Surg. Oncol.21, 58. 10.1186/s12957-023-02943-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi, F. et al. Pre-treatment prognostic nutritional index may serve as a potential biomarker in urinary cancers: a systematic review and meta-analysis. Cancer Cell. Int.18, 207. 10.1186/s12935-018-0708-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei, Y., Jiao, D., Yao, Z., Wang, L. & Zhao, Z. Prognostic values of preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte- to-monocyte ratio for patients with muscle- invasive bladder cancer undergoing radical cystectomy. Arch. Esp. Urol.75, 287–294 (2022). [PubMed] [Google Scholar]
- 16.Semler, M. W., Self, W. H. & Rice, T. W. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 378, (1951). (2018) 10.1056/NEJMc1804294 [DOI] [PMC free article] [PubMed]
- 17.McCluskey, S. A. et al. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth. Analg. 117, 412–421. 10.1213/ANE.0b013e318293d81e (2013). [DOI] [PubMed] [Google Scholar]
- 18.Toyonaga, Y. & Kikura, M. Hyperchloremic acidosis is associated with acute kidney injury after abdominal surgery. Nephrol. (Carlton). 22, 720–727. 10.1111/nep.12840 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Shafat, T., Novack, V., Barski, L. & Haviv, Y. S. Community-based serum chloride abnormalities predict mortality risk. PLoS One. 18, e0279837. 10.1371/journal.pone.0279837 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura, S. et al. Association of serum chloride concentration with outcomes in postoperative critically ill patients: a retrospective observational study. J. Intensive Care. 2, 39. 10.1186/2052-0492-2-39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita, S. et al. Impact of preoperative Sarcopenia and myosteatosis on prognosis after radical cystectomy in patients with bladder cancer. Int. J. Urol.28, 757–762. 10.1111/iju.14569 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Yamashita, S. et al. Myosteatosis as a novel prognostic biomarker after radical cystectomy for bladder cancer. Sci. Rep.10, 22146. 10.1038/s41598-020-79340-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akahori, T. et al. Prognostic significance of muscle attenuation in pancreatic Cancer patients treated with Neoadjuvant Chemoradiotherapy. World J. Surg.39, 2975–2982. 10.1007/s00268-015-3205-3 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Engelmann, S. U. et al. Body composition of patients undergoing radical cystectomy for bladder Cancer: Sarcopenia, low Psoas muscle index, and myosteatosis are independent risk factors for mortality. Cancers (Basel). 15. 10.3390/cancers15061778 (2023). [DOI] [PMC free article] [PubMed]
- 25.O’Sullivan, J., Lysaght, J., Donohoe, C. L. & Reynolds, J. V. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat. Rev. Gastroenterol. Hepatol.15, 699–714. 10.1038/s41575-018-0069-7 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Healy, L. A. et al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J. Thorac. Cardiovasc. Surg.134, 1284–1291. 10.1016/j.jtcvs.2007.06.037 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Miljkovic, I. et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obes. (Silver Spring). 21, 2118–2125. 10.1002/oby.20346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, C. S., Clark, J. & Wagenmakers, A. J. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr.30, 13–34. 10.1146/annurev.nutr.012809.104817 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Correa-de-Araujo, R. et al. Myosteatosis in the context of skeletal muscle function deficit: an Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol.11, 963. 10.3389/fphys.2020.00963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson, D. E. et al. Associations of computed tomography-based trunk muscle size and density with Balance and Falls in older adults. J. Gerontol. Biol. Sci. Med. Sci.71, 811–816. 10.1093/gerona/glv185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De La Cruz-Gongora, V., Salinas-Rodriguez, A. & Manrique-Espinoza, B. Prospective changes in anemia are associated with the incidence and persistence of Sarcopenia among older Mexican adults. Front. Nutr.11, 1323450. 10.3389/fnut.2024.1323450 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinke, J. S. J. et al. Iron deficiency is related to lower muscle mass in community-dwelling individuals and impairs myoblast proliferation. J. Cachexia Sarcopenia Muscle. 14, 1865–1879. 10.1002/jcsm.13277 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathak, R. A. & Hemal, A. K. Frailty and Sarcopenia impact surgical and oncologic outcomes after radical cystectomy in patients with bladder cancer. Transl Androl. Urol.7, S763–S764. 10.21037/tau.2018.08.06 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayr, R. et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle. 9, 505–513. 10.1002/jcsm.12279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirasawa, Y. et al. Sarcopenia as a Novel Preoperative Prognostic Predictor for Survival in patients with bladder Cancer undergoing Radical Cystectomy. Ann. Surg. Oncol.23, 1048–1054. 10.1245/s10434-016-5606-4 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. et al. Association of preoperative sarcopenia with the long-term prognosis of patients with bladder cancer undergoing radical cystectomy. J. Cancer Res. Clin. Oncol.150, 173. 10.1007/s00432-024-05705-6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillers, A. H., Bach, S. W., Saito, A. & Azawi, N. Muscle matters: skeletal muscle index and body mass index impact on complications and survival in renal cancer. BJUI Compass. 5, 783–790. 10.1002/bco2.405 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfortmueller, C. A., Uehlinger, D., von Haehling, S. & Schefold, J. C. Serum chloride levels in critical illness-the hidden story. Intensive Care Med. Exp.6, 10. 10.1186/s40635-018-0174-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astapenko, D., Navratil, P., Pouska, J. & Cerny, V. Clinical physiology aspects of chloremia in fluid therapy: a systematic review. Perioper Med. (Lond). 9, 40. 10.1186/s13741-020-00171-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veerakulwatana, S. et al. Perioperative factors and 30-day major complications following radical cystectomy: a single-center study in Thailand. Heliyon1010.1016/j.heliyon.2024.e33476 (2024). e33476. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are not deposited in a publicly accessible repository. However, the data will be made available upon request for academic purposes. Please contact T.H. (the corresponding author) for further details.