Abstract
Angiogenesis is an essential process during follicular development and corpora lutea (CL) formation. Recent studies have shown that vascular endothelial growth factor (VEGF) is an essential regulator of ovarian angiogenesis. Several lines of evidence have indicated that the production of VEGF is regulated by hypoxia inducible factor-1α (HIF-1α), especially under hypoxic conditions, but the expression of HIF-1α has not been well characterized in the porcine ovary. The present study examined the expression of HIF-1α mRNA and its localization in porcine ovaries at different stages of the estrous cycle. Northern blot analyses of total CL RNA indicated hybridization of the porcine HIF-1α probe to transcripts of approximately 3.8 kb. The mRNA expression of HIF-1α was highest in CL during the early luteal phase, followed by a decrease during the mid- and late-luteal phases. Using in situ hybridization, abundant HIF-1α mRNA was evident in follicles and CL. Within non-atretic follicles, HIF-1α mRNA was highly expressed in the granulosa cell layer, while weaker labeling was evident in the theca interna. These results suggest that HIF-1α may play a role in the regulation of cellular metabolism and blood supply during follicular growth and CL formation.
Résumé
L’angiogénèse est un processus essentiel durant le développement folliculaire et la formation du corpora lutea (CL). Des études récentes ont démontré que le facteur de croissance de l’endothélium vasculaire (VEGF) est un régulateur essentiel de l’angiogénèse ovarienne. Plusieurs évidences démontrent que la production de VEGF est régulée par le facteur 1-α inductible par l’hypoxie (HIF-1α), surtout lors de conditions hypoxiques, mais l’expression de HIF-1α par l’ovaire porcin n’est pas été bien caractérisée. L’expression de l’mRNA de HIF-1α et sa localisation dans des ovaires porcins à différents stades du cycle œstral a été examinée. Une analyse de Northern de l’ARN total du CL montrait une hybridation de la sonde pour HIF-1α à un transcrit d’environ 3,8 kb. L’expression du mRNA de HIF-1α était à son plus haut dans le CL durant la période initiale de la phase lutéale, suivie par une diminution durant les périodes médiane et tardive. Par hybridation in situ, une grande quantité d’ARNm de HIF-1α était détectée dans les follicules et le CL. À l’intérieur des follicules n’étant pas en atrésie, l’ARNm de HIF-1α était fortement exprimé dans la couche de cellules de la granulosa, alors qu’un marquage plus faible était noté dans la thèque interne. Ces résultats suggèrent que HIF-1α peut jouer un rôle dans la régulation du métabolisme cellulaire et de l’apport sanguin lors de la croissance folliculaire et de la formation du CL.
(Traduit par Docteur Serge Messier)
Introduction
Angiogenesis is a physiological component of follicle growth and formation of corpora lutea (CL) in the ovary during the reproductive cycle (1,2). Although several angiogenic factors were described for the ovary (3), vascular endothelial growth factor (VEGF) plays a critical role in follicle and CL angiogenesis. In pigs, VEGF mRNA and protein were evident in the periovulatory follicle, decreased after the LH surge, then increased during the forming of the CL (4). Gonadotropin-induced expression of VEGF in follicular (4) and luteal tissues was reported (5); however, a hypoxic environment likely is involved in angiogenesis during follicular development, selection, and ovulation (6). The theca compartment has greater vascularity than the granulosa compartment of the follicle, thereby predisposing granulosa cells to diminished local oxygen concentrations (7). Furthermore, partial separation of the theca and granulosa compartments after ovulation also decreases capillary contact with granulosa cells (8). Thus, hypoxic conditions presumably are involved in ovarian angiogenesis.
It was reported that hypoxia inducible factors (HIFs) mediate adaptive responses, such as angiogenesis, to low oxygen availability. Both 5′ and 3′ untranslated regions of the VEGF gene contain a hypoxia-sensing region, named the hypoxia responsive element (HRE) (9). The HIFs bind HRE and play an important role in the transcriptional regulation of VEGF by hypoxia (10). Hypoxia inducible factor-1 is a heterodimer, consisting of α- and β-subunits. Previous studies indicated that expression of HIF-1α and HIF-1β (aryl hydrocarbon receptor nuclear translocator [ARNT]) mRNA was almost ubiquitous (11), whereas HIF-2α, HIF-3α, ARNT2, and ARNT3 expression was shown to be restricted to specific cell types (12,13). The regulatory role of HIFs in VEGF expression and angiogenesis was demonstrated in several tumor tissues (14). However, a recent study showed that expression of HIF-1α and HIF-1β proteins did not correlate with VEGF protein expression in the normal mouse uterus (15). It is generally accepted that HIF-1β is constitutively expressed whereas HIF-1α is continuously synthesized and degraded by the ubiquitin-proteasome (16). Thus, HIF-1α is present only in hypoxic cells and regulation of HIF-1 activity is primarily determined by hypoxia-induced stabilization of the HIF-1α protein (16).
Control of steady-state protein levels is involved in HIF-1α activity under hypoxia; however, the regulation of HIF-1α expression and activity in vivo occurs at multiple levels, particularly mRNA expression (17,18). In the ovary, expression of the HIF-1α gene, as well as its association with VEGF expression and angiogenesis, was reported to occur in ovarian cancer cells (19). In contrast, few data exist on the expression of HIF-1α mRNA and its localization in the normal adult ovary. Therefore, the aims of the present study were to investigate the expression of HIF-1α mRNA in the porcine CL at different stages of the estrous cycle, to demonstrate the localization of HIF-1α mRNA, and to determine the distribution of VEGF and HIF-1α mRNA in the porcine ovary. The hypothesis was that HIF-1α mRNA expression would be similar to VEGF mRNA expression and reflect hypoxic conditions in the porcine ovary.
Materials and methods
Animals and tissue preparation
The experimental protocol was approved by the North Carolina State University Institutional Care and Use Committee whose guidelines are in accordance with the Canadian Council on Animal Care. Ovaries were collected from 15 pubertal gilts using previously described procedures (34). Briefly, 20 gilts (170 to 180 d of age) were treated with a combination of equine chorionic gonadotropin and human chorionic gonadotropin (PG600; Intervet, Millsboro, Deleware, USA), and subsequently checked daily for estrous with a mature boar. The 1st d of standing estrus was designated as day 0. Five animals were discarded from the study for failure to demonstrate estrus after the PG600 treatment.
Fifteen animals (n = 3 per day) were euthanized on days 4, 7, 10, 13, or 15 of the estrous cycle. Animals were euthanized by intravenous injections of 58.5 mg pentobarbital sodium and 7.5 mg phenytoin sodium/kg body weight (Beuthanasia-D Special; Schering-Plough Animal Health Corporation, Kenilworth, New Jersey, USA). The ovaries were collected immediately after euthanasia and 3 to 4 CL were dissected from the ovaries, snapfrozen in liquid nitrogen, and stored at −80°C for Northern blot analysis. Ovarian pieces containing follicles and CL were embedded in optimal cutting temperature (OCT) media on dry ice, and subsequently sectioned (8 μm) using a cryostat, mounted on slides, and stored at −80°C for in situ hybridization studies.
Cloning and synthesis of porcine HIF-1α cDNA and cRNA probes
The primer pairs for HIF-1α were based on human cDNA sequences available from Gen Bank (National Center for Biotechnology Information [NCBI], US National Library of Medicine, Bethesda, Maryland, USA). The primer sequences were 5′-AGG CTT ACC ATC AGC TAT TTG CG-3′ (sense) and 5′-TTC ATT CTG AGA AAA AAG CTT CGC-3′ (antisense), corresponding to nucleotides (nt) 180 to 202 and 491 to 514 of the human HIF-1α cDNA (GenBank accession no. XM_007373). Using previously described procedures (20), a polymerase chain reaction (PCR) of pig CL cDNA was used to generate a 335 base pair (bp) porcine HIF-1α DNA. The amplified products were isolated, cloned into a vector (pGEM-T Easy vector; Promega, Madison, Wisconsin, USA), and sequenced at the Iowa State University DNA Sequencing and Synthesis Facility (Iowa State University, Ames, Iowa, USA). After verification that the insert was correct, the cDNA clone was subsequently used as a template for radiolabeled probe synthesis. Based on comparisons of complete genomes (UniGene, NCBI) there is greater than 95% homology between bovine, human, and porcine sequences for HIF-1α.
The cDNA fragments used for the synthesis of probes for Northern blot analysis were a 355 bp EcoRI (restriction endonuclease; Promega) fragment of HIF-1α, 435 bp EcoRI fragment of VEGF (164 amino acid isoform), and a 689 kb EcoRI-NotI fragment of 18S (21). These cDNA fragments were randomly labeled with 32P-deoxycytidine 5′-triphosphate using the primer DNA labeling system (Random primer DNA labeling system; Gibco-BRL Life Technologies, Grand Island, New York, USA).
For in situ hybridization, 35S-labeled cRNA antisense and sense probes were prepared using an RNA transcription kit (MAXIscript SP6/T7 Kit; Ambion, Austin, Texas, USA), as previously described (22). Each cDNA template was linearized with PstI (restriction endonuclease; Promega) for T7 generation of the sense probe and with NcoI (restriction endonuclease; Promega) for SP6 generation of antisense probe of porcine HIF-1α and VEGF. The VEGF probe was previously developed and assessed (22). Each 35S-UTP labeled cRNA probe was filtered with sephadex spin columns (Sephadex G5-Quick Spin Columns; Roche, Indianapolis, Indiana, USA) to remove unincorporated nucleotides. All procedures were performed according to manufacturer’s recommendations.
RNA preparation and Northern blot analysis
RNA extraction and Northern blot hybridization were conducted as previously described (34). Briefly, total RNA was isolated from frozen CL using guanidine thiocyanate and phenol/chloroform extraction (Tri-Reagent; Molecular Research Center, Cincinnati, Ohio, USA). Samples of total CL RNA (20 μg of each sample) were denatured and separated in denaturing agarose gels (1% agarose and 6.6% [v/v] formaldehyde).
The gels were stained with ethidium bromide after electrophoresis and the ribosomal RNA bands were visualized under ultraviolet illumination to ensure the integrity of the RNA samples. The size of the mRNA transcripts was calculated based on molecular size markers run on the same gel after visualization by ethidium bromide staining.
Fractionated RNA were transferred to nylon membranes overnight using a transfer system (Turboblotter Rapid Downward Transfer System; Schleicher & Schuell, Keene, New Hampshire, USA). The blotted membrane was rinsed and dried at 80°C for 60 min. Afterward, the membranes were prehybridized at 65°C in prehybridization solution for 30 min, the section was then replaced with new prehybridization solution with denatured 32P-labeled HIF-1α cDNA probe (2 × 106 cpm/mL; total volume 5 mL) and the hybridization continued overnight. The membranes were then washed twice with 0.1× SSC, containing 0.1% sodium docedyl sulfate (SDS) and 1 mM ethylenediamine tertraacetic acid (EDTA), pH 8.0, at room temperature for 30 min, and twice with 20 mM NaHPO4, 1% SDS 1 mM EDTA, pH 8.0, at 65°C for 15 min. After a final wash, the membrane was covered with plastic wrap (Saran Wrap) and exposed to a phosphorimaging screen (Molecular Dynamics, Sunnyvale, California, USA) at room temperature for 10 h. Hybridization signals were scanned and quantified (ImageQuant software; Molecular Dynamics). Subsequently, the blots were stripped in a shaking chamber containing a boiling solution of 0.1× SSC and 0.5% SDS for 15 min and then boiling water for 15 min. The membrane was prehybridized as before and then hybridized with the 32P-labeled porcine 18S cDNA probe. The blot was washed and exposed to a phosphorimaging screen for 15 min. Quantification of hybridization signals was obtained by phosphorimage analysis using the same software. A pool of RNA was made by combining purified RNA extracted from days 4 to 15 CL. The pooled RNA was used as an internal control between each blot. The intensity for HIF-1α was adjusted according to the difference between the signals of the pooled RNA samples among blots. Therefore, the HIF-1α signals were adjusted before determining the RNA:18S ratios. A 32P-labeled probe for porcine 18S cDNA fragment was used as a control to assess sample loading. Values are reported as arbitrary units above background and normalized to the signal levels of 18S cDNA in each lane by expressing each value as a ratio to the 18S signal. Northern blot analysis on each CL stage (day 4 to 15) was performed from 3 different animals.
In situ hybridization
After the slides were removed from the −80°C, they were fixed in 4% paraformadehyde/phosphate buffered saline solution (PBSS), rinsed in PBSS, dehydrated gradually, and allowed to air-dry at room temperature. Acetylation and hybridization of the tissues were performed essentially as described previously (20). Briefly, sections were covered with prehybridization buffer and incubated in a moist chamber at room temperature for 2 h. After the prehybridization buffer was removed, the sections were covered with hybridization buffer with sense or antisense radiolabeled cRNA probe of HIF-1α and incubated at 55°C overnight for 18 h in a moist chamber. After hybridization, slides were washed under high stringency conditions and treated with ribonuclease A in order to remove unhybridized probe. The slides were then dipped in liquid emulsion (Kodak NTB2; Eastman Kodak, Rochester, New York, USA) and exposed for 3 wk at 4°C. The emulsion-coated slides were developed (Kodak-D19 developer; Eastman Kodak) and counterstained with hematoxylin and eosin or periodic acid Schiff stain before viewing and photography. In order to compare mRNA distribution within the same ovarian structures, consecutive sections of porcine ovaries were sequentially hybridized with either VEGF or HIF-1α cRNA probes.
Blood samples and hormone assays
Blood samples were collected by venipuncture of the jugular vein of each animal before administration of PG600 and before they were euthanized to determine serum progesterone concentrations. Progesterone was quantified using a described radioimmunoassay (20). The intra-assay coefficients of variation for both high (18 ng/mL) and low (0.3 ng/mL) progesterone reference sera were less than 5%. All samples were analyzed in 1 assay.
Statistical analysis
Northern blot data were expressed as the ratio of the signal intensity for HIF-1α probe to 18S rRNA and are shown as the mean arbitrary units + standard error of means (sχ̄). The mean for a specific day represents luteal mRNA expression from 3 animals. The data were initially analyzed for heterogeneity of variance using a univariate procedure (23). Since the data were homogeneous, the general linear model procedure was used for the analysis of variance (ANOVA) (23). Differences in means were compared by using Duncan’s multiple range tests.
Results
Serum progesterone concentrations
Prior to the administration of PG600, serum progesterone concentrations were less than 0.2 ng/mL in all animals. Serum progesterone concentrations were 10.4, 22.6, 39.9, 86, and 46.9 ng/mL (sχ̄ = 6.3) for days 4, 7, 10, 13, and 15, respectively. There were 13.4 CL/animal. The substantial progesterone concentrations in pigs at day 15 indicated that the CL were functionally active on this day.
Northern blot analysis
Northern blot analysis of mRNA showed a strong positive band of about 3.8 kb in porcine CL collected from different stages of the estrous cycle (Figure 1a). Expression of pig HIF-1α transcript in the corpora lutea was found at all stages of the cycle, with greater expression during the early luteal phase than in later stages (Figure 1a). The HIF-1α mRNA levels decreased with CL age (day 4 versus days 10 to 15) when normalized for 18S RNA (Figure 1b P, < 0.05); however, expression at days 7, 10, 13, and 15 did not differ.
Figure 1.
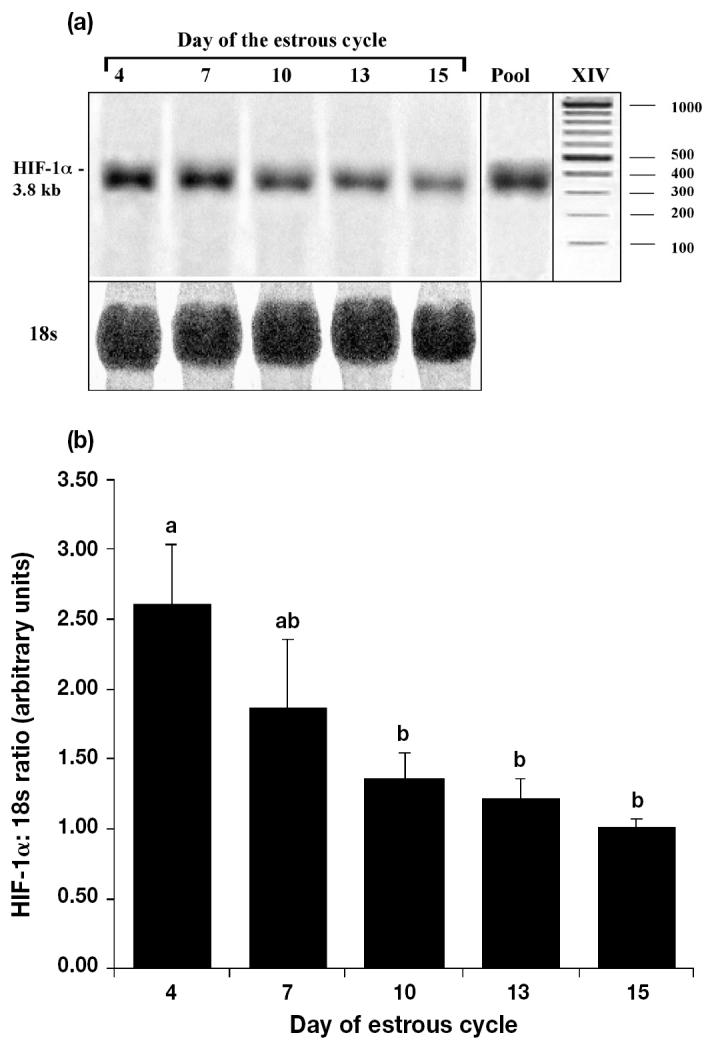
Expression of hypoxia inducible factor (HIF)-1α mRNA in pig corpora lutea (CL) obtained at days 4, 7, 10, 13, and 15 after the onset of estrous. (a) Representative autoradiograph of Northern blot analysis for HIF-1α (top) and 18s rRNA (bottom). The pooled RNA (Pool) was used as an internal control. Marker XIV (Roche Diagnostics, Indianapolis, Indiana, USA). (b) Relative amounts of HIF-1α mRNA to 18s rRNA (arbitary units + sχ̄ n = 3 animals day−1) in pig CL differed between days.
a, b Values with different letters differ; P < 0.05.
In situ hybridization
In order to characterize localization of HIF-1α in ovarian tissues, in situ hybridization with a HIF-1α RNA probe was performed. As shown in Figures 2 to 4, prominent expression of the HIF-1α mRNA was evident in luteal tissues from CL on days 4 and 7 of the estrous cycle. Expression of the HIF-1α mRNA also was observed in morphologically non-atretic follicles with apparently greater intensity in the granulosa compartment than in the theca compartment (Figures 3a, 3b, 3e, 3f, and 4c). Localization of HIF-1α (Figures 3a, 3b, and 4c) and VEGF mRNA (Figures 3c and 3d) appeared in similar locations of the ovarian CL and particularly in the granulosa cell layer of non-atretic follicles. The HIF-1α and VEGF were not evident in atretic follicles.
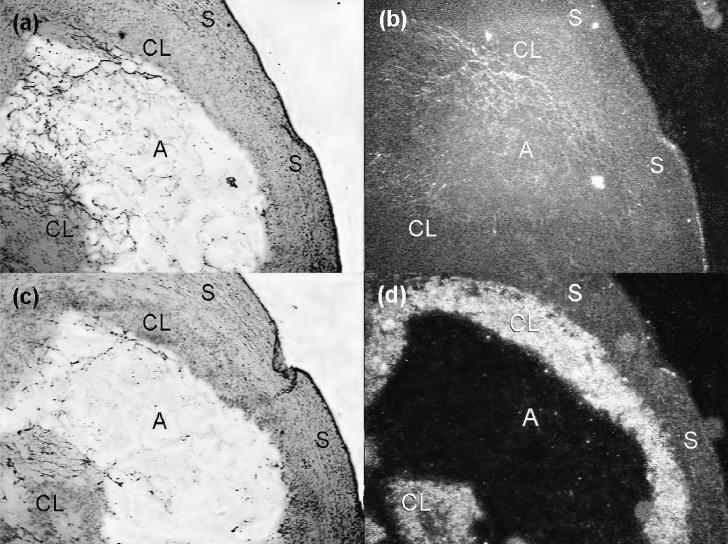
Figure 2.
Sections of pig ovary at day 4 of the estrous cycle. (a) and (b) are brightfield and darkfield images, respectively, of hybridization in the ovary using a sense hypoxia inducible factor (HIF)-1α RNA probe. (c) and (d) are brightfield and darkfield views, respectively, of adjacent sections hybridized with an antisense HIF-1α RNA probe. High amounts of hybridization signals for HIF-1α mRNA predominantly were observed in granulosa-derived luteal tissues.
CL — corpus luteum; S — luteal stroma; A — antrum. Scale bars represent 100 μm.
Figure 4.
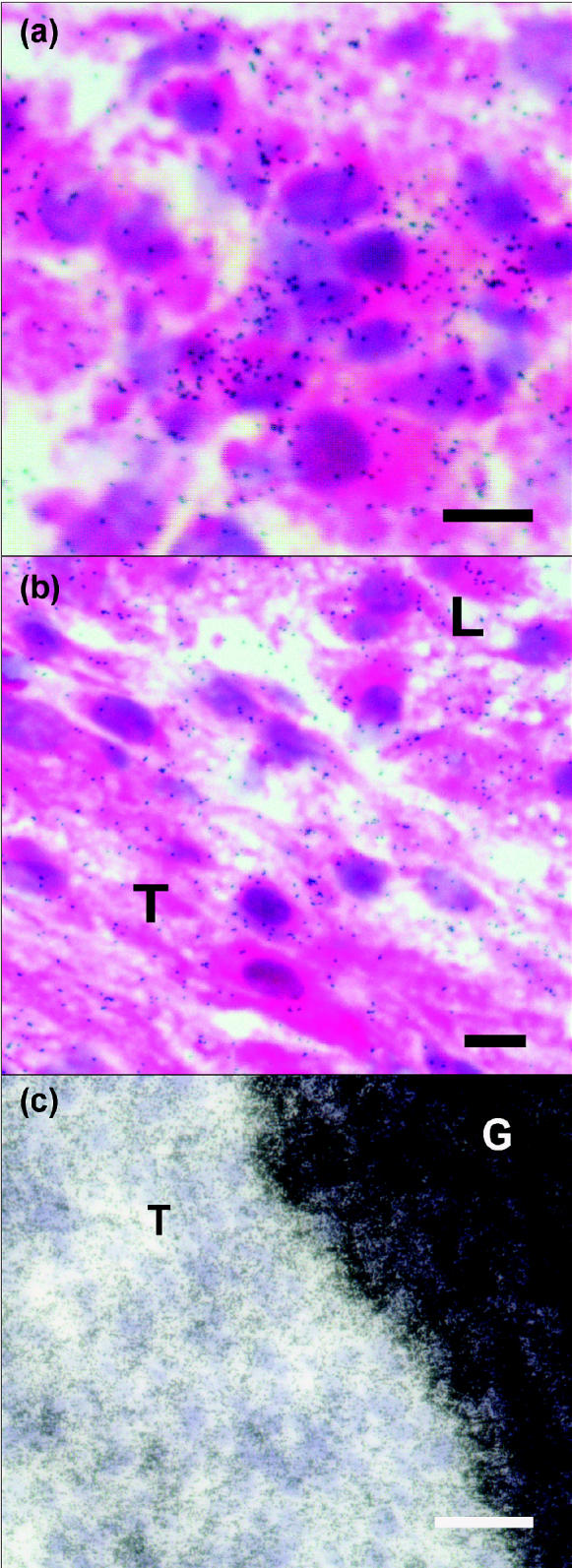
Photomicrographs of brightfield images of sections of pig corpus luteum at day 7 (a and b; scale bars represent 25 μm) and follicle at day 15 (c; scale bar represents 100 μm) of the estrous cycle. An antisense hypoxia inducible factor (HIF)-1α RNA probe was used for hybridization in the ovary. Slides were stained with hematoxylin-eosin (a,b) or periodic acid Schiff stain (c). (a) Hybridization signals, seen as silver grains (black dots), are present in most luteal cells. (b) Hybridization signals were dispersed in the thecal (T) and luteal (L) tissue interface. (c) The intense hybridization signals obscured the granulosa cells (G) and were evident in the thecal layers (T) of a follicle at day 15.
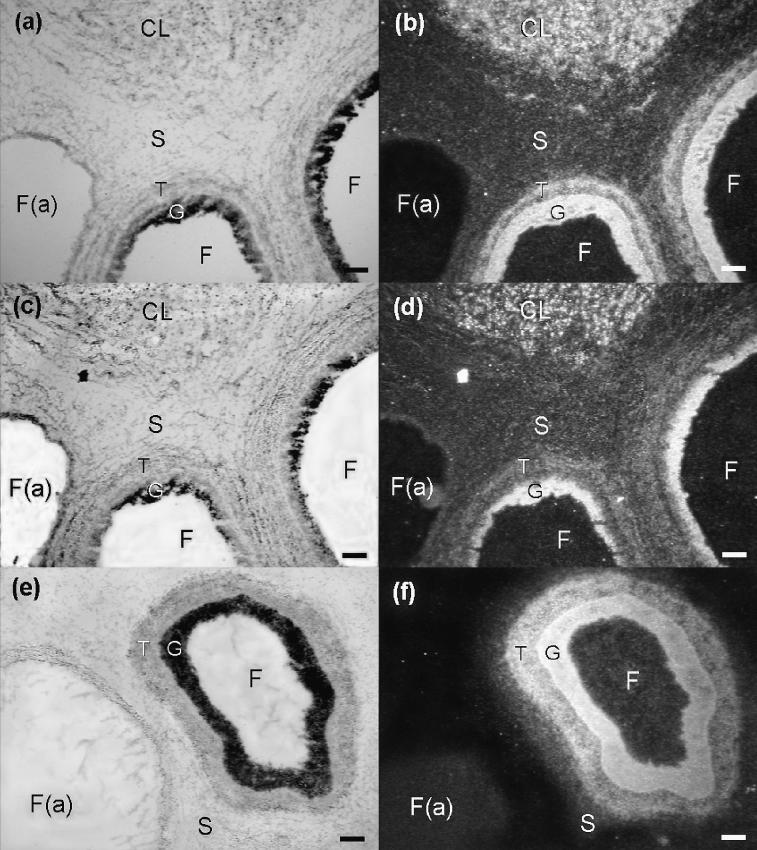
Figure 3.
Sections of pig ovary at days 7 (a to d) and day 15 (e and f) of the estrous cycle. Left and right panels are brightfield and darkfield images, respectively, of hybridization in the ovary using an antisense hypoxia inducible factor (HIF)-1α probe (a,b and e,f) or a vascular endothelial growth factor (VEGF) RNA probe (c,d). Hybridization signals for HIF-1α mRNA are seen predominantly in the corpora lutea (CL) and granulosa compartment, and to a lesser extent in the theca compartment of morphologically non-atretic follicles (a,b and e,f). Hybridization signals for VEGF mRNA (c,d) are seen in similar areas as the HIF-1α signals.
F — follicle; F(a) — atretic follicle; G — granulosa compartment; S — stroma; T — theca compartment. Scale bars represent 100 μm.
Discussion
The results of the present study demonstrated the presence of mRNA for HIF-1α in the porcine ovary. The expression of HIF-1α was described for bovine ovarian follicles and it was suggested that hypoxia may be encountered during follicular growth (24). In addition, strong expression of HIF-1α in tumor cells adjacent to areas of necrosis indicated that HIF-1 presumably contributes to VEGF expression and angiogenesis in human epithelial ovarian tumors (25). Our results provide additional evidence for the role of HIF-1 in development and angiogenesis during follicular growth and CL formation.
With in situ hybridization, mRNA of HIF-1α was expressed predominantly in non-atretic follicles and CL. In preovulatory follicles, expression of HIF-1α mRNA subjectively appeared to be more evident in the granulosa compartment than the theca compartment, suggesting different degrees of transcript regulation (Figures 3 and 4). Previous immunohistochemical studies demonstrated that the granulosa area of the Graffian follicle was avascular until the lutenizing hormone (LH) surge subsided (26). These observations concur with a subsequent study that reported a morphological analogy between the ovarian follicle and a solid tumor. Thus, it was speculated that hypoxic stress in the inner part of avascular multicellular structures induces angiogenesis through the VEGF system (6). Despite the expression of HIF-1α and VEGF mRNA in the granulosa cells, it is difficult to elucidate the relative lack of vasculature. Evidently, the LH surge triggers additional events that promote vascularization of the early CL (26).
The HIF-1 was identified as a transcription factor capable of mediating hypoxic adaptation at the cellular level. The HIF-1β protein is constitutively expressed in all cells, whereas HIF-1α protein primarily is present under hypoxic conditions, suggesting that transcriptional activity of the HIF-1 is tightly regulated by HIF-1α protein (27,28). Some reports indicated that hypoxia increases HIF-1α protein levels by inhibiting degradation, rather than acting on HIF-1α transcription (29,30). However, the induction of HIF-1 expression by hypoxia also was demonstrated in many cells and organs (18,19). Prominent expression of HIF-1α mRNA in the inner granulosa cell layers of the preovulatory follicle, as shown in the present study, may contribute to the modulation of oxygen homeostasis and physiological processes during follicular development. As HIF-1 activates genes encoding glycolytic enzymes like phosphofructokinase or enolase (31), HIF-1α transcripts may increase transport of glucose required for the metabolic adaptation in the avascular granulosa zone of the preovulatory follicle.
The present study showed that HIF-1α mRNA and VEGF mRNA were expressed in similar areas of non-atretic follicles and CL. Although the roles of hypoxic stress on the production of VEGF by follicles and CL remain controversial, Graffian follicles and early CL exist in a relatively hypoxic environment (8). Low O2 levels increased VEGF production in vitro in human granulosa cells (32). In contrast, VEGF production by macaque granulosa cells did not depend on hypoxia (33). Recently, it was shown that insulin-like growth factors (IGFs) and LH stimulated VEGF production in granulosa cells of the mature follicle of macaques (33), and previous studies demonstrated that production of VEGF by hypoxia or IGFs primarily is mediated by HIF-1 (34). The current study provides evidence of HIF-1α and VEGF transcripts in the same ovarian structure, suggesting that hypoxic stress may contribute to the production of ovarian VEGF.
As assessed by Northern blot analysis, HIF-1α mRNA expression was greater in luteal tissues collected early in the estrous cycle than in CL at days 10, 13, and 15. Based on our previous studies of VEGF (22), the present results indicate that an association does not exist between the expression patterns of VEGF and HIF-1α mRNA. While HIF-1α mRNA expression decreased with age of CL, expression of VEGF mRNA was consistent throughout the estrous cycle (22). If mRNA expression is indicative of protein quantity, these findings suggest that HIF-1α is sufficient for VEGF induction at all stages of the estrous cycle or additional factors are involved in the regulation of VEGF expression in the porcine CL.
Our results indicate that HIF-1α mRNA levels change in the porcine CL during the estrous cycle; however, it remains to be determined whether this reflects similar changes in HIF-1α protein. While incomplete, emerging data support the understanding that after ovulation, luteal tissue is avascular with low levels of oxygen or other essential nutrients, and so HIF-1α is highly expressed in an attempt to respond to the avascular conditions. In contrast, luteal tissue is highly vascular after neovascularization of the CL. After neovascularization in the early phases of the estrous cycle, the abundant vascular supply may be required to prevent cellular damage associated with hypoxia. Consequently, expression of HIF-1α is somewhat limited in CL in the later phases of the cycle.
A surge of LH and various growth factors may contribute to ovarian HIF-1α expression during the periovulatory period and early luteal phase. Indeed, the expression of IGF-1, IGF-2, and IGF-binding proteins was described for bovine preovulatory follicle and CL (35). Recent evidence suggested that IGF-1 and IGF-2 synergize with LH to promote VEGF secretion by monkey granulosa cells from preovulatory follicles (33), and HIF-1 protein mediates VEGF induction by insulin and IGF-1 (34). As the full transcriptional induction of VEGF gene expression by hypoxia requires activator protein (AP)-1 binding (36), factors such as FSH and LH, which up-regulate the Fos–Jun heterodimer, a AP-1 transcription factor (37), may contribute to ovarian VEGF gene expression in response to HIF-1 stimulation. Thus, a variety of factors including oxygen tension, gonadotropins and growth factors may regulate the HIF-1-modulated VEGF transcription and subsequent angiogenesis.
In conclusion, this study showed that HIF-1α mRNA is highly expressed in porcine ovarian follicles and CL, providing promising evidence for molecular mechanisms of metabolic adaptation and angiogenesis in these tissues. However, further studies are needed to determine whether HIF-1α mRNA levels correlate with protein levels and to establish the role of HIF-1 on VEGF production in the porcine ovary.
Acknowledgments
The authors thank Dr. Ron McNeel, Baylor College of Medicine, for the gift of cDNA for porcine 18s rRNA, and Drs. Barry Peters, William Flowers, Charlotte Farin, and William Miller for their technical advice and support. This research was supported by the Competitive State Research Support Program, NCSU College of Veterinary Medicine.
References
- 1.Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol. 2002;83:151–164. doi: 10.1046/j.1365-2613.2002.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modlich U, Kaup FJ, Augustin HG. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest. 1996;74:771–780. [PubMed] [Google Scholar]
- 3.Stouffer RL, Martinez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001;32:567–575. doi: 10.1016/s0188-4409(01)00323-x. [DOI] [PubMed] [Google Scholar]
- 4.Barboni B, Turriani M, Galeati G, et al. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod. 2000;63:858–864. doi: 10.1095/biolreprod63.3.858. [DOI] [PubMed] [Google Scholar]
- 5.Wulff C, Dickson SE, Duncan WC, Fraser HM. Angiogenesis in the human corpus luteum: simulated early pregnancy by HCG treatment is associated with both angiogenesis and vessel stabilization. Hum Reprod. 2001;16:2515–2524. doi: 10.1093/humrep/16.12.2515. [DOI] [PubMed] [Google Scholar]
- 6.Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C. Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. Int J Exp Pathol. 1997;78:57–70. doi: 10.1046/j.1365-2613.1997.d01-247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosden RG, Byatt-Smith JG. Oxygen concentration gradient across the ovarian follicular epithelium: model, predictions and implications. Hum Reprod. 1986;1:65–68. doi: 10.1093/oxfordjournals.humrep.a136362. [DOI] [PubMed] [Google Scholar]
- 8.Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol. 1999;28:157–166. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Minchenko A, Salceda S, Bauer T, Caro J. Hypoxia regulatory elements of the human vascular endothelial growth factor gene. Cell Mol Biol Res. 1994;40:35–39. [PubMed] [Google Scholar]
- 10.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Comm. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 13.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 15.Daikoku T, Matsumoto H, Gupta RA, et al. Expression of hypoxia-inducible factors in the periimplantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner: Evidence for differential function during early pregnancy. J Biol Chem. 2003;278:7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- 16.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 18.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi A, Imai T, Shimizu M, et al. Hypoxia-induced changes in the expression of VEGF, HIF-1α and cell cycle-related molecules in ovarian cancer cells. Anticancer Res. 2002;22:2697–2702. [PubMed] [Google Scholar]
- 20.Boonyaprakob U, Gadsby JE, Hedgpeth V, Routh P, Almond GW. Cloning of pig prostaglandin F2alpha FP receptor cDNA and expression of its mRNA in the corpora lutea. Reproduction. 2003;125:53–64. [PubMed] [Google Scholar]
- 21.Ding ST, McNeel RL, Mersmann HJ. Expression of porcine adipocyte transcripts: tissue distribution and differentiation in vitro and in vivo. Comp Biochem Physiol B Biochem Mol Biol. 1999;123:307–318. doi: 10.1016/s0305-0491(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 22.Boonyaprakob U, Gadsby JE, Hedgpeth V, Routh P, Almond GW. Expression and localization of vascular endothelial growth factor and its receptors in the porcine corpora lutea during the oestrous cycle. Reproduction. 2003;126:393–405. doi: 10.1530/rep.0.1260393. [DOI] [PubMed] [Google Scholar]
- 23.SAS. SAS User’s Guide: Statistics. Statistical Analysis System Institute Inc. Cary, North Carolina, USA. 1988.
- 24.Harvey AJ, Ritter LJ, Thompson JG. Expression of hypoxia-inducible factor-1 and -2α during bovine preimplantation embryo development. Proc Soc Reprod Biol. 2001;32:68. [Google Scholar]
- 25.Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res. 2001;7:1661–1668. [PubMed] [Google Scholar]
- 26.McClure N, Macpherson AM, Healy DL, Wreford N, Rogers PA. An immunohistochemical study of the vascularization of the human Graafian follicle. Hum Reprod. 1994;9:1401–1405. doi: 10.1093/oxfordjournals.humrep.a138718. [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 28.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 29.Wenger RH, Kvietikova I, Rolfs A, Gassmann M, Marti HH. Hypoxia-inducible factor-1 alpha is regulated at the post-mRNA level. Kidney Int. 1997;51:560–563. doi: 10.1038/ki.1997.79. [DOI] [PubMed] [Google Scholar]
- 30.Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 32.Friedman CI, Danforth DR, Herbosa-Encarnacion C, Arbogast L, Alak BM, Seifer DB. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68:607–612. doi: 10.1016/s0015-0282(97)00278-1. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Chequer JC, Stouffer RL, Hazzard TM, Patton PE, Molskness TA. Insulin-like growth factor-1 and -2, but not hypoxia, synergize with gonadotropin hormone to promote vascular endothelial growth factor: A secretion by monkey granulosa cells from preovulatory follicles. Biol Reprod. 2003;68:1112–1118. doi: 10.1095/biolreprod.102.011155. [DOI] [PubMed] [Google Scholar]
- 34.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schams D, Berisha B, Kosmann M, Amselgruber WM. Expression and localization of IGF family members in bovine antral follicles during final growth and in luteal tissue during different stages of estrous cycle and pregnancy. Domest Anim Endocrinol. 2002;22:51–72. doi: 10.1016/s0739-7240(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 36.Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327:419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma SC, Richards JS. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem. 2000;275:33718–33728. doi: 10.1074/jbc.M003555200. [DOI] [PubMed] [Google Scholar]