Contribution of Intrinsic Neuronal Factors in the Generation of Cortically Driven Electrographic Seizures
Timofeev I, Grenier F, Steriade M
J Neurophysiol 2004;92:1133–1143
Some electrographic seizures are generated intracortically. The cellular and ionic bases of cortically generated spontaneous seizures are not fully understood. Here we investigated spontaneously occurring seizures consisting of spike–wave complexes intermingled with fast runs in ketamine-xylazine anesthetized cats, by using dual intracellular recordings in which one pipette contained a control solution and another pipette contained blockers of K+, Na+, or Ca2+ currents. We show that closely located neocortical neurons display virtually identical fluctuations of the membrane potential during electrographic seizures, thus directly demonstrating a high degree of focal synchrony during paroxysmal activity. In addition to synaptic drives, the persistent Na+ current [INa(p)] and probably the high-threshold Ca2+ current contributed to the generation of paroxysmal depolarizing shifts (PDSs) during cortically driven seizures. Ca2+-activated K+ current [IK(Ca)] also took part in the control of the amplitude and duration of PDSs. The hyperpolarizing components of seizures largely depended on Cs+-sensitive K+ currents. IK(Ca) played a significant, although not exclusive, role in the mediation of hyperpolarizing potentials related to EEG “waves” during spike–wave seizures. We conclude that intrinsic cellular factors have a significant role in the generation of depolarizing and hyperpolarizing components of seizures.
COMMENTARY
Observing the cheering section at a football game after a touchdown is scored, one is struck by a traveling wave, whereby people rise to their feet with outstretched arms (spikes?); beginning at one point (the “focus”), the wave travels along the audience in relentless progression. The analogy to the development and spread of a neocortical seizure is striking. Nearby neurons become synchronized and then recruit ever more distant neurons into the hyperexcitable firing pattern, as the waves (and spikes) of excitation spread through the cortex. The mechanisms that underlie such paroxysmal firing and the motivation for otherwise sensible human beings to take part in the wave ritual continue to baffle scientists.
However, at least on the epilepsy front, progress is being made. Seizures originating in neocortex are quite common. Etiologies include acquired (e.g., cortical dysplasia, traumatic injury) and idiopathic/genetic causes. Neocortical seizures manifest differently, depending on the exact onset location and pattern of spread. Like a seizure in any brain region, the mechanisms underlying neocortical seizures entail disruption of the normal excitatory/inhibitory homeostasis, mediated by intrinsic and synaptic conductances.
For several decades, Professor Steriade and his colleagues have studied the mechanisms by which cortical and thalamic neurons cooperate to generate epileptic seizures. In a large body of experimental work in the cat (reviewed in ref. 1), they have built a convincing case that the cortex, not the thalamus, is the origin of seizures in both absence seizures (3-Hz spike–wave) and the epileptic encephalopathy, Lennox–Gastaut syndrome (LGS). The investigators have demonstrated that neocortical seizures do not require thalamic participation (the neocortical seizures persist after destruction of the thalamus or in isolated cortical slabs), although they are modified by thalamic input. These neocortically generated seizures are seen in several situations: (a) arising from slow oscillations of sleep, (b) in awake, active animals, and (c) under the condition of ketamine/xylazine anesthesia. In each condition, the cortical electroencephalogram (EEG) shows two reproducible patterns: slow spike–waves (SWs) or polyspike–waves (PSWs) at 1.5 Hz to 3 Hz and, sometimes, superimposed fast runs (FRs) of rhythmic discharges at 10 Hz to 20 Hz. These electrographic patterns resemble those seen in patients with LGS—a syndrome characterized by intractable seizures of multiple types (atypical absence, tonic, atonic, etc.). Seizures in LGS often have a multifocal neocortical origin, an EEG with slow SWs at 1.5 Hz to 3 Hz as well as fast runs of 10 Hz to 20 Hz activity, and cognitive impairment. Although acknowledging that this epilepsy syndrome is a complex clinical entity with multiple components, Steriade and colleagues have sought to understand the mechanisms underlying the electrographic seizures seen in their experimental preparation, as related to those of LGS. Dissection of the ionic and synaptic mechanisms underlying the SW/PSW and FR patterns might lead to novel therapeutic approaches.
The present study by Timofeev and colleagues aims to elucidate the ionic conductances mediating the hyperpolarizing (EEG wave) and depolarizing (EEG spike) components of electrographic seizures from neocortex of cats anesthetized with ketamine/xylazine. Thirty percent of these cats exhibit spontaneous electrographic seizures when recorded under this anesthetic condition, with SW/PSW and FR spikes. Simultaneous intracellular recordings from pairs of nearby neurons (<0.5 mm apart) demonstrate that the cells [which are mainly of the regular spiking type (2)] have identical membrane potential fluctuations, suggesting that neurons within the epileptogenic zone possess a high degree of focal synchrony. The implication is that these neurons (and many others in the area) fire synchronously during the electrographic seizure.
The authors exploit this observation to design experiments to test mechanistic hypotheses. With paired intracellular recordings (in conjunction with extracellular recordings of field potentials/EEG), one intracellular recording (using potassium acetate-filled micropipettes) is considered the control, whereas the other cell is manipulated by changing the pipette solution with a variety of agents that alter synaptic or ionic conductances (e.g., calcium, sodium, or potassium channel blockers).
The paroxysmal depolarization shift (PDS) has long been considered the intracellular correlate of the interictal EEG spike. In these experiments, the PDS also is regarded as the intracellular manifestation of the ictal firing, as the PDSs coalesce and fire in rapid succession to compose the electrographic seizure. The PDS has been shown to be a giant excitatory postsynaptic potential (EPSP) (3), mediated by intracellular influx of calcium (and to a lesser extent, sodium) ions through voltage-gated and N-methyl-d-aspartate (NMDA)-receptor channels. However, the PDS is modifiable by intrinsic ionic conductances. Here, the authors provide the first in vivo evidence of PDS modulation by ionic mechanisms (see Table 1). First, the inter-PDS interval (EEG wave or hyperpolarizing phase) is regulated by potassium conductances, not by γ-aminobutyric acid (GABA)-ergic mechanisms, as previously thought. (Most earlier studies of the PDS, done in brain slices, used GABA antagonists.) In the present experiments, without GABA blocked, PDS amplitude and duration are altered by blocking cesium-dependent potassium (i.e., leak) conductance or calcium-dependent potassium currents (with an intracellular calcium chelator). Therefore the wave or hyperpolarizing phase of the seizure discharge is mediated by a bevy of potassium conductances.
TABLE 1.
Comparison of EEG spikes and waves during neocortical electrographic seizures
| EEG spike (PDS) | EEG wave |
|---|---|
| Depolarizing phase | Hyperpolarizing phase |
| EEG (field potential) negativity | EEG (field potential) positivity |
| All neocortical cell types fire | No neocortical cell type fires |
| Conductance increases | Conductance decreases; disfacilitation |
| Chloride-dependent IPSPs are present | Not GABA mediated |
| Mediated by EPSPs (NMDA and non-NMDA); persistent sodium current controls PDS amplitude | Calcium-dependent potassium current controls PDS amplitude and duration (not affected by chloride injection from KCl-filled pipettes) |
EEG, electroencephalogram; PDS, paroxysmal depolarization shift; EPSP, excitatory postsynaptic potential; IPSP, inhibitory postsynaptic potential; NMDA, N-methyl-d-aspartate; GABA, γ-aminobutyric acid; KCl, potassium chloride.
In contrast, generation of the PDS (spike component of the spike–wave complex) during spontaneous seizures is modulated by the persistent sodium current INa(p) (4) and a high-threshold calcium conductance. INa(p) is a voltage-dependent sodium current, activated in the subthreshold voltage range, separate from the current that produces the action potential. In these neocortical seizures, blockade of either INa(p) or calcium currents led to decreased PDS amplitude. Therefore both sodium and calcium currents contribute to the PDS; as previously discussed, the post-PDS hyperpolarization (EEG wave) also is dependent on calcium entry. It must be remembered that ketamine is used as the anesthetic in these experiments, raising the possibility that NMDA-dependent processes are suppressed.
Certainly, other ionic and synaptic conductances also are involved in the complex sculpting of electrical activity during seizure discharges. For example, the hyperpolarization-activated cation current Ih is hypothesized to control the occurrence of subsequent cycles of spike–wave (see Fig. 1). These experiments successfully determined which conductances contribute to the various seizure phases but could not quantify their extent. The companion article presents a modeling study in which it is shown that increased extracellular potassium from seizure-induced neuronal firing is sufficient to modify intrinsic ionic currents to produce the range of neocortical paroxysmal oscillations seen in vivo (5). Sorting out the specific region- and age-dependent ionic and synaptic currents generating the various components of a seizure is critical to development of novel therapeutic strategies. Experiments such as these are pivotal because they use an intact brain and show the feasibility of studying ionic mechanisms previously attempted only in slice preparations. We should celebrate this feat by doing a wave.
FIGURE 1.
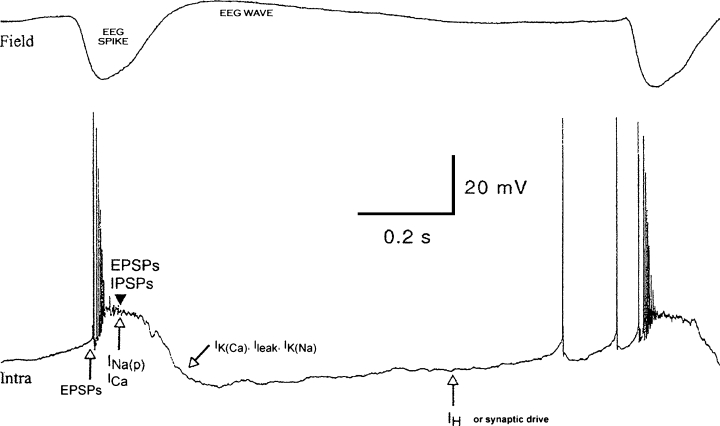
Synaptic and intrinsic currents hypothesized to be activated in neocortical neurons during paroxysmal activity. Typical EEG field potentials (Field) and paroxysmal depolarization shifts (PDSs; Intra) in neocortical neurons undergoing paroxysmal activity. The top trace shows the EEG spike and wave components. The lower trace shows hypothesized ionic mechanisms governing each phase. The PDS is initiated by EPSPs, and the plateau potential of the PDS is maintained by a combination of synaptic potentials (EPSPs, IPSPs) and ionic conductances (persistent sodium current and high-threshold calcium current). The post-PDS hyperpolarization is governed by multiple potassium currents, activated by calcium or sodium entry, as well as by leak current. The next cycle of depolarization is initiated by both synaptic drive and the hyperpolarization-activated IH current. Adapted from Neuroscience 2004;123:324, with permission.
References
- 1.Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 2.Steriade M. Neocortical cell classes are flexible entities. Nat Rev Neurosci. 2004;5:121–134. doi: 10.1038/nrn1325. [DOI] [PubMed] [Google Scholar]
- 3.Johnston D, Brown TH. Giant spike potential hypothesis for epileptiform activity. Science. 1981;211:294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- 4.Stafstrom CE, Schwindt PC, Crill WE. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982;236:221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- 5.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Potassium model for slow (2-3 Hz) in vivo neocortical paroxysmal oscillations. J Neurophysiol. 2004;92:1116–1132. doi: 10.1152/jn.00529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


