Abstract
Background
Supervised exercise programs improve walking impairment and quality of life (QoL) in patients with peripheral artery disease (PAD). However, such programs are underutilized, due to their limited accessibility. A feasible and effective exercise program is needed. This study assessed the feasibility of a hybrid onsite and home-based exercise program (HY-PAD) and changes in walking capacity and PAD-specific QoL following HY-PAD.
Methods
Due to recruitment challenges imposed by the COVID-19 pandemic, this randomized controlled trial was modified to a pre–post intervention design that excluded the control group. Eligible patients with PAD were assigned to HY-PAD, consisting of 1-hour, supervised onsite exercise sessions 3 days per week for 4 weeks, followed by a home-based program with weekly 15-minute telephone calls to discuss exercise planning for 8 weeks. Feasibility was determined based on recruitment, adherence, and adverse events. Walking capacity was measured by a 6-minute walk test. PAD-specific QoL was assessed using the Walking Impairment Questionnaire.
Results
Of 24 patients enrolled (3 women, aged 70 ± 8 years), 21 (87.5%) completed HY-PAD. Twenty participants attended ≥ 87.5% of prescribed sessions. Two participants experienced adverse events (foot injury and limb ischemia). The 6-minute walk test distance (357 ± 105 vs 413 ± 53 meters, P = 0.021), and 2 domains of the questionnaires (walking speed: 38.4 ± 24.1 vs 60.6 ± 26.6 points and stair-climbing: 60.6 ± 29.4 vs 74.0 ± 23.1 points, both P < 0.001) increased significantly.
Conclusions
High attendance and low dropout rates support the feasibility of using HY-PAD, which was associated with improved walking capacity and PAD-specific QoL. Future studies are warranted to confirm its efficacy.
Résumé
Contexte
Des programmes d’exercice supervisés atténuent la difficulté à la marche et améliorent la qualité de vie chez les patients atteints d’artériopathie périphérique. Cependant, de tels programmes sont sous-utilisés, car ils sont peu accessibles. Un programme d’exercice faisable et efficace est nécessaire. Cette étude visait à évaluer la faisabilité d’un programme d’exercice hybride, soit en centre et à domicile, ainsi que les modifications dans la capacité de marcher et dans la qualité de vie spécifique de l’artériopathie périphérique après un programme d’exercice hybride.
Méthodologie
En raison des difficultés d’inscription liées à la pandémie de COVID-19, le plan de cet essai contrôlé à répartition aléatoire a été modifié pour comprendre une évaluation pré- et post-intervention qui excluait le groupe témoin. Des patients atteints d’une artériopathie périphérique admissibles ont été inscrits au programme d’exercice hybride en centre et à domicile consistant en des séances d’exercice d’une heure supervisées dans un centre 3 jours par semaine durant 4 semaines, ainsi qu’un programme basé au domicile des patients, comportant des appels téléphoniques hebdomadaires de 15 minutes pour discuter de la planification des exercices, durant 8 semaines. La faisabilité a été déterminée sur la base des inscriptions, de l’adhésion des patients et des effets indésirables. La capacité de marcher a été mesurée à l’aide d’un test de marche de 6 minutes. La qualité de vie spécifique de l’artériopathie périphérique a été évaluée à l’aide du questionnaire WIQ (Walking Impairment Questionnaire).
Résultats
Parmi les 24 patients inscrits (3 femmes; âgés de 70 ± 8 ans), 21 (87,5 %) se sont rendus à la fin du programme d’exercice hybride en centre et à domicile. Vingt participants ont assisté à au moins 87,5 % des séances prévues. Deux participants ont connu des effets indésirables (lésion au pied et ischémie d’un membre). La distance au test de marche de 6 minutes (357 ± 105 vs 413 ± 53 mètres, p = 0,021) et 2 domaines des questionnaires (vitesse de la marche : 38,4 ± 24,1 vs 60,6 ± 26,6 points et montée des marches : 60,6 ± 29,4 vs 74,0 ± 23,1 points, p < 0,001 pour les deux) ont augmenté considérablement.
Conclusions
La participation élevée et le faible taux d’abandons appuient la faisabilité des programmes d’exercice hybride en centre et à domicile, lesquels ont été associés à une amélioration de la marche et de la qualité de vie liée à l’artériopathie périphérique. De futures études sont nécessaires pour en confirmer l’efficacité.
Peripheral artery disease (PAD) represents a complex pathophysiology, such as restricted arterial flow, endothelial dysfunction, inflammation, muscle structural, and metabolic abnormalities, resulting in significant functional limitations.1 Patients with PAD are burdened with poor quality of life (QoL),2 a high risk of ischemic limb symptoms (eg, intermittent claudication),3 amputations,4 and mortality.5 However, these patients are underdiagnosed and undertreated, compared to patients with coronary artery disease,2,5 and they experience suboptimal management of their condition.5,6 Supervised exercise programs improve functional status, leg symptoms, walking endurance,7, 8, 9 and QoL4,10,11 in patients with PAD. Societal guidelines4,10,12 recommend supervised exercise programs as a first-line therapy for patients with PAD who are experiencing intermittent claudication.
Despite robust evidence supporting the efficacy of supervised exercise programs, they are heavily underutilized in PAD,13 due to limited accessibility.14 Effective and accessible exercise programs for patients with PAD are needed. In patients with coronary artery disease, a combination of supervised centre-based and monitored home-based exercise programs (ie, hybrid cardiovascular [CV] rehabilitation) have been shown to increase adherence, reduce costs, and result in similar clinical outcomes, compared to centre-based CV rehabilitation.15 In those with PAD, monitored, home-based exercise programs improve walking capacity,16, 17, 18 and structured, home-based programs are recommended when supervised exercise is not feasible.12 Given the established benefits of supervised, centre-based and monitored, home-based exercise programs in PAD, a hybrid CV rehabilitation (ie, a biphasic exercise program starting with a lead-in supervised phase followed by a monitored, home-based phase) may be an effective program, with increased accessibility and adherence in patients with PAD.
The primary purpose of this pilot study was to examine the feasibility of a hybrid onsite and home-based exercise program in patients with PAD (HY-PAD) who are living with intermittent claudication. The secondary purpose was to examine the changes in pain-free and maximum walking distances, PAD-specific QoL, and CV risk indicators associated with HY-PAD.
Methods
Focus-group interview
To understand and accommodate the specific needs, barriers, and preferences of patients with PAD, and to deliver a program that is well-received, sustainable, and effective, we conducted a focus-group interview with patients with lived experiences of PAD and their caregivers. This interview reaffirmed the significant burden that PAD imposes on patients’ daily living and QoL, and all patients and caregivers highlighted improvements in their walking capacity as a top priority for their health and QoL. When asked about specific modes of program delivery that would best address their needs, some patients indicated a preference for being supervised, as a means to overcome their fears of exercise, learn their limits, and feel safe and comfortable to exercise in the hospital setting, whereas others indicated a need for a more-individualized and flexible program delivered at home. The findings from this focus group led to the development of HY-PAD to improve functional status, QoL, and CV disease-risk indicators in patients with PAD.
Study design
HY-PAD (ClinicalTrials.gov ID: ICT03649204) was designed as a pilot, single-centre randomized controlled trial with 2 parallel arms to compare HY-PAD and standard care in patients with PAD. In 2020, the trial was put on hold due to the COVID-19 pandemic, which precluded in-person contact for several months. This step was followed by permanent changes to our CV rehabilitation programs to adapt to the new regulations requiring increased distancing and reduced class size. During the pandemic, the study was deemed to be high risk, as the protocol required high-risk populations (ie, older populations with CV disease) to report to the hospital. To reduce the total number of patient visits and in-person interactions, the study team reviewed the design and removed the standard-care arm to allocate all participants to HY-PAD in January 2022. The modified study protocol (ie, pre/post-test design) was approved by the Ottawa Health Science Network Research Ethics Board (Protocol #: 20180658-01H) and updated on ClinicalTrials.gov. The current study is reported in accordance with the CONSORT and SPIRIT Extension for RCTs Revised in Extenuating Circunstabce (CONSERVE) guidance19 to improve the transparency and completeness of reporting important protocol modifications.
Study participants
Participants meeting the following criteria were included: patients with intermittent claudication with hemodynamic evidence of PAD characterized by ankle-brachial index (ABI) ≤ 0.90 or > 1.4020 or anatomic evidence of ≥ 50% lower-extremity arterial stenosis identified by lower-extremity angiography, computed tomography angiography, magnetic resonance angiography, or ultrasound; able to participate in the 6-minute walk test (6MWT; ie, ability to walk independently); and willingness to provide informed consent. Patients who had lower-extremity amputation, critical limb ischemia, open lower-extremity wounds, or difficulty walking or understanding English or French were excluded.
Recruitment
The target sample size for this pilot study was n = 25. Patients who consented to be contacted for research purposes were contacted for study participation. If patients were referred to the CV or PAD rehabilitation program, research staff contacted the treating physicians for permission to approach for the study. Interested patients met with the research staff who explained the study and obtained written informed consent.
Baseline assessments
Age, sex, past medical history, past surgical and interventional history, medications, most recent ABI, family history of PAD, socioeconomics (ie, education level, net household income), and employment status were collected by research staff. Participants’ smoking status, glucose and lipid profiles, Walking Impairment Questionnaire (WIQ; measure of PAD-specified QoL), and 6MWT results were extracted from clinical intake assessments for those with PAD. All participants underwent baseline assessments including arterial stiffness using an automated blood pressure instrument (Mobil-O-Graph, IEM, Stolberg, Germany) and anthropometric measures, such as height, body mass, and waist and hip circumference.
HY-PAD
Participants were randomized in a 1:1 ratio into the HY-PAD or standard-care arm before the protocol amendment. Following the amendment, all recruited participants were assigned to the HY-PAD program. Participants attended a 1-hour supervised onsite PAD program 3 days per week for 4 weeks (a total of 12 sessions). During the supervised sessions, participants either walked on the indoor rehabilitation track or on treadmills at a walking speed producing the pain-scale score of 3-4 leg pain out of 5 after 8 minutes. Participants then rested until the pain subsided, and then started again, repeating the cycle 4 times for a total of 32 minutes of walking. The clinical consensus statement by the European Society of Cardiology Working Group recommends this type of exercise12; it is considered the most-effective intervention to improve walking-related outcomes.21
Following the 4-week onsite program, participants attended an 8-week home-based exercise program with weekly telephone calls (a total of 8 calls). Participants were instructed to perform unsupervised exercise as performed during the onsite sessions 3 days per week. Physiotherapists called the participants once a week (each call was approximately 15 minutes in duration), during which participants engaged in exercise-planning, goal-setting, action-planning, problem-solving, skill-building, and tips for exercise maintenance.
Follow-up assessments
Research staff collected the following: medications used, smoking status, WIQ responses, glucose and lipid profiles, and 6MWT results, if they were assessed as part of the clinical practice at our institute. If any of the measures were not completed, the study team performed the assessments. Participants also completed anthropometric and arterial-stiffness measures.
Primary outcome
Feasibility
Recruitment ratio, drop-out and reasons, adherence, and adverse events were assessed for feasibility. Recruitment rate was determined by the number of participants who enrolled during the study period. The dropout level was assessed as the number of participants who discontinued their participation in the HY-PAD program after first consenting to participate. Adherence to the onsite program was assessed by the number of prescribed exercise sessions completed by participants. For the home-based program, adherence was assessed by the number of phone calls participants completed.
Secondary outcomes
6MWT
Participants were instructed to walk on an indoor truck as far as possible for 6 minutes. With the onset of claudication, participants were permitted to rest until the pain subsided. The time of the onset of claudication and the distance covered before the onset of claudication were recorded, in addition to the total walking distance. When participants did not experience claudication during the 6MWT, the data were excluded from the analysis. The minimal clinically important differences (MCIDs) reported for patients with PAD completing home-based exercise were used. For small, moderate, and large changes in pain-free walking distance, the MCIDs were 24, 61, and 97 meters, respectively.22 The MCIDs for small, moderate, and large changes in 6MWT distance were 11, 28, and 45 meters, respectively.22
WIQ
WIQ assesses a PAD-specific measure of QoL, including self-perceived limitations in walking distance, walking speed, and stair-climbing.23 Each domain was scored on a scale of 0-100, for which 0 represented the greatest difficulty, and 100 represents the minimum restriction. The WIQ is a valid measure of walking ability in heterogeneous groups, including PAD.24 Published MCIDs were 6, 14, and 23 points for WIQ distance; 5, 12, and 19 points for WIQ speed score; and 5, 13, and 22 points for WIQ stair-climbing score, respectively, for small, moderate, and large differences.22
Blood glucose and lipid concentrations
Glycated hemoglobin A1C (A1C), cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride concentrations were retrieved from medical charts. If they were not completed clinically, the glucose and lipid concentrations were collected by research staff.
Cardiovascular hemodynamics
Noninvasive CV hemodynamics were measured by brachial and central blood pressure, cardiac output, stroke volume, augmentation index normalized for a heart rate of 75 beats per minute (AIx@75), augmentation pressure, total vascular resistance, and estimated pulse wave velocity using an ambulatory blood pressure monitoring system (Mobil-O-Graph). These measures were repeated 3 times, following 10 minutes of seated rest. While measurements were being taken, the participant was instructed to remain still, in a comfortably seated position. The 3 consecutive measures were averaged for analyses.
Statistical analysis
Statistical analyses were conducted with SPSS Statistics, version 28 (IBM, Armonk, NY). Intention-to-treat analysis was performed. For feasibility, descriptive statistics were used. For our secondary outcomes, the Shapiro-Wilk test was used to test for normality. Walking distance, claudication distance and time, WIQ distance, WIQ stair, total vascular resistance, augmentation pressure augmentation index, A1C, high-density lipoprotein cholesterol, and triglycerides measures violated the normality assumption. These data were normalized using a 2-step approach.25 Because the transformed data showed results consistent with those for the nontransformed data, outputs from the nontransformed data are reported. To evaluate whether outcomes were missing at random, or due to a systemic error, Little’s test of missing completely at random was performed. As data were found to be missing at random, a linear mixed-effects model with an unstructured covariance matrix was used to examined changes in walking time and distance, cardiovascular hemodynamics, blood glucose, and lipid concentrations over time. The maximum likelihood–estimation method was used to handle missingness. A 2-sided P value < 0.05 was considered statistically significant. Data are presented separately for female and male participants, as a previous study reported sex differences in WIQ scores in response to supervised exercise.26 However, no sex-specific analysis or between-sex comparisons were performed, owing to the limited number of female participants.
Results
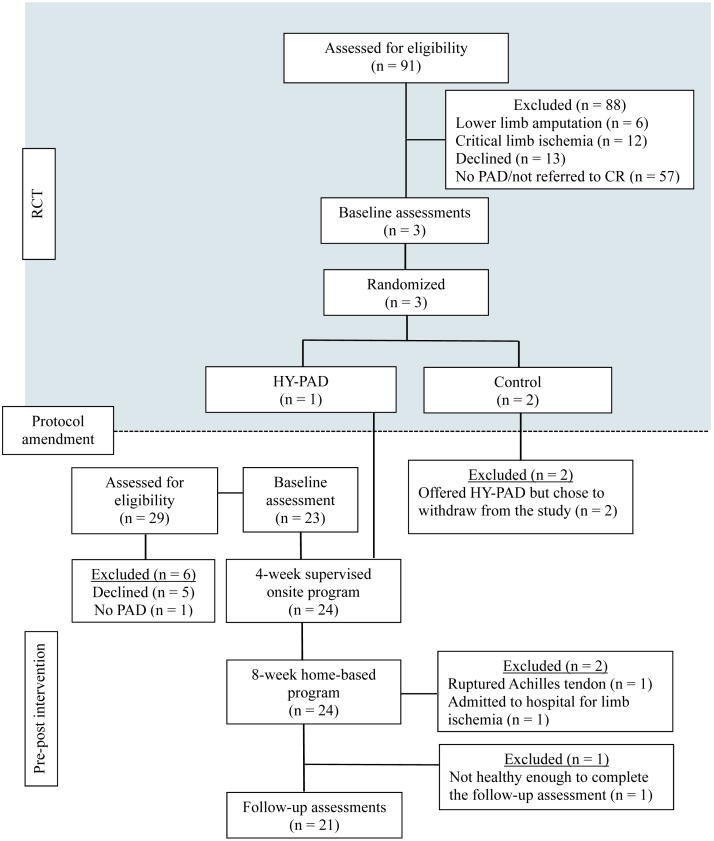
Figure 1 shows the flow of participants through each stage of the trial. Briefly, of the 120 patients assessed for eligibility, 24 enrolled in HY-PAD. Most of the participants were male (n = 21; 87.5%). Baseline characteristics are summarized in Table 1.
Figure 1.
Flow chart of participants through each stage. CR, cardiovascular rehabilitation; HY-PAD, hybrid onsite and home-based exercise program; PAD, peripheral artery disease; RCT, randomized controlled trial.
Table 1.
Baseline characteristics
| Characteristic | N = 24 | Female (n = 3) | Male (n = 21) |
|---|---|---|---|
| Age, y | 70 [8] | 71 [13] | 70 [7] |
| Height, cm | 170.0 [9.6] | 160.0 [5.3] | 171.5 [9.2] |
| Body mass, kg | 77.9 [19.1] | 70.8 [17.7] | 78.9 [19.5] |
| Ankle-brachial index | 0.68 [0.17] | 0.71 [0.14] | 0.68 [0.18] |
| Current employment status | |||
| Full-time employed | 5 (21) | 0 (0) | 5 (24) |
| Disability leave | 3 (13) | 1 (33) | 2 (10) |
| Retired | 13 (54) | 1 (33) | 12 (57) |
| Unknown | 3 (13) | 0 (0) | 3 (14) |
| Net annual household income, CAD | |||
| < 39,000 | 4 (17) | 0 (0) | 4 (19) |
| 40,000–99,999 | 6 (25) | 3 (100) | 3 (14) |
| > 100,000 | 11 (46) | 0 (0) | 11 (52) |
| Unknown | 3 (13) | 0 (0) | 3 (14) |
| Highest level of education attained | |||
| High school | 6 (25) | 2 (67) | 4 (19) |
| College | 5 (21) | 1 (33) | 4 (19) |
| Associate’s and/or bachelor’s degree | 7 (29) | 0 (0) | 7 (33) |
| Master’s, doctoral, or professional degree | 4 (17) | 0 (0) | 4 (19) |
| Unknown | 2 (8) | 0 (0) | 2 (10) |
| Risk factors | |||
| Family history of PAD | 8 (33) | 1 (33) | 7 (33) |
| Current smoking | 1 (4) | 0 (0) | 1 (5) |
| Past smoking | 22 (92) | 3 (100) | 19 (91) |
| Hypertension | 19 (79) | 2 (67) | 17 (81) |
| Diabetes | 11 (46) | 1 (33) | 10 (48) |
| Obstructive sleep apnea | 6 (25) | 2 (67) | 4 (19) |
| Coexisting cardiovascular conditions | |||
| Myocardial infarction | 8 (33) | 0 (0) | 8 (38) |
| Percutaneous coronary intervention | 6 (25) | 0 (0) | 6 (29) |
| Coronary artery bypass graft surgery | 11 (46) | 0 (0) | 11 (52) |
| Stroke | 3 (13) | 0 (0) | 3 (14) |
| Heart failure | 4 (17) | 0 (0) | 4 (19) |
| Medications | |||
| ACE-inhibitor | 12 (50) | 1 (33) | 11 (52) |
| β-blocker | 18 (75) | 1 (33) | 17 (81) |
| Angiotensin-receptor blocker | 5 (21) | 0 (0) | 5 (24) |
| Calcium-channel blocker | 7 (29) | 1 (33) | 6 (29) |
| Diuretics | 13 (54) | 2 (67) | 11 (52) |
| Aspirin | 17 (71) | 3 (100) | 14 (67) |
| Metformin | 6 (25) | 0 (0) | 6 (29) |
| Insulin | 6 (25) | 0 (0) | 6 (29) |
Values are mean [standard deviation] or n (%). CAD, Canadian dollar; PAD, peripheral artery disease; ACE, angiotensin-converting enzyme inhibitor.
Feasibility (adherence, attrition, and adverse events)
Two participants randomized into the control group prior to the protocol amendment decided not to attend HY-PAD following the baseline assessments. Of the remaining 24 enrolled in HY-PAD (20% recruitment ratio), the onsite program was completed by all participants. A total of 21 participants completed all 12 supervised sessions (ie, 100% adherence); 2 completed 11 sessions (92% adherence); and 1 participant completed 2 sessions (17% adherence). No adverse events occurred during the supervised program.
Although attending the home-based program, 1 participant was admitted to the hospital for limb ischemia during week 5. Of the remaining 23 participants, 19 attended all 8 phone-call meetings (100% adherence); 1 attended 7 meetings (88% adherence); 1 attended 4 meetings (50% adherence); 1 attended 3 meetings (38% adherence); and 1 did not attend any meetings (0% adherence). One participant ruptured their Achilles tendon during the last week and was not able to complete the follow-up assessments. Another participant who had a low level of adherence (ie, 17% for onsite, and 0% for home-based) was unable to complete the follow-up assessments, owing to non-PAD health issues. Altogether, 17 participants attended all onsite sessions and telephone meetings. Overall adherence was 93% ± 19%.
6MWT
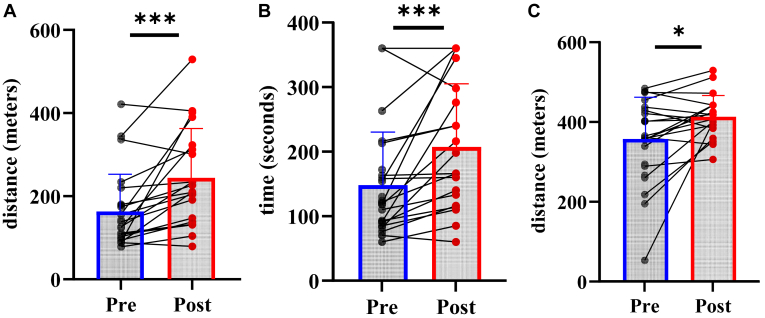
Pre- and post-6MWTs were completed by 21 participants. Following completion of the HY-PAD program, the onset of claudication distance (157 ± 90 vs 236 ± 122 meters; P < 0.001) and time (139 ± 70 vs 199 ± 94 seconds; P < 0.001) increased significantly (Fig. 2). Seven participants (33.3%) achieved a small MCID; 1 (4.8%) achieved a moderate MCID; and 6 (28.6%) achieved a large MCID for pain-free walking distance, respectively.
Figure 2.
6-minute walk test (6MWT) outcomes at baseline and 12 weeks. (A) Pain-free walking distance; (B) Pain-free walking time; (C) 6MWT distance. ∗P < 0.05, ∗∗∗P < 0.001.
Total 6MWT distance increased from 357 ± 105 to 413 ± 53 meters (P = 0.021; Fig. 2). Two participants (9.5%) achieved a small MCID; 2 (9.5%) achieved a moderate MCID; and 9 (42.9%) achieved a large MCID, respectively.
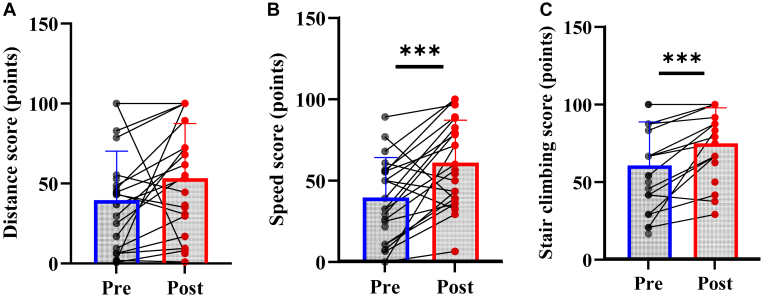
WIQ
Changes in the WIQ outcomes are presented in Figure 3. The WIQ speed score increased from 38.4 ± 25.1 to 60.6 ± 26.6 points (P < 0.001), and the WIQ stair-climbing score increased from 60.6 ± 29.4 to 74.0 ± 23.1 points (P < 0.001). The WIQ distance score did not increase significantly (39.8 ± 30.6 to 53.6 ± 34.0 points, P = 0.072). Three (14%), one (5%), and 14 (64%) achieved small, moderate, and large MCIDs for the WIQ speed score. For the WIQ stair-climbing score, 5 (28%), 3 (17%), and 5 (28%), respectively, achieved the small, moderate, and large MCIDs. For the WIQ speed score, 3 (14%), 1 (5%), and 14 (64%) achieved small, moderate, and large MCIDs, respectively.
Figure 3.
Changes in self-perceived walking limitations assessed by Walking Impairment Questionnaire (WIQ). (A) WIQ distance score; (B) WIQ speed score; (C) WIQ stair-climbing score. ∗∗∗P < 0.001.
Noninvasive CV hemodynamics
Noninvasive CV hemodynamics parameters measured at baseline and following completion of the HY-PAD program are summarized in Table 2. No significant changes occurred in the parameters.
Table 2.
Baseline and follow-up noninvasive cardiovascular hemodynamic measures
| Measure | n | Baseline | n | 12-week | Pre vs post, P | |
|---|---|---|---|---|---|---|
| Systolic blood pressure, mm Hg | O | 23 | 127 (19) | 17 | 128 (19) | 0.761 |
| F | 3 | 133 (20) | 3 | 134 (8) | ||
| M | 20 | 127 (19) | 14 | 127 (20) | ||
| Diastolic blood pressure, mm Hg | O | 23 | 73 (9) | 17 | 71 (9) | 0.183 |
| F | 3 | 75 (11) | 3 | 73 (9) | ||
| M | 20 | 73 (9) | 14 | 71 (9) | ||
| Stroke volume, mL | O | 23 | 79.6 (10.1) | 17 | 77.8 (12.2) | 0.646 |
| F | 3 | 81.1 (11.8) | 3 | 74.3 (11.6) | ||
| M | 20 | 79.4 (10.1) | 14 | 78.5 (12.6) | ||
| Cardiac output, L/min | O | 23 | 5.3 (0.9) | 17 | 5.1 (1.0) | 0.480 |
| F | 3 | 5.6 (1.0) | 3 | 4.8 (0.8) | ||
| M | 20 | 5.2 (0.9) | 14 | 5.1 (1.1) | ||
| Total vascular resistance, dyn/cm5 | O | 23 | 1.2 (0.3) | 17 | 1.3 (0.4) | 0.607 |
| F | 3 | 1.1 (0.4) | 3 | 1.3 (0.2) | ||
| M | 20 | 1.2 (0.3) | 14 | 1.3 (0.4) | ||
| Augmentation pressure, mm Hg | O | 23 | 15.7 (12.3) | 17 | 16.0 (12.0) | 0.282 |
| F | 3 | 18.4 (16.8) | 3 | 22.6 (9.5) | ||
| M | 20 | 15.3 (12.0) | 14 | 14.6 (12.3) | ||
| Augmentation index @ 75 bpm | O | 23 | 15.7 (12.3) | 17 | 15.0 (11.0) | 0.859 |
| F | 3 | 18.4 (16.8) | 3 | 21.8 (9.6) | ||
| M | 20 | 15.3 (12.0) | 14 | 13.5 (11.1) | ||
| Aortic pulse wave velocity, 90 m/sec | O | 23 | 10.1 (1.3) | 17 | 10.1 (1.8) | 0.986 |
| F | 3 | 10.4 (1.9) | 3 | 10.3 (2.6) | ||
| M | 20 | 10.1 (1.3) | 14 | 10.1 (1.7) |
bpm, beats per minute; F, females; M, males; O, overall.
Blood glucose and lipid concentrations
Changes in glucose and lipids measures are presented in Table 3. No significant changes occurred in glucose or lipid concentrations.
Table 3.
Blood glucose and lipid concentrations, at baseline and 12 weeks
| Concentration | n | Baseline | n | 12-week | Pre vs post, P | |
|---|---|---|---|---|---|---|
| Glycated hemoglobin A1C, % | O | 23 | 6.2 (1.1) | 19 | 6.0 (0.8) | 0.378 |
| F | 2 | 7.3 (1.8) | 2 | 6.1 (0.4) | ||
| M | 21 | 6.1 (1.0) | 17 | 6.0 (0.8) | ||
| Cholesterol, mmol/L | O | 21 | 3.2 (0.8) | 21 | 3.3 (0.7) | 0.515 |
| F | 2 | 3.7 (0.2) | 3 | 3.6 (0.7) | ||
| M | 19 | 3.1 (0.8) | 18 | 3.2 (0.7) | ||
| HDL-C, mmol/L | O | 21 | 1.1 (0.4) | 21 | 1.2 (0.4) | 0.218 |
| F | 2 | 1.3 (0.0) | 3 | 1.6 (0.5) | ||
| M | 19 | 1.1 (0.4) | 18 | 1.2 (0.4) | ||
| LDL-C, mmol/L | O | 21 | 1.5 (0.6) | 20 | 1.4 (0.6) | 0.325 |
| F | 2 | 1.6 (0.2) | 3 | 1.1 (0.7) | ||
| M | 19 | 1.4 (0.6) | 17 | 1.4 (0.5) | ||
| Triglycerides, mmol/L | O | 21 | 1.4 (0.6) | 21 | 1.6 (1.1) | 0.279 |
| F | 2 | 1.7 (1.1) | 3 | 1.9 (1.3) | ||
| M | 19 | 1.3 (0.6) | 18 | 1.5 (1.0) |
F, females; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; M, males; O, overall.
Discussion
This study is the first to report that a hybrid exercise program consisting of onsite and home-based components is feasible and associated with a significant increase in pain-free walking time and distance, and maximum 6MWT distance. Our results also showed that HY-PAD program completion was associated with significant improvements in self-perceived limitations, including walking speed and stair-climbing, but not with changes in CV hemodynamics, blood glucose or lipid concentrations. Walking capacity is an important predictor of the later morbidity and mortality incidence in patients with PAD.27 Increasing exercise performance with a corollary improvement in QoL and functional status is the primary goal of PAD treatment.2
The combination of acceptable recruitment ratio, low dropout rates, high attendance rates, and a small number of adverse events suggests that the HY-PAD program is a feasible intervention associated with improved walking capacity in patients with PAD. Of 120 individuals assessed for eligibility, 57 did not have PAD or were not referred to CV rehabilitation, 6 had lower-limb amputation, and 12 had critical-limb ischemia. Of the remaining 44 patients with PAD, 24 consented to participate in the HY-PAD program. Although our recruitment ratio (20%) was slightly better than that in previous RCTs (11%-15%),28,29 it was relatively low considering that the intervention was designed to increase accessibility. This difference may be attributable partly to the inclusion of individuals without PAD and those not referred to CV rehabilitation during their initial contact. The adherence level to the program was high for both onsite supervised and home-based, remote programs, except for 1 participant with health issues unrelated to PAD that negatively affected attendance. Because patient monitoring, patient education, self-efficacy, goal-setting, feedback, and training plan are critical for successful outcomes for patients with PAD enrolled in home-based programs,30 weekly phone calls may have contributed to a low level of attrition, a high level of adherence, and significant increases in walking capacity. No adverse events occurred during the onsite supervised sessions. However, rupture of the Achilles tendon and limb ischemia were reported during remote sessions, suggesting that an enhanced level of caution may be required for unsupervised programs. The dropout rate was low, with only those who experienced adverse events withdrawing from the study (n = 3; 13%). This rate was lower than the rates of withdrawal from onsite CV rehabilitation programs in patients with PAD (22%), and in those with concomitant PAD and coronary artery disease (25%).31
The mean change in 6MWT distance (49 ± 94 meters) following HY-PAD program participation was greater than the change induced by a previous 12-week, supervised program (15 ± 52 meters), and comparable to a home-based program (45 ± 53 meters).18 A previous meta-analysis showed that home-based walking exercise is associated with greater improvements in 6MWT distance, compared to supervised exercise.16 Our results may have been similar to those from a previous home-based program18 because a large part of our HY-PAD program (8 of 12 weeks) was home-based. The 6MWT distance is associated closely with physical activity during daily life in patients with PAD.32 Given that more than half of the participants achieved a moderate-to-large MCID for changes in 6MWT distance, the HY-PAD program may improve the functional capacity of patients with PAD who are living with claudication. Similarly, the significant increases in claudication walking distance and time support the potential benefits of HY-PAD program participation. The change in pain-free walking distance following HY-PAD participation (74 ± 89 meters) was similar to the effects of exercise, compared with usual care, reported in a previous meta-analysis (82 meters, 95% confidence interval: 72-92 meters).33 Although the change in pain-free walking time following HY-PAD program participation (60 ± 77 seconds) was less than that reported in the review (2.9 minutes, 95% confidence interval: 1.8-4.1 minutes),33 this difference is likely due to the fact that pain-free time was assessed during the 6MWT in this study, compared to treadmill walking in other studies. Leg pain during activity is a major determinant of functional limitation among patients with PAD, negatively affecting their ability to perform activities of daily living and their QoL.4 Although physiologic adaptations mediating the changes in walking ability were not examined in this study, one possibility is that HY-PAD participation increased walking capacity by improving arterial flow, endothelial function, inflammation, muscle structural, and metabolic abnormalities.1 The improvements in PAD-specific QoL may be attributable to the improvements in functional capacity and pain-free walking ability.
The mean changes in WIQ distance (13.4 ± 35.1 points) and stair-climbing (16.9 ± 16.8 points) scores observed in our study were similar to the changes seen following a 12-week supervised program (WIQ distance score, 13 ± 28 points; WIQ stair-climbing score, 12 ± 15 points), whereas the change in WIQ speed score observed in our study was larger (22.5 ± 25.4 vs 9 ± 15 points).34 This result suggests that a hybrid program can have similar or greater effects on the WIQ scores, compared to those of supervised programs. Why the only the improvement in WIQ speed score was greater in our HY-PAD program, compared to the score in the previous study is unclear. Given that home-based exercise may be superior to supervised exercise to increase community-based ambulation,34 our HY-PAD program that prescribed home-based exercise following supervised exercise sessions may have had a positive impact on the WIQ speed score. Combined with the outcomes showing that most participants achieved the MCID for WIQ scores, HY-PAD participation may be beneficial in improving self-perceived limitations in walking ability.
The lack of changes in blood glucose or lipid concentrations observed in our study is consistent with results in previous exercise studies in PAD.7 Blood glucose and lipid concentrations of our participants at baseline were well controlled, leaving little room for improvement. This situation may have resulted in no change following HY-PAD program participation. Similarly, CV hemodynamics, an independent predictor for CV events and mortality,35 did not change over 12 weeks. Because changes in CV hemodynamics depend on exercise intensity,35 our exercise program may have lacked the intensity required to produce significant changes. Another possibility is that our 12-week program was not sufficient for the vascular system remodelling, as exercise training load is a major determinant of structural change.36 Important to note is that because these measures were collected at rest, we cannot disregard the possibility that CV responses may have differed during exercise.
A major limitation of the study was that we had to modify the design from a randomized trial to a pre/post design, as a means to reduce the number of patients’ hospital visits during the COVID-19 pandemic. This approach resulted in a smaller-than-anticipated number of participants. The small sample size precluded us from establishing the feasibility of participation in the HY-PAD program, compared to receipt of standard of care. Additionally, the efficacy of the HY-PAD program on the important changes was not established, as we lacked a control group. Given the promising results, future randomized trials are warranted to examine the efficacy of hybrid programs in patients with PAD. Second, although we were sensitive to the issue of under-recruitment of female participants in clinical trials, our study participants were predominantly male, limiting the generalizability of our findings to the female population. An important result is that, of 41 potential female participants screened, only 3 (7%) enrolled in the HY-PAD program. This participation level is markedly lower than the 18% enrollment level of male participants. Strategies to increase female participation are warranted. Third, adherence to the home-based program was assessed based on the number of phone calls completed by the participants. The number of sessions completed by the participants was not available in our study. Further, data on exercise intensity were not available. This limitation is major, given that exercise intensity is a determinant of walking performance.37 Future studies should measure exercise intensity of interventions to develop more-specific exercise prescriptions for patients with PAD. Last, a ceiling effect occurred on the onset of claudication time and distance, because they were assessed during the 6MWT. For example, one participant did not experience claudication during the baseline 6MWT, and this did not change over time. Improvements that may have occurred in this participant were not captured.
Conclusion
The finding from this pre/post-intervention study showed that the HY-PAD program was feasible and was associated with significantly increased walking capacity and decreased self-perceived walking limitations in patients with PAD. Given the challenges in participating in onsite CV rehabilitation programs,38 the hybrid program may offer a feasible and effective intervention to improve the functional capacity and QoL of patients suffering from PAD. Future randomized controlled trials are warranted to confirm the efficacy of the HY-PAD program in patients with PAD.
Acknowledgements
The authors thank Sandra Black, Irene Toonders, and Alexandra Dicks for their support for this study.
Data Availability
A data-sharing agreement can be requested from our legal team.
Ethics Statement
The modified study protocol (ie, pre/post-test design) was approved by the Ottawa Health Science Network Research Ethics Board (Protocol #: 20180658-01H) and updated on ClinicalTrials.gov.
Patient Consent
The authors confirm that a patient consent form(s) has been obtained for this article.
Funding Sources
This investigator-initiated research was supported by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario (UOH-18-005). The funder had no role in the design, data collection, data analysis, or reporting of the study.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 118 for disclosure information.
References
- 1.Hiatt W.R., Armstrong E.J., Larson C.J., Brass E.P. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116:1527–1539. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 2.Olin J.W., White C.J., Armstrong E.J., et al. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol. 2016;67:1338–1357. doi: 10.1016/j.jacc.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 3.McDermott M.M. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116:1540–1550. doi: 10.1161/CIRCRESAHA.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson B.L., Al-Omran M., Anand S.S., et al. Canadian Cardiovascular Society 2022 guidelines for peripheral arterial disease. Can J Cardiol. 2022;38:560–587. doi: 10.1016/j.cjca.2022.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Rehring T.F., Sandhoff B.G., Stolcpart R.S., et al. Atherosclerotic risk factor control in patients with peripheral arterial disease. J Vasc Surg. 2005;41:816–822. doi: 10.1016/j.jvs.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E., Hirsch A.T. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999-2004. Atherosclerosis. 2008;201:425–433. doi: 10.1016/j.atherosclerosis.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy T.P., Cutlip D.E., Regensteiner J.G., et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leng G.C., Fowler B., Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000990. [DOI] [PubMed] [Google Scholar]
- 9.Gardner A.W., Poehlman E.T. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 10.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott M.M., Ades P., Guralnik J.M., et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzolai L., Belch J., Venermo M., et al. Exercise therapy for chronic symptomatic peripheral artery disease. Eur Heart J. 2024;45:1303–1321. doi: 10.1093/eurheartj/ehad734. [DOI] [PubMed] [Google Scholar]
- 13.Gupta T., Manning P., Kolte D., et al. Exercise therapy referral and participation in patients with peripheral artery disease: insights from the PORTRAIT registry. Vasc Med. 2021;26:654–656. doi: 10.1177/1358863X211033649. [DOI] [PubMed] [Google Scholar]
- 14.Cetlin M.D., Polonsky T., Ho K., et al. Barriers to participation in supervised exercise therapy reported by people with peripheral artery disease. J Vasc Surg. 2023;77:506–514. doi: 10.1016/j.jvs.2022.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heindl B., Ramirez L., Joseph L., et al. Hybrid cardiac rehabilitation—the state of the science and the way forward. Prog Cardiovasc Dis. 2022;70:175–182. doi: 10.1016/j.pcad.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Thangada N.D., Zhang D., Tian L., et al. Home-based walking exercise and supervised treadmill exercise in patients with peripheral artery disease: an individual participant data meta-analysis. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.34590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott M.M. Exercise rehabilitation for peripheral artery disease: a review. J Cardiopulm Rehabil Prev. 2018;38:63–69. doi: 10.1097/HCR.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner A.W., Parker D.E., Montgomery P.S., Blevins S.M. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orkin A.M., Gill P.J., Ghersi D., et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA. 2021;326:257–265. doi: 10.1001/jama.2021.9941. [DOI] [PubMed] [Google Scholar]
- 20.Anderson J.L., Halperin J.L., Albert N.M., et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 21.Peñín-Grandes S., López-Ortiz S., Maroto-Izquierdo S., et al. Winners do what they fear: exercise and peripheral arterial disease—an umbrella review. Eur J Prev Cardiol. 2024;31:380–388. doi: 10.1093/eurjpc/zwad261. [DOI] [PubMed] [Google Scholar]
- 22.Gardner A.W., Montgomery P.S., Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med. 2018;23:349–357. doi: 10.1177/1358863X18762599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regensteiner J.G., Steiner J.F., Panzer R.J., Hiatt W.R. Evaluation of walking impairment by questionnaire in patients with peripheral arterial-disease. Clin Res. 1990;38:A515. [Google Scholar]
- 24.McDermott M.M., Liu K., Guralnik J.M., et al. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–1081. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 25.Templeton G. A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research. Commun Assoc Information Syst. 2011;28:41–58. [Google Scholar]
- 26.Lanzi S., Pousaz A., Calanca L., Mazzolai L. Sex-based differences in supervised exercise therapy outcomes for symptomatic peripheral artery disease. Vasc Med. 2023;28:147–149. doi: 10.1177/1358863X221149454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott M.M., Liu K., Ferrucci L., et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011;57:962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott M.M., Liu K., Guralnik J.M., et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy T.P., Cutlip D.E., Regensteiner J.G., et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pymer S., Ibeggazene S., Palmer J., et al. An updated systematic review and meta-analysis of home-based exercise programs for individuals with intermittent claudication. J Vasc Surg. 2021;74:2076–2085.e2020. doi: 10.1016/j.jvs.2021.03.063. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen C.H., Marzolini S. Pre-participation withdrawal and noncompletion of cardiac rehabilitation in peripheral artery disease: matched comparisons to coronary artery disease. J Cardiopulm Rehabil Prev. 2024;44:55–63. doi: 10.1097/HCR.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 32.McDermott M.M., Ades P.A., Dyer A., et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–1237.e1231. doi: 10.1016/j.jvs.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane R., Harwood A., Watson L., Leng G.C. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12:CD000990. doi: 10.1002/14651858.CD000990.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner A.W., Parker D.E., Montgomery P.S., et al. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–498. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashor A.W., Lara J., Siervo M., et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e110034. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green D.J., Smith K.J. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harb Perspect Med. 2018;8:a029819. doi: 10.1101/cshperspect.a029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fassora M., Calanca L., Jaques C., et al. Intensity-dependent effects of exercise therapy on walking performance and aerobic fitness in symptomatic patients with lower-extremity peripheral artery disease: a systematic review and meta-analysis. Vasc Med. 2022;27:158–170. doi: 10.1177/1358863X211034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Divakaran S., Carroll B.J., Chen S., et al. Supervised exercise therapy for symptomatic peripheral artery disease among Medicare beneficiaries between 2017 and 2018: participation rates and outcomes. Circ Cardiovasc Qual Outcomes. 2021;14:e007953. doi: 10.1161/CIRCOUTCOMES.121.007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing agreement can be requested from our legal team.