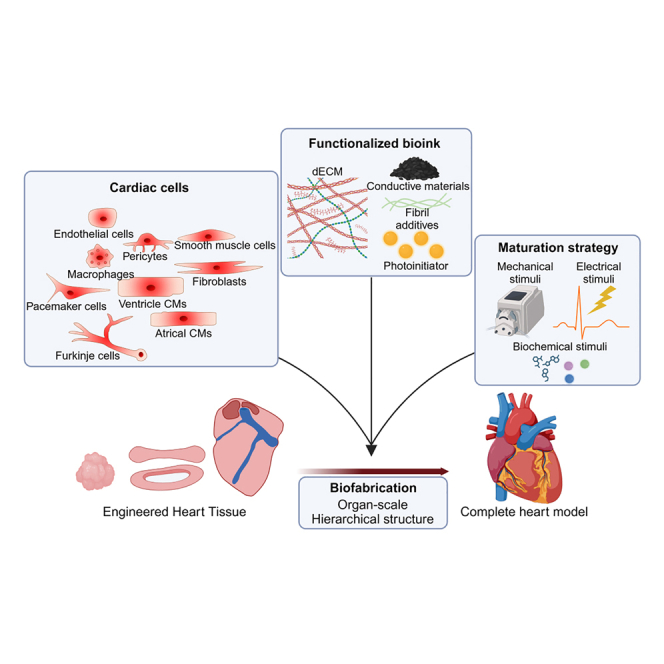
Summary
The heart, with its complex structural and functional characteristics, plays a critical role in sustaining life by pumping blood throughout the entire body to supply nutrients and oxygen. Engineered heart tissues have been introduced to reproduce heart functions to understand the pathophysiological properties of the heart and to test and develop potential therapeutics. Although numerous studies have been conducted in various fields to increase the functionality of heart tissue to be similar to reality, there are still many difficulties in reproducing the blood-pumping function of the heart. In this review, we discuss advancements in cells, biomaterials, and biofabrication in cardiac tissue engineering to achieve cardiac models that closely mimic the pumping function. Moreover, we provide insight into future directions by proposing future perspectives to overcome remaining challenges, such as scaling up and biomimetic patterning of blood vessels and nerves through bioprinting.
Subject areas: Cardiovascular medicine, Bioengineering, Tissue engineering, Biomaterials
Graphical abstract
Cardiovascular medicine; Bioengineering; Tissue engineering; Biomaterials
Introduction
The heart plays an essential role in maintaining life by supplying blood to all organs.1 Its pumping function is facilitated by several key structural and functional components, including the contraction of the myocardial walls to generate sufficient force for blood ejection, the conduction system to regulate the timing of contractions, the vascular system to deliver blood, and the valves to ensure unidirectional blood flow by preventing backflow. Replicating these complex cardiac functions in vitro enables a deeper understanding of the pathophysiology of cardiovascular disease and the development of novel therapeutic strategies.2,3,4 Three-dimensional (3D) engineered heart tissue (EHT) models, from self-assembled spheroids to bioprinted chamber-like tissues, have emerged as a promising platform to recapitulate the contractility and electrophysiological properties of the myocardium.5,6
Major components in generating EHT include cells, biomaterials, and biofabrication techniques.7,8 Cells serve as the fundamental units of function of EHTs. Parenchymal cells directly execute specific functions, while mesenchymal cells provide essential support to enhance parenchymal cell function.9 Consequently, the development of cell sources that constitute the heart forms the foundation for constructing EHT capable of replicating cardiac function. Biomaterials offer a 3D space where cells can reside while providing mechanical and biochemical cues that affect cellular behaviors.10 When the cells are surrounded by tissue-specific biomaterials, they reproduce more native-like behaviors including functionality and genetic expression. Simultaneously, biomaterials must possess suitable shape fidelity to enable precise fabrication. However, these properties involve trade-offs, introduced by the concept of biofabrication window, and ongoing efforts are focused on addressing both properties.11 Biofabrication provides geometrical cues to cells by replicating the structural patterns inherent to native tissue architecture, thereby regulating cellular functions and facilitating the formation and maturation of EHTs.12,13 EHTs in strip or ring shapes produce contractility and electrophysiological properties; however, EHTs in chamber-like structures, can produce more chamber-like structures such as volume-pressure relationship or perfusability between chambers.14,15,16,17
Advances across various fields have enabled the development of individual components replicating critical cardiac functions, including the conduction system, vascular system, and valves, alongside EHTs that mimic the hierarchical structural characteristics of the heart.18,19,20,21,22,23 However, the integration of these elements into a comprehensive, all-in-one heart model capable of replicating the actual pumping function remains an unmet challenge.
In this review, we discuss the current status of cardiac models, focusing on advancements in their functionalities and the development of diverse tissue engineering strategies. We explored various approaches that considered diverse aspects, such as cellular composition, biomaterials, and geometrical cues (Figure 1). Finally, we addressed the remaining challenges and key milestones required to achieve blood-pumping function.
Figure 1.
Key elements necessary for achieving blood-pumping heart model
Enhanced heart functions obtained by reproducing cellular heterogeneity
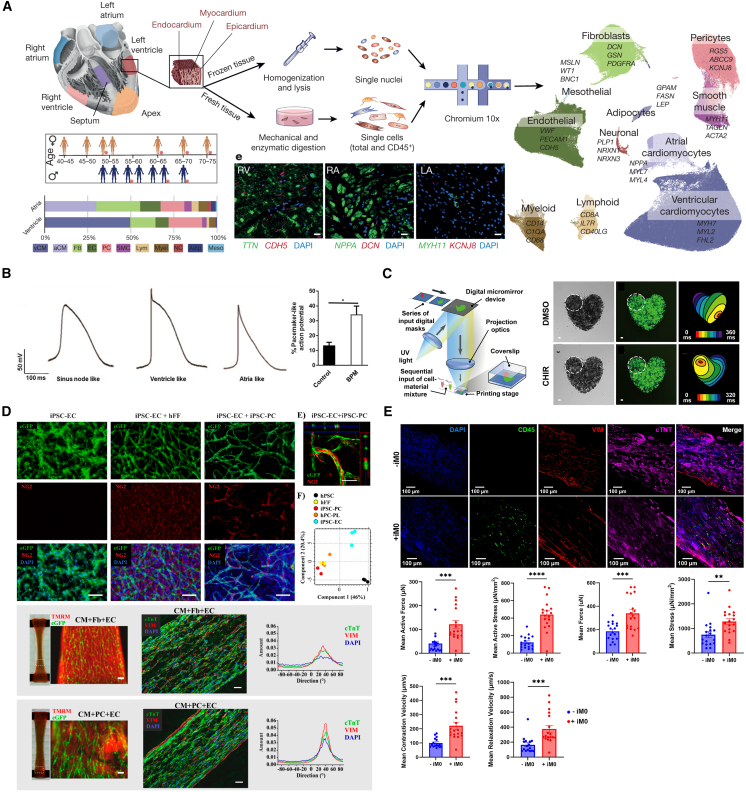
The heart contains a complex cell population. Litviňuková et al. revealed a cellular atlas of the adult human heart via single-cell transcriptomes that illustrates the interactions and networks of various cell types, including cardiomyocytes (CMs), pericytes, fibroblasts (FBs), cardiovascular, as well as immune cells (Figure 2A).24 CMs, the functional units of the heart, produce contractility along with electrophysiological properties and are categorized as working CMs (e.g., atrial and ventricular CMs), pacemaker cells, or conducting CMs. They are often derived from stem cells or primary cells, exhibiting immature characteristics in in vitro culture compared to adult CMs. In this context, non-myocytes support working CMs to produce mature functionality by remodeling the extracellular matrix (ECM) and interacting via paracrine and juxtacrine effects, which enhance structural, metabolic, and electrophysiological development, resulting in cell alignment, improved calcium ion signaling, and ultimately greater contractile force.25,26 In addition, the cells comprising the heart valves, arteries, veins, and coronary arteries not only play distinct roles but also contribute to the heart’s optimal blood-pumping functionality through their integration within their structures, preventing backflow, delivering blood outward, and supplying oxygen to the myocardium. Therefore, various cardiac cells have been utilized to generate EHT, demonstrating the cellular ensembles contribute significantly to precisely replicating cardiac physiology.
Figure 2.
Reproduction of cellular heterogeneity by developing engineered heart tissue models consisting of various cardiac cells
(A) Schematic illustrating heterogeneous cellular composition of adult human heart. (Reproduced with permission.24 Copyright 2020, Springer Nature.).
(B) Distinct action potential waveform for different cardiomyocytes (Reproduced with permission.27 Copyright 2020, BioMed Central Ltd.).
(C) Bioprinting of pacemaker cells to control the initial point of contraction (Reproduced with permission.28 Copyright 2019, Elsevier B.V.).
(D) EHT developed using iPSC derived endothelial cells and pericytes and the role of pericytes in pathophysiological properties (Reproduced with permission.29 Copyright 2020, MDPI.).
(E) EHT developed using iPSC derived macrophages, which improves cardiac functions (Reproduced with permission.30 Copyright 2024, Elsevier Inc.).
Generation of chamber-specific EHT from sub-type CMs
The working CMs, which are characterized by a striated myofibrillar actin structure, are essential for generating EHTs because they produce the most important cardiac functions such as contractile force and electrophysiological properties. Ventricular (vCM) and atrial (aCM) CMs exhibit distinct structural and functional properties based on their roles in the heart. vCMs have a larger cell body and longer sarcomere than aCMs, enabling them to generate the greater contractile force needed to pump blood out of the heart.25 Functionally, they display a longer contraction-relaxation time and slower beating frequency compared to aCMs. Additionally, vCMs and aCMs differ in ion channel types, leading to specific responses to drugs.31 Given its central role and unique properties, selecting the appropriate type of working CM is essential for generating chamber-specific EHT.
Early EHTs were created by culturing CMs isolated from animals—such as rats, chickens, and pigs—in a 3D environment to engineer tissue-like functions.26,32,33,34 However, isolated CMs show different characteristics, such as susceptibility to contamination with non-myocytes and vulnerability to long-term culture.35,36 Advances in induced pluripotent stem cell (iPSC) technology and tissue engineering have enabled the study of CMs as an unlimited cell source with long-term survival at the tissue level. Initial differentiation protocols primarily yielded vCMs that corresponded to the earliest stages of cardiac development.37 Consequently, the EHT models produced using iPSC-derived CMs (iPSC-CMs) exhibited ventricle-like characteristics, making them suitable for cardiotoxicity assessment in drug development and potential therapeutic applications in vitro.
The development of differentiation techniques has enabled directed differentiation of aCMs. The addition of retinoic acid at the differentiation stage of the cardiac mesoderm resulted in the population of aCM becoming dominant, characterized by the expression of myosin regulatory light chain 2 atrial isoform, and exhibiting shorter and more frequent contractile activity.38 These distinctive properties of aCMs have been incorporated into EHTs, enabling them to demonstrate atrial-specific cardiac functionality. EHTs composed of aCMs exhibited upregulated atrial genes (GJA5, KCNA5, KCNJ3, NPPA, MYL7, NR2F2) and downregulated ventricular genes (MYL2, MYH7, HEY2). Moreover, atrial EHTs displayed distinct contractility and electrophysiological properties, including action potential amplitude, duration, and conduction velocity. When ventricular and atrial EHTs were employed for pharmacological testing, they showed chamber-specific responsiveness following treatment with lidocaine, a sodium channel blocker used only for treating ventricular arrhythmias.16 Likewise, Zhao et al. utilized the Biowire platform to produce EHTs compartmentalized into atrial and ventricular regions using aCMs and vCMs, respectively, demonstrating distinct chamber-specific functions in each region.39 Each part exhibited distinct myosin regulatory light chain 2 expressions, as well as, calcium transient and action potential profiles. Furthermore, serotonin and ranolazine, a 5-HT agonist, increased calcium transients exclusively in atrial regions, while ranolazine, a rapid voltage-gated sodium channel blocker, selectively reduced conduction velocity in atrial regions without affecting the ventricular area.
Protocols for differentiating nodal-like CMs (i.e., pacemaker cells) have been established through the modulation of complex signaling pathways.27,28,40,41 These nodal-like CMs were characterized by the expression profile NKX2-5-, cTnT+, and SHOX2+, as well as their specific action potential characteristics, such as faster beating rate, gradual depolarization, short action potential duration, and small action potential amplitude (Figure 2B).27,28 Moreover, the nodal-like CMs acted as biological pacemakers when integrated into both the mouse heart and printed EHT model (Figure 2C).28,41 In CM monolayers lacking nodal-like cardiomyocytes, the initiation point of electrical activity shifted continuously. However, it stabilized following the addition of nodal-like CMs. Furthermore, when human nodal-like CMs were transplanted into a rat heart, the beating rate slowed to match the typical beating rate of a human heart, indicating that electrical activity is regulated by nodal-like CM.41 In addition, the initiation point of electrical activity can be precisely modulated by strategically positioning nodal-like CMs within EHTs using bioprinting technology.28
Improved functionality of EHT models by co-culturing non-myocytes with CMs
In recent years, the significance of non-myocytes has been increasingly recognized, with studies revealing their indispensable roles in cardiac development and disease through their interactions with myocytes.42 Non-myocytes primarily consist of cardiac FBs and endothelial cells (ECs), along with additional populations of immune cells and autonomic neurons.43 Integrating these non-myocyte cell populations into cardiac modeling systems is expected to enhance the structural, metabolic, and electrophysiological development of iPSC-CMs, contributing to the creation of a microenvironment that closely resembles physiological conditions.
FBs are representative supporting cells responsible for ECM remodeling and linkage between CMs, as connective tissues. Initially, alternative stromal cells such as mesenchymal stem cells or FBs from other tissues were utilized to replace FBs.44,45,46,47 The addition of these cells resulted in tissue compaction and the emergence of a structurally improved phenotype compared to that observed with CMs alone. In addition, they improved cell viability, sarcomeric organization, and contractility of EHTs. In particular, cardiac FBs showed similar interactions with CMs compared to other FBs.48 Giacomelli et al. have shown that cardiac FBs promote greater structural, electrical, and metabolic maturation than skin FBs. This effect was attributed to the formation of gap junctions and the activation of the cyclic adenosine 3,5-monophosphate-pathway.49
ECs and pericytes are responsible for vascular formation in the heart by supplying oxygen and nutrients to the thick myocardium.29 In addition, ECs secrete various cytokines (e.g., basic fibroblast growth factor , vascular endothelial growth factor, and hepatocyte growth factor), and signaling factors (e.g., neuregulin, PDGF-β, nitric oxide, and endothelin-1), which affect viability and functionality of CMs and FBs.50,51,52 Initially, human umbilical vein endothelial cells, a well-established and commonly used source of ECs, were employed for cardiac tissue engineering.53,54 Moreover, adipose-derived stromal and stem cells and human cardiac microvascular ECs have been used to create vascular networks.55,56 Recently, research on EHT production using iPSC-ECs has been actively conducted, and it has been reported that ECs and CMs have a synergistic effect on mutual maturation.49,57,58
Advancements in iPSC differentiation technology have enabled the development and utilization of various cell types, including pericytes, epicardial cells, SMCs, and immune cells, in addition to major non-myocyte FBs and ECs for EHT production.3,29,30,59,60,61 For example, iPSC pericytes support the angiogenesis of ECs and participate in fibrotic processes alongside FBs under fibrotic conditions (Figure 2D).29,62 Recently, the impact of the immune system on cardiac function has been investigated.30,60,63 Macrophage incorporation has been shown to enhance the contractile force and contraction kinetics of EHTs, enabling long-term vascularization (Figure 2E).30 The mechanism of this phenomenon was elucidated to be the stimulation of the β-adrenergic signaling pathway by cytokines and calcium signaling, and the reduction of stress by the ingestion of apoptotic CM.
Improving heart functions through maturation strategies
While the functionality of EHTs has become sophisticated with the development of diverse cell sources, they still exhibit fetal-like phenotypes due to the use of iPSC-derived cells. Therefore, various maturation strategies have been studied to achieve adult-like functional characteristics.64
Mechanical stress to improve structural properties
Mechanical stimulation replicates the preload and afterload in the heart, caused by the cardiac cycle, wall stress, myocardial stretch, and blood pressure.65,66,67 The mechanical stimuli facilitate the structural development of EHTs, as well as load-dependent ion channels and intracellular ion concentrations, influencing overall cardiac functions.66,68 To replicate the native myocardium, many EHT platforms incorporate controlled uniaxial tension to develop tissues with aligned myofibril structures. Leonard et al. engineered an EHT system suspended between a rigid and a flexible post for passive mechanical stimulation, where afterload conditions could be tuned from 0.09 up to 9.2 μN/μm by adjusting brace lengths.69 Increased afterload deflected the flexible post, revealing that auxotonic twitch forces increased with higher afterload levels. Notably, EHTs subjected to higher afterload exhibited elongated sarcomeres, increased CM area, and improved calcium handling and contractile function, thereby promoting cardiac maturation. However, excessive afterload induced pathological hypertrophic response characterized by fibrosis and fetal-like metabolism, suggesting its potential utility as an in vitro cardiac disease model.
Electrical stimulation to improve electrophysiological properties
Electrical stimulation has been widely employed to promote the maturation of EHTs by developing cell-cell coupling via gap junctions and voltage-gated ion channels.70 Min et al. introduced a micropillar electrode array platform that delivered intermittent biphasic electrical pulses mimicking a natural heartbeat.71 Electrically stimulated spheroids elevated the expression of cardiac-specific markers, such as α-actinin and cTnT, and exhibited well-defined sarcomeric striations with a prominent expression of connexin-43, a key cardiac gap junction protein. Enhanced maturation led to a significantly higher normalized peak amplitude compared to non-stimulated spheroids, as evidenced by the upregulated expression of channel proteins associated with calcium flux. Ronaldson-Bouchard et al. demonstrated that electrical stimulation was significantly more effective during the early stages of differentiation (day 12), when CMs exhibit high plasticity, compared to later stages (day 28). Furthermore, a protocol involving the gradual increase of stimulation frequency over time was shown to be superior to a constant-frequency approach. This strategy facilitated the development of adult-like phenotypes in CMs within EHTs, characterized by mature gene expression profiles, highly organized ultrastructure, physiological sarcomere length, mitochondrial development, the formation of transverse tubules, oxidative metabolism, a positive force-frequency relationship, and functional calcium handling. In addition, the resulting adult-like CMs demonstrated appropriate physiological responses to isoproterenol treatment and pathological hypertrophy induction.72
Biochemical cues for metabolic maturation
Biochemical cues mimic the metabolic and hormonal maturation of EHT.73,74,75 Growth hormones, such as tri-iodo-L-tyronine (T3), which is essential for optimal heart growth, have been shown to facilitate hiPSC-CM maturation.73 Following T3 treatment, hiPSC-CMs exhibited larger cell sizes, and longer sarcomere lengths, resulting in reduced proliferative activity compared to untreated cells. These translated into significantly improved contractile force and kinetics, as evidenced by higher twitch force and shorter time to peak contraction. Additionally, regulating the cardiac energy metabolism has emerged as a promising strategy for CM maturation. Among the molecular pathways involved in this process during cardiac development is the transition of postnatal CMs to higher oxygen tension environments. This transition is characterized by the downregulation of hypoxia-inducible factor 1α (HIF-1α) and the activation of fatty acid oxidation (FAO), promoting CM maturation.76 In this context, treating hiPSC-CMs with HIF-1α inhibitors upregulated the expression of genes involved in FAO. This treatment enhanced mitochondrial quantity and maturation, resulting in significantly higher calcium transient rates and improved contraction and relaxation velocities.74 Feyen et al. developed a metabolic maturation medium enriched with high levels of fatty acids and reduced glucose concentrations to supply oxidative substrates. This approach promoted the maturation of EHTs, enhancing their contractile force. Moreover, the metabolic medium improved the long-term stability and structural development of EHTs, facilitating the generation of reliable disease models, such as long QT syndrome and dilated cardiomyopathy.77
Designing biomaterials to increase structural and functional properties of EHTs
Biomaterials are employed to provide necessary biochemical and biophysical microenvironments to cells that can modulate cellular behavior during tissue morphogenesis and regeneration.78 Synthetic polymers (e.g., polylactic acid, polyglycolic acid, polylactic-co-glycolic acid, poly(ethylene glycol), and polyvinyl alcohol) are commonly used to fabricate 3D tissue scaffolds that support rigid tissue structures due to their high mechanical strength, enabling precisely controlled architectures.79 In addition, they are advantageous for their tunable properties and well-established structures that allow for easy modification.80 However, they often lack cell-interactive characteristics due to their bio-inert nature and limited surface functional groups for cell adhesion, which can inhibit cell proliferation and function.81,82 In contrast, natural biomaterials (e.g., collagen, fibrinogen, gelatin, silk, and decellularized ECM (dECM)) offer better bioactivities and have been widely used for cardiac tissue engineering.83 In particular, type I collagen and fibrin have been employed to generate EHTs. These biomaterials have the advantage of creating a 3D matrix by providing a favorable environment for cells.26,84,85,86 Moreover, these materials are often supplemented with mixtures of basement membrane proteins, such as Matrigel and Geltrex, to provide a more native-like microenvironment.34,87 Nevertheless, they are fall short in reproducing the intricate constitution and structure of the native heart ECM.58,88
Providing a tissue-specific microenvironment with tissue-derived biomaterials
Decellularization of native tissues has attracted significant interest as a promising method for obtaining a microenvironment that can closely mimic native tissues by eliminating cellular components while preserving the ECM. This approach not only offers a straightforward yet intricate environment but also ensures a tissue-specific microenvironment for incorporation, given that each tissue constitutes a distinct ECM.89,90,91 Pati et al. developed three distinct dECM hydrogels and demonstrated their tissue-specific gene expression following cell encapsulation (Figure 3A).88 Moreover, the authors utilized these dECM hydrogels mixed with cells as a bioink for bioprinting, marking the first application of these hydrogels in bioprinting. These dECM hydrogels contained tissue-specific protein compositions, and their effects were highlighted through an in-depth analysis of tissue-specific cell behavior and gene expression profiles during cell culture.92 Specifically, a heart tissue-derived ECM (hdECM) hydrogel demonstrated efficacy by upregulating the differentiation and maturation of cells.33 Direct reprogramming of cardiac FBs into CMs was promoted in 3D cultures encapsulated in hdECM, and recovery was promoted when cells were delivered to the site of myocardial infarction.93 Moreover, iPSC-derived CMs, FBs, and ECs showed enhanced structural arrangement and upregulated cardiac-specific genes in hdECM, as compared to type I collagen and other types of ECM hydrogels, emphasizing their tissue specificity (Figure 3B).58
Figure 3.
Functional biomaterials to provide native-like microenvironment and improve cardiac function
(A) Decellularized extracellular matrix (dECM)-based hydrogel, faithfully replicating the structures and composition of the natural heart ECM, showing high cell viability and functionality (Reproduced with permission.88 Copyright 2014, Springer Nature.).
(B) Superiority of decellularized extracellular matrix in EHT fabrication compared to other biomaterials (Reproduced with permission.58 Copyright 2024, Springer Nature.).
(C) Visible-light activated photoinitiator enabling the fabrication of dECM-based bioink into large and complex constructs with high shape fidelity (Reproduced with permission.94 Copyright 2021, John Wiley & Sons.).
(D) Incorporation of conductive materials enhancing the electrophysiological functions of the tissue (Reproduced with permission.95 Copyright 2021, Elsevier B.V.).
Improvement of crosslinking properties for superior shape fidelity
While natural biomaterials offer superior biofunctionality, they exhibit inferior mechanical properties, leading to low shape fidelity. Increasing polymer concentration is one method to enhance shape fidelity; however, the resulting dense polymer network can impede cell growth and function, creating a trade-off between these properties.11,96 To simultaneously achieve high shape fidelity while preserving the biofunctionality of natural polymers, diverse crosslinking strategies have been developed, including chemical crosslinking (e.g., photo and enzymatic crosslinking) and physical crosslinking (e.g., ionic and thermal crosslinking).
Ionic crosslinking is a rapid physical crosslinking method, with alginate being extensively utilized biomaterial. Alginate crosslinks via multivalent ions (e.g., calcium (Ca2+) or magnesium (Mg2+) ions), forming an “egg-box” structure that imparts gelling ability and mechanical strength.97 However, it is difficult to control crosslinking kinetics because they are governed by intrinsic and rapid binding, resulting in inhomogeneity within structures.98 Furthermore, alginate lacks cell-binding sites, limiting cell migration and adhesion, which affects long-term cell function.99,100 In addition, the stability of ionically crosslinked hydrogels can be disrupted by exchange reactions occurring between natural monovalent cations and the multivalent ions in the crosslinked scaffold.97,101 Therefore, the composite of alginate with other natural biomaterials has attracted attention as a strategy to overcome the limitations of each material.100,102 Optimization of an alginate-dECM composite hydrogel has achieved both high cell functionality and enhanced mechanical properties, in which alginate contributes improved mechanical properties, while dECM provides cell-binding sites and enables further thermal gelation of the hydrogel, preventing the ionic disruption of crosslinked structures.20,103
In contrast to ionic crosslinking, light-activated photocrosslinking enhances gelation kinetics with spatiotemporal control by simply adjusting the working parameters, including the light source, light intensity, exposure time, and illumination area.104,105,106 Photocrosslinking systems comprise photoinitiators, light sources, and photoreactive polymers. Photoinitiators (e.g., Irgacure 2959, lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), riboflavin, ruthenium/sodium persulfate (Ru/SPS), and Eosin Y) are compounds that generate reactive species (e.g., free radicals) by electronic excitation through the absorption of light within a given spectral range..94,107,108,109,110,111,112 For instance, LAP and Irgacure 2959 activate under ultraviolet (UV) exposure, triggering the crosslinking of methacrylate groups in hydrogels.105 Recent studies have focused on the synthesis of methacryloyl groups in various types of biomaterials (e.g., collagen, gelatin, chitosan, hyaluronic acid, and dECM) to enable UV photocrosslinking via chemical modifications, thereby improving shape fidelity.113,114,115,116,117 However, UV light can induce cell damage by forming reactive oxygen species with oxygen in the environment.118 In addition, UV light is known to have not only a limited penetration depth due to its short wavelength but also slow crosslinking kinetics caused by oxygen inhibition from the high reactivity of oxygen with UV-induced radicals.119,120 Alternatively, visible light-activated photoinitiators have been developed, which offer deeper penetration depth and enhanced biocompatibility without oxygen inhibition, making them more effective for fabricating thick tissue constructs.118 Lim et al. utilized Ru/SPS, a visible light-activated photoinitiator, to fabricate thick GelMA hydrogel constructs that exhibited superior cell viability and metabolic activity, demonstrating the effectiveness of the Ru/SPS photopolymerization system in comparison to the conventional UV crosslinking method.118 This strategy has also been applied to ECM-based hydrogels, such as collagen, fibrin, and dECM, owing to the presence of phenolic residues (e.g., tyrosine) in ECM molecules.94,107,108,109,121,122 Kim et al. created large freestanding constructs with high shape fidelity and complex architectures, including a cardiac chamber, using dERS bioinks, simultaneously securing cell viability and functionality by utilizing the bioactivity of dECM (Figure 3C).94
Incorporation of conductive materials for enhancing electrophysiological properties
Another approach to enhancing cardiac functionality is to provide a conductive microenvironment to stimulate electrophysiological properties and facilitate the transmission of electrical signals to cells by adding conductive materials (e.g., graphene, MXene, gold nanorods, and PEDOT:PSS).95,123,124,125,126 Roshanbinfar et al. incorporated PEDOT:PSS in a hydrogel composed of collagen and alginate to enhance electrical conductivity.127 This results in CM elongation and sarcomere organization, along with enhanced connexin 43 expression, suggesting maturation of the tissue. In particular, the sarcomeric length was comparable to that of adult CMs with enhanced contraction speed and amplitude. Tsui et al. augmented the electroconductivity of dECM hydrogels by adding reduced graphene oxide (rGO) (Figure 3D).95 This approach enhances the electroconductivity of the dECM hydrogel while maintaining its bioactivity. dECM-rGO tissues exhibited significantly increased electrophysiological functions, such as greater Ca2+ influx, longer action potential duration, and upregulation of expression levels related to ion channels.
Biofabrication of structural and functional features of the heart
Recent studies have suggested that the functionality of living tissues is closely related to their intricate and highly specialized architectures, implying the importance of capturing the shape-function relationship within engineered tissues.11 The heart, in particular, features complex geometrical structures, integrating multiple cardiac tissue components across various scales in a hierarchical spatial arrangement. These structures include anisotropic myocardial fibers and their orientation within chamber-like structures, along with heart valves and multiscale blood vessel networks. Advancements in biofabrication technology allow the production of diverse functional constructs by mimicking the distinct structural properties of native architecture (Table 1).128 This section explores the diverse structural components of the heart and employs biofabrication methods to reconstruct them, examining how these structures affect tissue organization and overall functionality.
Table 1.
Advancements in biofabrication strategies for diverse tissue engineered cardiac tissues
| Geometries | Key features | Fabrication technique | Reference | |
|---|---|---|---|---|
| Engineered heart tissues | Spheroid |
|
Hanging drop | Polonchuk et al.129 |
| Ultra-low adhesion plates | Giacomelli et al.49, Min et al.58, Kahn-Krell et al.61, Jin et al.,93, and Tan et al.130 | |||
| Suspension culture | Chen et al.131 and Kahn-Krell et al.132 | |||
| Bioprinting | Khoury et al.133 and Kang et al.107 | |||
| Strip or ring-shape |
|
Two-post | Mannhardt et al.5, Szepes et al.29, Lock et al.30, Tulloch et al.85, Tsui et al.95, Schaaf et al.134, Querdel et al.135, Ruan et al.136, Ronaldson-Bouchard et al.72, and Hansen et al.137 | |
| Biowire | Zhao et al.39 | |||
| Mold casting (ring) | Goldfracht et al.16, Xu et al.17, and Zimmermann et al.34 | |||
| Bioprinting | Noor et al.21, Finkel et al.138, and Ahrens et al.139 | |||
| Tubular structure |
|
Mold casting | Andrée et al.140 | |
| Bioprinting | Arai et al.44, Bliley et al.141, Kawai et al.142, and Arai et al.143 | |||
| Chamber |
|
Mold casting | Li et al.144 | |
| Fiber-spinning | Chang et al.145 | |||
| Bioprinting | Michas et al.14, Lee et al.19, Noor et al.21, Choi et al.22, Hwang et al.23, and Kupfer et al.146 | |||
| Engineered blood vesselsrowhe | Micro vessels |
|
Tissue assembly | Pretorius et al.147 |
| Macro vessels |
|
Bioprinting | Gao et al.20 and Noor et al.21 | |
| Engineered heart valvesr |
|
Fiber-spinning | Motta et al.18 | |
| Melt Electrowriting | Saidy et al.148 | |||
| Bioprinting | Lee et al.19 and Jafari et al.149 |
Anisotropic myocardial fibers generating contractility and electrophysiological properties
Uniaxial anisotropic EHT models, such as strip- or ring-shaped EHTs, replicate the precise architecture of native myocardial structures, applying a unidirectional force of contraction that enhances cellular alignment and tissue maturation.5,16,17,39,69,134,150 These structures can be readily fabricated using mold casting methods, wherein silicone posts or nanowires embedded within the mold enable cells to remodel and compact along with these constructs, thereby enhancing cellular alignment.39,150 These EHTs exhibit CMs that are more mature and aligned, with sarcomeric structures, contractility, and electrophysiological properties closely resembling those of human ventricular tissues.17,69,135 Therefore, these models have been utilized to study the pathophysiological phenomena in cardiac tissues, including the interactions of CMs with other cells, disease modeling, and drug testing.5,151,152,153 Furthermore, this anisotropic feature was also demonstrated in the patch-like EHTs. The patch, which guided the cellular arrangement through surface patterning or bioprinting to have an anisotropic structure, exhibited more physiologically relevant characteristics and a notable regenerative capacity.135,154
Mimicking the macro- and micro-structure of the cardiac chamber to generate volumetric functions
Uniaxial models remain flat and limited to the recreation of chamber-like functions.155 The contraction of CMs comprising the heart chamber induces changes in the volume and pressure of the internal space and pumping blood. Therefore, fabricating CMs into chamber-like structures is essential to reproduce this function.15 Advancements in biofabrication allow the generation of chamber-like structures, departing from the flat structures. Initially, a mold using a balloon was used to fabricate the chamber structure (Figure 4A).144 Moreover, an electrospun cup-like scaffold was developed, and CMs were seeded onto the scaffold to create tissue-engineered ventricles.15 The advent of bioprinting-based approaches has facilitated the development of chamber structures. Embedded bioprinting promotes the generation of freestanding constructs with more biomimetic architecture (Figure 4B).21,156 The freeform reversible embedding of suspended hydrogel (FRESH) bioprinting approach has been widely adopted for fabricating cardiac chambers.138 Lee et al. created a cup-like chamber that exhibited spontaneous contraction and calcium propagation throughout the tissue.19 Recently, Kupfer et al. employed FRESH printing to develop multichambered tissue models, with discrete vessels serving as perfusion conduits that recapitulated the intricate anatomical structures of the heart.146 The electrical signal propagated throughout the entire tissue construct, generating pressure-volume loops. The maximum volume moved through the chambers was 5.0 μL, which is approximately 25% of the average stroke volume of an adult murine heart. The recapitulation of cardiac chamber structures enables pump-like functions to be performed. This advancement provided new insights by presenting physiological properties, such as changes in pressure-volume dynamics with drug treatment.15,144
Figure 4.
Reconstruction of diverse heart chamber structure exhibiting chamber-like functions
(A) Chamber-like model created by using molding method, producing volume-pressure dynamics (Reproduced with permission.144 Copyright 2018, Elsevier B.V.).
(B) In-bath printed chamber-like model display thick cardiac tissue with perfusable vasculatures (Reproduced with permission.21 Copyright 2019, John Wiley & Sons.).
(C) Cardiac chamber-like structure formed by rolling cell sheets with diverse orientations, demonstrating volume-pressure dynamics (Reproduced with permission.157 Copyright 2022, John Wiley & Sons.).
(D) Heart chamber tissue mimicking hierarchical myocardial fiber orientation through bioprinting-assisted tissue assembly, resulting in the reproduction of left ventricular twists (Reproduced with permission.23 Copyright 2024, John Wiley & Son).
In addition to the chamber-like structure, the left ventricle shows a microscopic structural feature, myocardial fiber orientation, gradually changing fiber orientation depending on the depth of the myocardium. These unique features produce multiaxial fiber contraction, resulting in twisting chambers that play a critical role in efficient blood pumping.23 Therefore, significant efforts have been devoted to controlling fiber alignment and creating multi-oriented fiber structures within chamber-like constructs. Electrospinning can generate highly aligned fiber scaffolds and the orientation can be controlled by rotating the collector.158 Chang et al. utilized rotary jet spinning to produce highly aligned fibers and controlled fiber orientation by adjusting the angle of the collecting mandrel to create two-chamber models with circumferential and helical patterns.145 Compared to circumferentially aligned EHTs, the helically aligned chamber exhibited a rotating apex and improved pressure-volume dynamics. This approach also creates a multilayer and multichamber structure that mimics the native architecture of the heart, by rotating the orientation of the scaffold and stacking fibers layer-by-layer. Moreover, bioprinting methods have also been devised to guide the cellular orientation in situ by increasing the shear stress during the printing process. The inclusion of fibril-like additives, such as collagen, gelatin, and anisotropic cellular building blocks, in bioinks has been demonstrated to generate highly aligned printed structures following the printing path.22,139,159 Choi et al. incorporated prefabricated gelatin fibers into a 3D bioprinted scaffold.22 The addition of prefabricated gelatin fibers not only increased shear stress but also tailored rheological properties, achieving the printing of freestanding chamber-like structures without a supporting bath.
Sequential approaches have been developed to recreate hierarchical orientation changes with myocardial depth. These approaches involve seeding cells into compartmentalized scaffolds patterned in different directions, and then rolling them into a conical shape (Figure 4C).157 Alternatively, anisotropic tissue modules can be bioprinted and assembled in multilayer and different orientations.23 Hwang et al. suggested bioprinting-assisted tissue assembly to recapitulate hierarchical myocardial fiber orientation in the left ventricle (Figure 4D).23 Each module exhibited anisotropic cellular structure and cardiac function, and the EHT modules were functionally and structurally integrated after the assembly. Therefore, an intricate ventricular structure, comprising multiple layers and orientations, was generated, and the resemblance of the hierarchical structure resulted in the reproduction of left ventricular twists, which is the opposite rotation of the base and apex.
Heart valves to control the blood flow direction
Heart valves play a crucial role in ensuring unidirectional blood flow through the heart chambers and preventing the backward flow of blood. The repeated opening and closing of the valves in response to pressure changes during the cardiac cycle maintain the pressure gradients necessary within the heart chamber.160 The influence of the heart valve was highlighted by showing a native-like pressure-volume loop in a microfluidic chip-based study.14 Therefore, research has increasingly focused on fabricating engineered heart valves (EHVs) and recapitulating the heart valve functions.
The focused rotary jet spinning utilizing a valve-like collecting mandrel rapidly fabricated a trileaflet valve that mimics native valve structure and functions (Figure 5A).18 The dimensions, including the leaflet curvature, leaflet length, and leaflet height, were optimized to reproduce an adequate opening-closing function under pulmonary pressure. The optimized EHV showed approximately 30% regurgitation, which decreased to approximately 13% depending on the polymer material. FRESH printing was also employed to create an EHV that closely mimicked the anatomical structures based on micro–computed tomographic images.19 This collagen-based EHV showed proper opening-closing function, achieving regurgitation of less than 15%, and the maximum transvalvular pressure was approximately 40 mmHg, exceeding the physiologic pressures for the tricuspid and pulmonary valves but less than the aortic and mitral valves.
Figure 5.
Engineered heart component tissues supporting cardiac function
(A) Electrospun engineered heart valves, showing native valve-like fluidic flow functions under hemodynamic pressures (Reproduced with permission.18 Copyright 2023, Elsevier B.V.).
(B) Scalable and perfusable vascular tissues generated via coaxial-based bioprinting, allowing for diversity in various structures (Reproduced with permission.161 Copyright 2018, John Wiley & Sons. Reproduced with permission; 162 Copyright 2020, John Wiley & Sons.).
(C) Complex microvasculatures created by bioprinting enabling adequate oxygen supply and exhibiting compartmentalized EC layers (Reproduced with permission.21 Copyright 2019, John Wiley & Sons.).
Multiscale vascular systems for blood circulation
The heart has complex and hierarchical vascular networks. The great vessels, which encompass the aorta, vena cava, and pulmonary artery and vein, are responsible for transporting blood to and from the heart, playing a vital role in blood circulation.163 Various tissue-engineered blood vessels (TEBVs) have been developed to mimic these structures and their function in substance transport. Techniques such as rolling cell sheets, seeding cells on electrospun scaffolds, and mold casting have been employed to generate hollow and perfusable EBVs. Although these methods can generate multilayered vascular structures by stacking different types of cell sheets and rolling them into cylindrical structures, their shapes differ from those of native vascular tissues.164 Native blood vessels have geometrical complexities with varying curvatures and tortuosities that govern the biophysical behavior of circulating blood.165 The introduction of bioprinting for large vessels allows the creation of blood vessels of various shapes and geometries. Tabriz et al. created a range of vascular structures, from simple cylindrical to complex branched architectures, in a layer-by-layer manner using extrusion-based bioprinting techniques.166 In particular, coaxial-based bioprinting enabled the direct printing of cells to form vessel walls, facilitating the generation of perfusable and scalable vascular structures.20,103,161,162,165 Gao et al. demonstrated its versatility by mimicking a multilayered blood vessel wall and increasing the number of coaxial nozzles to form functional endothelial and smooth muscle layers.20 Moreover, the width and shape of the EBVs were easily controlled by adjusting printing parameters such as the nozzle diameter, printing path, and moving speed (Figure 5B).161,162,165
While the great vessels are responsible for blood circulation, the coronary vessels, including coronary arteries and veins, are responsible for providing oxygen and nutrients to the thick myocardium. The coronary vessels root into the great vessels, creating hierarchically branched vascular networks.167 The microvasculature was generated to evenly supply oxygen and nutrients to the thick myocardium for improved long-term viability and functionality.21,168,169,170 Microfluidic chip-based approaches have been used to create the microvasculature. Zhang et al. fabricated 3D microchannel networks by patterning scaffolds into various intricate structures with bifurcating conduits and 3D branching networks.170 ECs were seeded in microporous scaffolds to create endothelialized scaffolds, and EHTs were generated surrounding these endothelialized structures. It has been proven that nutrients and oxygen are supplied through these vascular structures by maintaining a thick muscle layer. Moreover, a positive chronotropic response of the myocardium was confirmed by supplying epinephrine through the microvasculature. Noor et al. created physiologically relevant complex vascular networks using bioprinting approaches. The blood vessels were generated based on a computed tomography image of the coronary arteries, enabling an evenly distributed oxygen supply to the entire area of the thick cardiac patch (Figure 5C).21 Recently, successful integration of the vasculature into bulk cardiac tissue that meets the requisite cellular density was achieved. Skylar-Scott et al. developed the sacrificial writing into functional tissue (SWIFT) printing technique to create tissue constructs with vasculatures, thereby enhancing overall tissue functionality.171 Perfusable vascular channels were introduced via embedded printing, which involved the dense assembly of patient-specific iPSC-derived organoids to produce bulk vascularized tissue. These cardiac constructs exhibited spontaneous and synchronous beating, with rhythmic and rapid calcium wave propagation following perfusion, ensuring cell viability and tissue functionality throughout the thick tissue.
Current challenges and future perspectives
Key elements to be addressed in the several challenges that must be overcome in the generation of scalable heart substitutes for emulating a real heart are as follows.
Reproducing cardiac cellular heterogeneity and density for functional cardiac tissues
Achieving physiologically relevant levels of functional cardiac models necessitates the precise replication of the cellular heterogeneity of the heart. In particular, incorporating cardiac-specific lineage-cell types is essential for fabricating relevant organ constituents without introducing ectopic genes into tissues.172 In this context, stem cells have shown great promise for generating cardiac-specific lineage-cell types. Various differentiation protocols have been developed to produce CMs, pacemaker CMs, and non-CMs, including FBs, ECs, pericytes, SMCs, epicardial cells, endocardial cells, and macrophages.3,29,30,39,49,59,60,61,173 However, further development of currently underrepresented cardiac cellular compositions, such as Purkinje fibers, myeloid cells, and lymphoid cells, remains necessary for heart function. In addition, the differentiated cells exhibit a heterogeneous population, which poses challenges to reproducibility and may result in unintended outcomes.174,175 Hence, a cell sorting process is essential for obtaining a uniform and consistent cell composition, allowing for the controlled utilization of various cell types in EHT fabrication. Furthermore, advanced techniques are required to promote the full maturation of stem cell-derived cells as their biological and physiological characteristics often remain at an early fetal stage.136 As immature cells hinder the generation of functional cardiac models, various maturation strategies such as electrical, mechanical, and metabolic stimulation are required to enhance cell maturity.69,71,74,130,136,176
Furthermore, the large-scale production of cells to meet the estimated requirement of 2–3 billion cardiac muscle cells in the human heart poses another significant obstacle.177 Current EHTs contain only a few hundred million cells, with the non-proliferative and non-migratory nature of CMs hindering the achievement of high cell densities.19,146,152 Recently, Ho et al. successfully developed a scalable production pipeline for iPSC aggregates using automated bioreactors, yielding approximately 4 billion cells from a 1 L bioreactor culture.177 Moreover, a strategy that bioprints stem cells directly to form chamber-like structures, leveraging their proliferative properties to achieve sufficient cell numbers before differentiating them into CMs in situ, has enabled the creation of high-density cardiac constructs.146 While this method effectively increases the number of cells in the tissue, it poses challenges in precisely regulating the distribution and proportion of the different cell types required to achieve the correct cardiac configuration. These challenges may be addressed by incorporating recently developed orthogonally induced differentiation methods, which involve printing stem cells programmed to differentiate into specific lineages in desired locations, resulting in programming cellular complexity in bioprinted tissue.178 Continued exploration of differentiation and large-scale production methods for generating diverse cardiac-specific lineage cell types are imperative.
Customizing dECM bioinks for mechanical diversity and its automation
The biochemical and mechanical properties of the heart vary, based on the tissue regions and their specific functions, with Young’s modulus ranging from to 8–15 kPa for the adult human myocardium to 16 MPa for pulmonary valves.179,180 Proper stiffness is a critical factor in regulating cellular behavior and function,181 whereas other mechanical properties such as elasticity, durability, and toughness are also essential for fabricating suitable tissue constructs and maintaining their shapes, thereby expanding the biofabrication window.182 Recently, various strategies have been developed to increase the intrinsic strength of hydrogels or enhance the crosslinking kinetics of dECM, leveraging remarkable biochemical advantages to achieve desired properties. Functionalizing dECM can enhance and tailor its mechanical properties to match those of the native heart and improve printability through several crosslinking strategies, such as adjusting the degree of crosslinking or employing sequential crosslinking methods.109,110,183 However, unlike other pure native polymers, dECMs have a complex protein composition, which leads to low efficiency and reproducibility in synthesis for functionalization and batch-to-batch variations in fabrication. To minimize these variations, the development of an automated decellularization and synthesis system represents a significant advancement in improving the consistency of functionalized dECM production.
Advancing biofabrication techniques for integrated and scalable cardiac tissue models
Currently, EHTs encounter significant limitations in achieving the size and intricacy of an actual human heart. They are only a few centimeters in diameter, whereas the human heart is approximately the size of a fist. To generate scalable cardiac models, it is imperative to implement advanced biofabrication strategies. For instance, a multi-nozzle system can significantly enhance efficiency within a short time frame.184 Skylar-Scott et al. Developed a multi-materials multi-nozzle 3D printing technique that programs the composition, function, and structure of materials as the voxel scale.184 This approach enables seamless junctions with high-frequency switching between multiple materials within multi-nozzle printheads, achieving enhanced complexity and fabrication speed. Additionally, light-assisted bioprinting techniques, such as digital light processing (DLP) and tomographic bioprinting, offer notable advantages in effective, volumetric tissue construction.183,185,186,187,188 Conventional light-based bioprinting techniques, such as DLP printing, have limitations requiring layer-by-layer printing for volumetric tissues. However, a recent study by Gabriel et al. demonstrated the rapid fabrication of layerless tissues using tomographic bioprinting, which enhances design freedom beyond traditional light-based methods.188 Besides, despite substantial technical advancements, the development of multiple anatomical components such as blood vessels, multiaxially aligned myocardial fibers, a thick muscular wall, valves, and chambers have been achieved independently.19,20,21,145,147,149,145,147,149 Integrating these various scale constructs into a cohesive system remains a formidable challenge. To advance the fabrication of integrated multicomponent, functional cardiac models, it is essential to refine biofabrication strategies to achieve the multimodality of diverse micro- and macro-constructs in a unified system. Potential approaches include the bioprinting-assisted tissue assembly of diverse components into a single system or the crosslinking of multiple materials at various wavelengths to pattern each structural component into a sophisticated organizational structure.189,190
Engineering vascular networks to support sustained cardiac tissue functionality
Developing scalable cardiac substitutes, particularly those with a robust myocardium, requires effective vascularization to ensure a sufficient supply of oxygen and nutrients to the thick myocardial tissue.191 Various vascularization strategies have been developed to recapitulate the function and physiology of heart tissue. Initial approaches involved biological methods, where the inclusion of biofactors (e.g., vascular endothelial growth factor) accelerates vessel formation by ECs in cardiac tissues and enhances long-term tissue survival.192,193 These methods attempt to stimulate endogenous blood vessels to grow within cardiac tissue; however, the resulting randomly self-assembled networks often exhibit a highly disorganized architecture.192,194 In this regard, biofabrication technologies allow the creation of vasculature with defined network patterns using techniques such as soft lithography and sacrificial molding.169,170 In particular, 3D bioprinting technology can be used to deposit multiple cells at precise locations to create larger and more complex vascular structures.161,165,191 Despite the implementation of various strategies, the development of a mature and well-organized vascular network that can adequately support scalable and contractile thick cardiac tissue with physiological CM density remains challenging.171 In addition, engineering the entire vascular hierarchy within cardiac chamber-like structures remains a significant challenge. Achieving proper distribution of the microvasculature throughout the thick myocardial tissue, along with the integration of great vessels to allow blood inflow and outflow within the chamber-like tissue, is required. Furthermore, recreating the intrinsic hierarchical networks between the microvasculature and great vessels such as the coronary arteries originating from the aorta is essential for recreating the metabolic features and functions of the heart.195
Creating a cardiac conduction system for synchronized contraction in organ-scale cardiac tissue
Functional contraction of the heart is orchestrated by its electrical conduction system, which is crucial for synchronized heartbeats that are essential for efficient blood pumping and maintaining consistent blood pressure.196,197,198 The contraction process starts with pacemaker cells in the sinoatrial node (SAN) which initiate the heartbeat spontaneously199,200 and are transmitted to the atria, where the aCM pushes blood into the ventricles. The action potential is then transmitted to the atrioventricular node, where it is transmitted via Purkinje fibers, which constitute the cardiac conduction system and are responsible for transmitting electrical signals201 to the vCM, which pumps blood out of the heart.202 They exhibit varying conduction velocities; the cardiac muscle conducts at approximately 0.3–0.4 m/s, while Purkinje fibers conduct impulses at significantly higher velocities, around 2–3 m/s throughout the ventricles.203 These neuronal cells play a significant role in maintaining the overall cardiac rhythmic function, with Purkinje fibers being crucial in regulating arrhythmogenesis in heart failure.204
As the size of the cardiac model increases, conduction velocities must be enhanced to ensure synchronized beating across the entire tissue. Contemporary approaches, such as incorporating conductive materials into biomaterials or applying electrical stimulation to early-stage CM through intensity training, have demonstrated the potential to enhance electrophysiological properties.72,95 Recently, the effect of neurons on CM maturation and function has been explored through neurocardiac co-culture studies, primarily using 2D models.205,206,207 However, current EHTs exhibit suboptimal conduction velocities compared to the native human heart owing to the absence of conduction systems (Table 2).208,209,210 Integrating the cardiac conduction system into large-scale cardiac tissues is necessary to achieve sufficient conduction velocity for synchronized contraction throughout the volumetric tissue constructs. In this regard, advancements in stem cell technology are required to produce cardiac-specific neuronal cells such as Purkinje fibers. Furthermore, the development of advanced biofabrication technology is crucial for creating complex and interconnected structures between nerves and cardiac tissues by accurately patterning each tissue in the appropriate positions within large-scale constructs.
Table 2.
Conduction velocity value in current engineered heart tissues
| EHT geometry | Cell types | Conduction velocity | Reference |
|---|---|---|---|
| Spheroid | iPSC-CM | 9.4 ± 5.7 cm/s | Mattapally et al.211 |
| Strip | iPSC-CM | 2.76 ± 0.61 cm/s | Ruan et al.136 |
| hECM-CM iPSC-CM |
5.6 ± 1.0 cm/s (atrial) 13.0 ± 5.5 cm/s (ventricular) |
Zhao et al.39 | |
| ESC/iPSC-CM, CF | 10 cm/s (longitudinal) | Bliley et al.212 | |
| hiPSC-CM, hiPSC-EC, hCFs | 15.98 ± 3.47 cm/s (longitudinal) | Finkel et al.138 | |
| hiPSC-CM, human dermal fibroblasts | 25.0 ± 0.9 cm/s | Ronaldson-Bouchard et al.72 | |
| hiPSC-CM | 41 ± 2 cm/sa | Tzatzalos et al.135 | |
| hPSC-CM NRVMs |
25.8 ± 2 cm/s 52.5 cm/sa |
Jackman et al.213 | |
| Ring | hESC-CM | 4.1 ± 0.2 cm/s (atrial)a 21.4 ± 4.4 cm/s (ventricle)a |
Goldfracht et al.16 |
| Tubular | hESC-CM, hCFs | 5.44 ± 1.04 cm/s | Bliley et al.141 |
| Chamber | hESC-CM, FB | 2.0 cm/s (longitudinal) | Lee et al.19 |
| NRVMs | 8.3 cm/s (circumferentially aligned) 19.1 cm/s (helically aligned) |
Chang et al.145 |
Under electrical pacing.
Key elements for practical applications of engineered heart tissues
EHTs with blood-pumping functionality can be utilized across various fields of tissue engineering and translational medicine, including drug screening, disease modeling, and therapeutic applications. However, the specific demands of each application require tailored approaches to ensure their effectiveness. For example, cardiac tissues may serve as advanced in vitro models for drug screening, which requires high-throughput system rather than precisely mimicking construct.137 Thus, EHTs for this purpose may omit some of its detailed features to provide fast and accurate readout with reproducibility.
For disease modeling, this can be accomplished by utilizing patient-derived cells or genetically engineered cell lines, enabling personalized treatment strategies. Furthermore, more complex EHTs are capable of replicating intricate pathological characteristics. However, current methodologies face limitations in accurately interpreting outputs from such complex EHTs.35,214,215 To address this, biohybrid models, which integrate biological tissue with embedded sensors, offer a promising approach for facilitating the measurement and interpretation of data from complex tissues.
Lastly, therapeutic applications require several safety considerations, such as potential genetic and epigenetic abnormalities and potential tumorigenicity of the cell and biomaterial sources. In principle, these risks should be mitigated by manufacturing biomaterials and cells using GMP-compliant manufacturing facilities.216 This may also include methods to eliminate the immunogenicity of biomaterials and cells through an engineering approach.217
Conclusion
The heart is essential for survival, and cardiovascular diseases are the leading cause of death globally. Various approaches have evolved to emulate the intrinsic structures and functions of the heart for application in therapeutics, drug screening, and disease modeling. Significant progress has been made, including the development of various cardiac cell types co-cultured to replicate the cellular heterogeneity of the heart, based on stem cell technology. Biomaterials have also evolved to enhance their biochemical and mechanical functionality. Tissue-specific biomaterials have been developed to more accurately represent microenvironmental cues, and by supplementing them with other materials, the mechanical and functional properties of bioinks have been improved, thereby enabling the fabrication of structurally advanced cardiac models. Advances in biofabrication technology have made it feasible to replicate the unique geometry and highly organized hierarchy of the heart, leading to the development of diverse cardiac models.
These interdisciplinary advances have allowed EHTs to develop into increasingly larger and more complex structures, thereby attaining the maturity and metabolic properties of fetal hearts. Furthermore, the implementation of both micro- and macro-structures of the heart, ranging from myocardial fiber orientation to chamber-like structures, has enabled the achievement of heart-specific rotational movements, such as the left ventricular twist. In addition, advances in 3D bioprinting technology have improved structural complexity, allowing for the production of diverse heart components such as heart valves and multiscale vasculatures.
Substantial advancements in multidisciplinary studies have enabled the creation of large-scale, functional, cardiac tissue. However, several challenges must be addressed to achieve blood-pumping function. To generate physiologically relevant cardiac tissues, further research is required to accurately mimic the intrinsic cellular heterogeneity and population in the heart. Additionally, replicating the varied mechanical properties of heart tissue is essential, necessitating suitable bioinks and automated large-scale production to ensure bioink quality. Moreover, integrating multiple anatomical components of the heart, such as multiaxially aligned myocardial fibers, thick cardiac muscle tissue, chambers, valves, and vasculatures, into a single cardiac tissue construct through advanced biofabrication technology is essential for achieving comprehensive functionality. In addition, the vascular and nervous systems are key elements in reproducing the physiological functions of the heart, particularly its ultimate blood-pumping function, making their incorporation indispensable. Finally, the practical application of EHTs should be carefully tailored to meet the specific requirements of drug screening, disease modeling, and therapeutic applications, ensuring their effective use across diverse fields.
Acknowledgments
This research was supported by Korean Fund for Regenerative Medicine funded by Ministry of Science and ICT, and Ministry of Health and Welfare (21A0104L1, Republic of Korea). This work was also supported by the National Research Foundation of Korea grant funded by the Korea government (MSIT) (No. 2022M3C1A3081359).
Author contributions
Writing – original draft, M.K. and D.G.H.; Writing – review and editing, J.J.; Visualization, M.K. and D.G.H.; Investigation, M.K. and D.G.H.; Data curation, M.K. and D.G.H.; Conceptualization, M.K. and D.G.H.; Supervision, J.J.; Funding acquisition, J.J.
Declaration of interests
J.J. is one of the Guest Editors of the Special Issue “Advanced biomanufacturing of cardiovascular tissues” at iScience.
References
- 1.Tortora G.J., Derrickson B. 15th ed. John Wiley & Sons, Inc.; 2014. Principles of Anatomy and Physiology. [Google Scholar]
- 2.Cho S., Discher D.E., Leong K.W., Vunjak-Novakovic G., Wu J.C. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat. Methods. 2022;19:1064–1071. doi: 10.1038/s41592-022-01591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min S., Cho S.W. Engineered human cardiac tissues for modeling heart diseases. BMB Rep. 2023;56:32–42. doi: 10.5483/BMBRep.2022-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vunjak Novakovic G., Eschenhagen T., Mummery C. Myocardial tissue engineering: In vitro models. Cold Spring Harb. Perspect. Med. 2014;4 doi: 10.1101/cshperspect.a014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannhardt I., Breckwoldt K., Letuffe-Brenière D., Schaaf S., Schulz H., Neuber C., Benzin A., Werner T., Eder A., Schulze T., et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016;7:29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao X., Wang Z. Research Progress of Three-Dimensional Bioprinting Artificial Cardiac Tissue. Tissue Eng. Regen. Med. 2023;20:1–9. doi: 10.1007/s13770-022-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama K. Biofabrication. Elsevier; 2013. In Vitro Biofabrication of Tissues and Organs; pp. 1–21. [Google Scholar]
- 8.Lu T.Y., Xiang Y., Tang M., Chen S. 3D Printing Approaches to Engineer Cardiac Tissue. Curr. Cardiol. Rep. 2023;25:505–514. doi: 10.1007/s11886-023-01881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluguermann C., Wu L., Petrigliano F., Mcallister D., Miriuka S., Evseenko D.A. Novel aspects of parenchymal-mesenchymal interactions: From cell types to molecules and beyond. Cell Biochem. Funct. 2013;31:271–280. doi: 10.1002/cbf.2950. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Liu Y., Zhang Y., Yao B., Enhejirigala B., Li Z., Song W., Wang Y., Duan X., Yuan X., et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.640388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levato R., Jungst T., Scheuring R.G., Blunk T., Groll J., Malda J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020;32:e1906423. doi: 10.1002/adma.201906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callens S.J.P., Uyttendaele R.J.C., Fratila-Apachitei L.E., Zadpoor A.A. Substrate curvature as a cue to guide spatiotemporal cell and tissue organization. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119739. [DOI] [PubMed] [Google Scholar]
- 13.You S., Xiang Y., Hwang H.H., Berry D.B., Kiratitanaporn W., Guan J., Yao E., Tang M., Zhong Z., Ma X., et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michas C., Karakan M.Ç., Nautiyal P., Seidman J.G., Seidman C.E., Agarwal A., Ekinci K., Eyckmans J., White A.E., Chen C.S. Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 2022;8:3791. doi: 10.1126/sciadv.abm3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macqueen L.A., Sheehy S.P., Chantre C.O., Zimmerman J.F., Pasqualini F.S., Liu X., Goss J.A., Campbell P.H., Gonzalez G.M., Park S.J., et al. A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2018;2:930–941. doi: 10.1038/s41551-018-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldfracht I., Protze S., Shiti A., Setter N., Gruber A., Shaheen N., Nartiss Y., Keller G., Gepstein L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-13868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Qi J., Zhou W., Liu X., Zhang L., Yao X., Wu H. Generation of ring-shaped human iPSC-derived functional heart microtissues in a Möbius strip configuration. Biodes. Manuf. 2022;5:687–699. doi: 10.1007/s42242-022-00204-4. [DOI] [Google Scholar]
- 18.Motta S.E., Peters M.M., Chantre C.O., Chang H., Cera L., Liu Q., Cordoves E.M., Fioretta E.S., Zaytseva P., Cesarovic N., et al. On-demand heart valve manufacturing using focused rotary jet spinning. Matter. 2023;6:1860–1879. doi: 10.1016/j.matt.2023.05.025. [DOI] [Google Scholar]
- 19.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S., Bliley J.M., Campbell P.G., Feinberg A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 20.Gao G., Kim H., Kim B.S., Kong J.S., Lee J.Y., Park B.W., Chae S., Kim J., Ban K., Jang J., et al. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019;6:041402. doi: 10.1063/1.5099306. [DOI] [Google Scholar]
- 21.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019;6 doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S., Lee K.Y., Kim S.L., MacQueen L.A., Chang H., Zimmerman J.F., Jin Q., Peters M.M., Ardoña H.A.M., Liu X., et al. Fibre-infused gel scaffolds guide cardiomyocyte alignment in 3D-printed ventricles. Nat. Mater. 2023;22:1039–1046. doi: 10.1038/s41563-023-01611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang D.G., Choi H., Yong U., Kim D., Kang W., Park S.M., Jang J. Bioprinting-assisted Tissue Assembly for Structural and Functional Modulation of Engineered Heart Tissue Mimicking Left Ventricular Myocardial Fiber Orientation. Adv. Mater. 2024;36 doi: 10.1002/adma.202400364. [DOI] [PubMed] [Google Scholar]
- 24.Litviňuková M., Talavera-López C., Maatz H., Reichart D., Worth C.L., Lindberg E.L., Kanda M., Polanski K., Heinig M., Lee M., et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D’Avila A., Dobrev D., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eschenhagen T., Fink C., Remmers U., Scholz H., Wattchow J., Weil J., Zimmermann W., Dohmen H.H., Schäfer H., Bishopric N., et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb. J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 27.Liu F., Fang Y., Hou X., Yan Y., Xiao H., Zuo D., Wen J., Wang L., Zhou Z., Dang X., et al. Enrichment differentiation of human induced pluripotent stem cells into sinoatrial node-like cells by combined modulation of BMP, FGF, and RA signaling pathways. Stem Cell Res. Ther. 2020;11 doi: 10.1186/s13287-020-01794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren J., Han P., Ma X., Farah E.N., Bloomekatz J., Zeng X.X.I., Zhang R., Swim M.M., Witty A.D., Knight H.G., et al. Canonical Wnt5b Signaling Directs Outlying Nkx2.5+ Mesoderm into Pacemaker Cardiomyocytes. Dev. Cell. 2019;50:729–743. doi: 10.1016/j.devcel.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szepes M., Melchert A., Dahlmann J., Hegermann J., Werlein C., Jonigk D., Haverich A., Martin U., Olmer R., Gruh I. Dual function of ipsc-derived pericyte-like cells in vascularization and fibrosis-related cardiac tissue remodeling in vitro. Int. J. Mol. Sci. 2020;21:8947–9020. doi: 10.3390/ijms21238947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lock R.I., Graney P.L., Tavakol D.N., Nash T.R., Kim Y., Sanchez E., Morsink M., Ning D., Chen C., Fleischer S., et al. Macrophages enhance contractile force in iPSC-derived human engineered cardiac tissue. Cell Rep. 2024;43 doi: 10.1016/J.CELREP.2024.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lickiss B., Hunker J., Bhagwan J., Linder P., Thomas U., Lotay H., Broadbent S., Dragicevic E., Stoelzle-Feix S., Turner J., Gossmann M. Chamber-specific contractile responses of atrial and ventricular hiPSC-cardiomyocytes to GPCR and ion channel targeting compounds: A microphysiological system for cardiac drug development. J. Pharmacol. Toxicol. Methods. 2024;128 doi: 10.1016/j.vascn.2024.107529. [DOI] [PubMed] [Google Scholar]
- 32.Hansen A., Eder A., Bönstrup M., Flato M., Mewe M., Schaaf S., Aksehirlioglu B., Schwörer A., Uebeler J., Eschenhagen T. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 33.Das S., Kim S.W., Choi Y.J., Lee S., Lee S.H., Kong J.S., Park H.J., Cho D.W., Jang J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann W.H., Fink C., Kralisch D., Remmers U., Weil J., Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 2000;68:106–114. doi: 10.1002/(SICI)1097-0290(20000405)68:1<106::AID-BIT13>3.0.CO. [DOI] [PubMed] [Google Scholar]
- 35.Hwang D.G., Kang W., Park S.M., Jang J. Biohybrid printing approaches for cardiac pathophysiological studies. Biosens. Bioelectron. 2024;260 doi: 10.1016/J.BIOS.2024.116420. [DOI] [PubMed] [Google Scholar]
- 36.Sander V., SuñE G., Jopling C., Morera C., Izpisua Belmonte J.C. Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat. Protoc. 2013;8:800–809. doi: 10.1038/nprot.2013.041. [DOI] [PubMed] [Google Scholar]
- 37.Lyra-Leite D.M., Gutiérrez-Gutiérrez Ó., Wang M., Zhou Y., Cyganek L., Burridge P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 2022;3 doi: 10.1016/J.XPRO.2022.101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J.H., Protze S.I., Laksman Z., Backx P.H., Keller G.M. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell. 2017;21:179–194.e4. doi: 10.1016/j.stem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y., Rafatian N., Feric N.T., Cox B.J., Aschar-Sobbi R., Wang E.Y., Aggarwal P., Zhang B., Conant G., Ronaldson-Bouchard K., et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell. 2019;176:913–927.e18. doi: 10.1016/j.cell.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang W., Han P., Kim E.H., Mak J., Zhang R., Torrente A.G., Goldhaber J.I., Marbán E., Cho H.C. Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells. Stem Cell. 2020;38:352–368. doi: 10.1002/STEM.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Protze S.I., Liu J., Nussinovitch U., Ohana L., Backx P.H., Gepstein L., Keller G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017;35:56–68. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 42.Tian Y., Morrisey E.E. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ. Res. 2012;110:1023–1034. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumberland M.J., Euchner J., Azad A.J., T N Vo N., Kirchhof P., Holmes A.P., Denning C., Gehmlich K. Generation of a human iPSC-derived cardiomyocyte/fibroblast engineered heart tissue model. F1000Res. 2024;12 doi: 10.12688/f1000research.139482.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arai K., Murata D., Verissimo A.R., Mukae Y., Itoh M., Nakamura A., Morita S., Nakayama K. Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiburcy M., Hudson J.E., Balfanz P., Schlick S., Meyer T., Chang Liao M.L., Levent E., Raad F., Zeidler S., Wingender E., et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135:1832–1847. doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H., Shao N., Holmström A., Zhao X., Chour T., Chen H., Itzhaki I., Wu H., Ameen M., Cunningham N.J., et al. Transcriptome analysis of non human primate-induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture vs. 3D engineered heart tissue. Cardiovasc. Res. 2021;117:2125–2136. doi: 10.1093/CVR/CVAA281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., Kong C.W., Tong M.H., Chooi W.H., Huang N., Li R.A., Chan B.P. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation. Acta Biomater. 2017;49:204–217. doi: 10.1016/J.ACTBIO.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 48.Hall C., Gehmlich K., Denning C., Pavlovic D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacomelli E., Meraviglia V., Campostrini G., Cochrane A., Cao X., van Helden R.W.J., Krotenberg Garcia A., Mircea M., Kostidis S., Davis R.P., et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell. 2020;26:862–879. doi: 10.1016/j.stem.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang F., Mohr F., Ullrich N.D., Hecker M., Wagner A.H. Endothelial cell modulation of cardiomyocyte gene expression. Exp. Cell Res. 2019;383 doi: 10.1016/j.yexcr.2019.111565. [DOI] [PubMed] [Google Scholar]
- 51.Zamani M., Karaca E., Huang N.F. Multicellular Interactions in 3D Engineered Myocardial Tissue. Front. Cardiovasc. Med. 2018;5:147. doi: 10.3389/fcvm.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narmoneva D.A., Vukmirovic R., Davis M.E., Kamm R.D., Lee R.T. Endothelial Cells Promote Cardiac Myocyte Survival and Spatial Reorganization. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y.S., Arneri A., Bersini S., Shin S.R., Zhu K., Goli-Malekabadi Z., Aleman J., Colosi C., Busignani F., Dell’Erba V., et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/J.BIOMATERIALS.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiullari F., Costantini M., Milan M., Pace V., Chirivì M., Maiullari S., Rainer A., Baci D., Marei H.E.S., Seliktar D., et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018;8:13532–13615. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsukamoto Y., Akagi T., Akashi M. Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer. Sci. Rep. 2020;10:5484–5511. doi: 10.1038/s41598-020-59371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrissette-McAlmon J., Ginn B., Somers S., Fukunishi T., Thanitcul C., Rindone A., Hibino N., Tung L., Mao H.Q., Grayson W. Biomimetic Model of Contractile Cardiac Tissue with Endothelial Networks Stabilized by Adipose-Derived Stromal/Stem Cells. Sci. Rep. 2020;10:8387. doi: 10.1038/s41598-020-65064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanjare M., Hou L., Nakayama K.H., Kim J.J., Mezak N.P., Abilez O.J., Tzatzalos E., Wu J.C., Huang N.F. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci. 2017;5:1567–1578. doi: 10.1039/C7BM00323D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min S., Kim S., Sim W.S., Choi Y.S., Joo H., Park J.H., Lee S.J., Kim H., Lee M.J., Jeong I., et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat. Commun. 2024;15 doi: 10.1038/s41467-024-46928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannerman D., Pascual-Gil S., Wu Q., Fernandes I., Zhao Y., Wagner K.T., Okhovatian S., Landau S., Rafatian N., Bodenstein D.F., et al. Heart-on-a-Chip Model of Epicardial–Myocardial Interaction in Ischemia Reperfusion Injury. Adv. Healthcare Mater. 2024;13 doi: 10.1002/adhm.202302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamidzada H., Pascual-Gil S., Wu Q., Kent G.M., Massé S., Kantores C., Kuzmanov U., Gomez-Garcia M.J., Rafatian N., Gorman R.A., et al. Primitive macrophages induce sarcomeric maturation and functional enhancement of developing human cardiac microtissues via efferocytic pathways. Nat. Cardiovasc. Res. 2024;3:567–593. doi: 10.1038/s44161-024-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn-Krell A., Pretorius D., Guragain B., Lou X., Wei Y., Zhang J., Qiao A., Nakada Y., Kamp T.J., Ye L., Zhang J. A three-dimensional culture system for generating cardiac spheroids composed of cardiomyocytes, endothelial cells, smooth-muscle cells, and cardiac fibroblasts derived from human induced-pluripotent stem cells. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.908848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Windt L.M., Wiendels M., Dostanić M., Bellin M., Sarro P.M., Mastrangeli M., Mummery C.L., van Meer B.J. Miniaturized engineered heart tissues from hiPSC-derived triple cell type co-cultures to study human cardiac function. Biochem. Biophys. Res. Commun. 2023;681:200–211. doi: 10.1016/J.BBRC.2023.09.034. [DOI] [PubMed] [Google Scholar]
- 63.Landau S., Zhao Y., Hamidzada H., Kent G.M., Okhovatian S., Lu R.X.Z., Liu C., Wagner K.T., Cheung K., Shawky S.A., et al. Primitive macrophages enable long-term vascularization of human heart-on-a-chip platforms. Cell Stem Cell. 2024;31:1222–1238. doi: 10.1016/J.STEM.2024.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karbassi E., Fenix A., Marchiano S., Muraoka N., Nakamura K., Yang X., Murry C.E. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020;17:341–359. doi: 10.1038/s41569-019-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scuderi G.J., Butcher J. Naturally engineered maturation of cardiomyocytes. Front. Cell Dev. Biol. 2017;5 doi: 10.3389/fcell.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toischer K., Rokita A.G., Unsöld B., Zhu W., Kararigas G., Sossalla S., Reuter S.P., Becker A., Teucher N., Seidler T., et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voorhees A.P., Han H.C. Biomechanics of cardiac function. Compr. Physiol. 2015;5:1623–1644. doi: 10.1002/cphy.c140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song M., Jang Y., Kim S.-J., Park Y. Cyclic Stretching Induces Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes through Nuclear-Mechanotransduction. Tissue Eng. Regen. Med. 2022;19:781–792. doi: 10.1007/s13770-021-00427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leonard A., Bertero A., Powers J.D., Beussman K.M., Bhandari S., Regnier M., Murry C.E., Sniadecki N.J. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J. Mol. Cell. Cardiol. 2018;118:147–158. doi: 10.1016/j.yjmcc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stoppel W.L., Kaplan D.L., Black L.D. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv. Drug Deliv. Rev. 2016;96:135–155. doi: 10.1016/j.addr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Min S., Lee H.J., Jin Y., Kim Y.H., Sung J., Choi H.J., Cho S.W. Biphasic Electrical Pulse by a Micropillar Electrode Array Enhances Maturation and Drug Response of Reprogrammed Cardiac Spheroids. Nano Lett. 2020;20:6947–6956. doi: 10.1021/acs.nanolett.0c01141. [DOI] [PubMed] [Google Scholar]
- 72.Ronaldson-Bouchard K., Ma S.P., Yeager K., Chen T., Song L., Sirabella D., Morikawa K., Teles D., Yazawa M., Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X., Rodriguez M., Pabon L., Fischer K.A., Reinecke H., Regnier M., Sniadecki N.J., Ruohola-Baker H., Murry C.E. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gentillon C., Li D., Duan M., Yu W.M., Preininger M.K., Jha R., Rampoldi A., Saraf A., Gibson G.C., Qu C.K., et al. Targeting HIF-1α in combination with PPARα activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2019;132:120–135. doi: 10.1016/j.yjmcc.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Funakoshi S., Fernandes I., Mastikhina O., Wilkinson D., Tran T., Dhahri W., Mazine A., Yang D., Burnett B., Lee J., et al. Generation of mature compact ventricular cardiomyocytes from human pluripotent stem cells. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-23329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopaschuk G.D., Jaswal J.S. Energy Metabolic Phenotype of the Cardiomyocyte During Development, Differentiation, and Postnatal Maturation. J. Cardiovasc. Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 77.Feyen D.A.M., McKeithan W.L., Bruyneel A.A.N., Spiering S., Hörmann L., Ulmer B., Zhang H., Briganti F., Schweizer M., Hegyi B., et al. Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim M., Hwang D.G., Jang J. 3D Pancreatic Tissue Modeling in vitro: Advances and Prospects. BioChip J. 2020;14:84–99. doi: 10.1007/s13206-020-4108-4. [DOI] [Google Scholar]
- 79.Place E.S., George J.H., Williams C.K., Stevens M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009;38:1139–1151. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- 80.Terzopoulou Z., Zamboulis A., Koumentakou I., Michailidou G., Noordam M.J., Bikiaris D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules. 2022;23:1841–1863. doi: 10.1021/acs.biomac.2c00047. [DOI] [PubMed] [Google Scholar]
- 81.Ciolacu D.E., Nicu R., Ciolacu F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines. 2022;10 doi: 10.3390/biomedicines10051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Satchanska G., Davidova S., Petrov P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers. 2024;16 doi: 10.3390/polym16081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majid Q.A., Fricker A.T.R., Gregory D.A., Davidenko N., Hernandez Cruz O., Jabbour R.J., Owen T.J., Basnett P., Lukasiewicz B., Stevens M., et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/FCVM.2020.554597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antoine E.E., Vlachos P.P., Rylander M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014;20:683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tulloch N.L., Muskheli V., Razumova M.V., Korte F.S., Regnier M., Hauch K.D., Pabon L., Reinecke H., Murry C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nunes S.S., Miklas J.W., Liu J., Aschar-Sobbi R., Xiao Y., Zhang B., Jiang J., Massé S., Gagliardi M., Hsieh A., et al. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zimmermann W.H., Didié M., Wasmeier G.H., Nixdorff U., Hess A., Melnychenko I., Boy O., Neuhuber W.L., Weyand M., Eschenhagen T. Cardiac Grafting of Engineered Heart Tissue in Syngenic Rats. Circulation. 2002;106:151. doi: 10.1161/01.CIR.0000032876.55215.10. [DOI] [PubMed] [Google Scholar]
- 88.Pati F., Jang J., Ha D.H., Won Kim S., Rhie J.W., Shim J.H., Kim D.H., Cho D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5 doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giobbe G.G., Crowley C., Luni C., Campinoti S., Khedr M., Kretzschmar K., De Santis M.M., Zambaiti E., Michielin F., Meran L., et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019;10:5658–5714. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim S., Min S., Choi Y.S., Jo S.-H., Jung J.H., Han K., Kim J., An S., Ji Y.W., Kim Y.-G., Cho S.W. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022;13:1692. doi: 10.1038/s41467-022-29279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim B.S., Das S., Jang J., Cho D.W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020;120:10608–10661. doi: 10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- 92.Han W., Singh N.K., Kim J.J., Kim H., Kim B.S., Park J.Y., Jang J., Cho D.W. Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119496. [DOI] [PubMed] [Google Scholar]
- 93.Jin Y., Kim H., Min S., Choi Y.S., Seo S.J., Jeong E., Kim S.K., Lee H.-A., Jo S.-H., Park J.-H., et al. Three-dimensional heart extracellular matrix enhances chemically induced direct cardiac reprogramming. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abn5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H., Kang B., Cui X., Lee S.H., Lee K., Cho D.W., Hwang W., Woodfield T.B.F., Lim K.S., Jang J. Light-Activated Decellularized Extracellular Matrix-Based Bioinks for Volumetric Tissue Analogs at the Centimeter Scale. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202011252. [DOI] [Google Scholar]
- 95.Tsui J.H., Leonard A., Camp N.D., Long J.T., Nawas Z.Y., Chavanachat R., Smith A.S.T., Choi J.S., Dong Z., Ahn E.H., et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malda J., Visser J., Melchels F.P., Jüngst T., Hennink W.E., Dhert W.J.A., Groll J., Hutmacher D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 97.Kuo C.K., Ma P.X. Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro. J. Biomed. Mater. Res. 2008;84:899–907. doi: 10.1002/jbm.a.31375. [DOI] [PubMed] [Google Scholar]
- 98.Bassett D.C., Håti A.G., Melø T.B., Stokke B.T., Sikorski P. Competitive ligand exchange of crosslinking ions for ionotropic hydrogel formation. J. Mater. Chem. B. 2016;4:6175–6182. doi: 10.1039/c6tb01812b. [DOI] [PubMed] [Google Scholar]
- 99.Neves M.I., Moroni L., Barrias C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahoo D.R., Biswal T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021;3 doi: 10.1007/s42452-020-04096-w. [DOI] [Google Scholar]
- 101.Adhikari J., Roy A., Das A., Ghosh M., Thomas S., Sinha A., Kim J., Saha P. Effects of Processing Parameters of 3D Bioprinting on the Cellular Activity of Bioinks. Macromol. Biosci. 2021;21:e2000179. doi: 10.1002/mabi.202000179. [DOI] [PubMed] [Google Scholar]
- 102.Hu T., Lo A.C.Y. Collagen–alginate composite hydrogel: Application in tissue engineering and biomedical sciences. Polymers. 2021;13 doi: 10.3390/polym13111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao G., Lee J.H., Jang J., Lee D.H., Kong J.S., Kim B.S., Choi Y.J., Jang W.B., Hong Y.J., Kwon S.M., Cho D. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201700798. [DOI] [Google Scholar]
- 104.Naranjo-Alcazar R., Bendix S., Groth T., Gallego Ferrer G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels. 2023;9 doi: 10.3390/gels9030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qin X.H., Ovsianikov A., Stampfl J., Liska R. Additive manufacturing of photosensitive Hydrogels for tissue engineering applications. BioNanoMaterials. 2014;15:49–70. doi: 10.1515/bnm-2014-0008. [DOI] [Google Scholar]
- 106.Zennifer A., Manivannan S., Sethuraman S., Kumbar S.G., Sundaramurthi D. 3D bioprinting and photocrosslinking: emerging strategies & future perspectives. Biomater. Adv. 2022;134 doi: 10.1016/j.msec.2021.112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang B., Park Y., Hwang D.G., Kim D., Yong U., Lim K.S., Jang J. Facile Bioprinting Process for Fabricating Size-Controllable Functional Microtissues Using Light-Activated Decellularized Extracellular Matrix-Based Bioinks. Adv. Mater. Technol. 2022;7 doi: 10.1002/admt.202100947. [DOI] [Google Scholar]
- 108.Han H., Park Y., Choi Y.M., Yong U., Kang B., Shin W., Min S., Kim H.J., Jang J. A Bioprinted Tubular Intestine Model Using a Colon-Specific Extracellular Matrix Bioink. Adv. Healthcare Mater. 2022;11:e2101768. doi: 10.1002/adhm.202101768. [DOI] [PubMed] [Google Scholar]
- 109.Han H., Kim M., Yong U., Jo Y., Choi Y.-M., Kim H.J., Hwang D.G., Kang D., Jang J. Tissue-specific gelatin bioink as a rheology modifier for high printability and adjustable tissue properties. Biomater. Sci. 2024;12:2599–2613. doi: 10.1039/d3bm02111d. [DOI] [PubMed] [Google Scholar]
- 110.Jang J., Kim T.G., Kim B.S., Kim S.W., Kwon S.M., Cho D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016;33:88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z., Kumar H., Tian Z., Jin X., Holzman J.F., Menard F., Kim K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces. 2018;10:26859–26869. doi: 10.1021/acsami.8b06607. [DOI] [PubMed] [Google Scholar]
- 112.Lu B., Ye M., Xia J., Zhang Z., Xiong Z., Zhang T. Electrical Stimulation Promotes the Vascularization and Functionalization of an Engineered Biomimetic Human Cardiac Tissue. Adv. Healthcare Mater. 2023;12:e2300607. doi: 10.1002/adhm.202300607. [DOI] [PubMed] [Google Scholar]
- 113.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 114.Izadifar M., Chapman D., Babyn P., Chen X., Kelly M.E. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. C Methods. 2018;24:74–88. doi: 10.1089/ten.tec.2017.0346. [DOI] [PubMed] [Google Scholar]
- 115.Amsden B.G., Sukarto A., Knight D.K., Shapka S.N. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules. 2007;8:3758–3766. doi: 10.1021/bm700691e. [DOI] [PubMed] [Google Scholar]
- 116.Lee H., Kim W., Lee J., Yoo J.J., Kim G.H., Lee S.J. Effect of Hierarchical Scaffold Consisting of Aligned dECM Nanofibers and Poly(lactide- co-glycolide) Struts on the Orientation and Maturation of Human Muscle Progenitor Cells. ACS Appl. Mater. Interfaces. 2019;11:39449–39458. doi: 10.1021/acsami.9b12639. [DOI] [PubMed] [Google Scholar]
- 117.Yoon J., Han H., Jang J. Nanomaterials-incorporated hydrogels for 3D bioprinting technology. Nano Converg. 2023;10 doi: 10.1186/s40580-023-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim K.S., Klotz B.J., Lindberg G.C.J., Melchels F.P.W., Hooper G.J., Malda J., Gawlitta D., Woodfield T.B.F. Visible Light Cross-Linking of Gelatin Hydrogels Offers an Enhanced Cell Microenvironment with Improved Light Penetration Depth. Macromol. Biosci. 2019;19:e1900098. doi: 10.1002/mabi.201900098. [DOI] [PubMed] [Google Scholar]
- 119.Lim K.S., Schon B.S., Mekhileri N.V., Brown G.C.J., Chia C.M., Prabakar S., Hooper G.J., Woodfield T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016;2:1752–1762. doi: 10.1021/acsbiomaterials.6b00149. [DOI] [PubMed] [Google Scholar]
- 120.Decker C., Jenkins A.D. Kinetic Approach of 02 Inhibition in Ultraviolet-and Laser-Induced Polymerizations. Macromolecules. 1985;18:1241–1244. doi: 10.1021/ma00148a034. [DOI] [Google Scholar]
- 121.Gulzar A., Yıldız E., Kaleli H.N., Nazeer M.A., Zibandeh N., Malik A.N., Taş A.Y., Lazoğlu I., Şahin A., Kizilel S. Ruthenium-induced corneal collagen crosslinking under visible light. Acta Biomater. 2022;147:198–208. doi: 10.1016/j.actbio.2022.05.040. [DOI] [PubMed] [Google Scholar]
- 122.Bjork J.W., Johnson S.L., Tranquillo R.T. Ruthenium-catalyzed photo cross-linking of fibrin-based engineered tissue. Biomaterials. 2011;32:2479–2488. doi: 10.1016/j.biomaterials.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Asaro G.A., Solazzo M., Suku M., Spurling D., Genoud K., Gonzalez J.G., Brien F.J.O., Nicolosi V., Monaghan M.G. MXene functionalized collagen biomaterials for cardiac tissue engineering driving iPSC-derived cardiomyocyte maturation. NPJ 2D Mater. Appl. 2023;7:44. doi: 10.1038/s41699-023-00409-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roshanbinfar K., Schiffer M., Carls E., Angeloni M., Koleśnik-Gray M., Schruefer S., Schubert D.W., Ferrazzi F., Krstić V., Fleischmann B.K., et al. Electrically Conductive Collagen-PEDOT:PSS Hydrogel Prevents Post-Infarct Cardiac Arrhythmia and Supports hiPSC-Cardiomyocyte Function. Adv. Mater. 2024;36 doi: 10.1002/ADMA.202403642. [DOI] [PubMed] [Google Scholar]
- 125.Navaei A., Saini H., Christenson W., Sullivan R.T., Ros R., Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016;41:133–146. doi: 10.1016/j.actbio.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 126.Elkhoury K., Kodeih S., Enciso-Martínez E., Maziz A., Bergaud C. Advancing Cardiomyocyte Maturation: Current Strategies and Promising Conductive Polymer-Based Approaches. Adv. Healthcare Mater. 2024;13 doi: 10.1002/adhm.202303288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roshanbinfar K., Vogt L., Greber B., Diecke S., Boccaccini A.R., Scheibel T., Engel F.B. Electroconductive Biohybrid Hydrogel for Enhanced Maturation and Beating Properties of Engineered Cardiac Tissues. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201803951. [DOI] [Google Scholar]
- 128.Moroni L., Boland T., Burdick J.A., De Maria C., Derby B., Forgacs G., Groll J., Li Q., Malda J., Mironov V.A., et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018;36:384–402. doi: 10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 129.Polonchuk L., Chabria M., Badi L., Hoflack J.C., Figtree G., Davies M.J., Gentile C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-06385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tan Y., Coyle R.C., Barrs R.W., Silver S.E., Li M., Richards D.J., Lin Y., Jiang Y., Wang H., Menick D.R., et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen V.C., Ye J., Shukla P., Hua G., Chen D., Lin Z., Liu J.c., Chai J., Gold J., Wu J., et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015;15:365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kahn-Krell A., Pretorius D., Ou J., Fast V.G., Litovsky S., Berry J., Liu X.M., Zhang J. Bioreactor Suspension Culture: Differentiation and Production of Cardiomyocyte Spheroids From Human Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.674260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khoury R.E., Nagiah N., Mudloff J.A., Thakur V., Chattopadhyay M., Joddar B. 3D Bioprinted Spheroidal Droplets for Engineering the Heterocellular Coupling between Cardiomyocytes and Cardiac Fibroblasts. Cyborg Bionic Syst. 2021;2021 doi: 10.34133/2021/9864212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schaaf S., Shibamiya A., Mewe M., Eder A., Stöhr A., Hirt M.N., Rau T., Zimmermann W.H., Conradi L., Eschenhagen T., Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Querdel E., Reinsch M., Castro L., Köse D., Bähr A., Reich S., Geertz B., Ulmer B., Schulze M., Lemoine M.D., et al. Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation. 2021;143:1991–2006. doi: 10.1161/CIRCULATIONAHA.120.047904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruan J.L., Tulloch N.L., Razumova M.V., Saiget M., Muskheli V., Pabon L., Reinecke H., Regnier M., Murry C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation. 2016;134:1557–1567. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hansen A., Eder A., Bönstrup M., Flato M., Mewe M., Schaaf S., Aksehirlioglu B., Schwörer A., Uebeler J., Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 138.Finkel S., Sweet S., Locke T., Smith S., Wang Z., Sandini C., Imredy J., He Y., Durante M., Lagrutta A., et al. FRESHTM 3D bioprinted cardiac tissue, a bioengineered platform for in vitro pharmacology. APL Bioeng. 2023;7 doi: 10.1063/5.0163363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahrens J.H., Uzel S.G.M., Skylar-Scott M., Mata M.M., Lu A., Kroll K.T., Lewis J.A. Programming Cellular Alignment in Engineered Cardiac Tissue via Bioprinting Anisotropic Organ Building Blocks. Adv. Mater. 2022;34:e2200217. doi: 10.1002/adma.202200217. [DOI] [PubMed] [Google Scholar]
- 140.Andrée B., Voß N., Kriedemann N., Triebert W., Teske J., Mertens M., Witte M., Szádocka S., Hilfiker A., Aper T., et al. Fabrication of heart tubes from iPSC derived cardiomyocytes and human fibrinogen by rotating mold technology. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-64022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bliley J., Tashman J., Stang M., Coffin B., Shiwarski D., Lee A., Hinton T., Feinberg A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac58be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kawai Y., Tohyama S., Arai K., Tamura T., Soma Y., Fukuda K., Shimizu H., Nakayama K., Kobayashi E. Scaffold-Free Tubular Engineered Heart Tissue From Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in vivo. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.806215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arai K., Murata D., Takao S., Nakamura A., Itoh M., Kitsuka T., Nakayama K. Drug response analysis for scaffold-free cardiac constructs fabricated using bio-3D printer. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-65681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li R.A., Keung W., Cashman T.J., Backeris P.C., Johnson B.V., Bardot E.S., Wong A.O.T., Chan P.K.W., Chan C.W.Y., Costa K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. doi: 10.1016/j.biomaterials.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang H., Liu Q., Zimmerman J.F., Lee K.Y., Jin Q., Peters M.M., Rosnach M., Choi S., Kim S.L., Ardoña H.A.M., et al. Recreating the heart’s helical structure-function relationship with focused rotary jet spinning. Science. 2022;377:180–185. doi: 10.1126/science.abl6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kupfer M.E., Lin W.H., Ravikumar V., Qiu K., Wang L., Gao L., Bhuiyan D.B., Lenz M., Ai J., Mahutga R.R., et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020;127:207–224. doi: 10.1161/CIRCRESAHA.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pretorius D., Kahn-Krell A.M., Lou X., Fast V.G., Berry J.L., Kamp T.J., Zhang J. Layer-By-Layer Fabrication of Large and Thick Human Cardiac Muscle Patch Constructs With Superior Electrophysiological Properties. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.670504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saidy N.T., Fernández-Colino A., Heidari B.S., Kent R., Vernon M., Bas O., Mulderrig S., Lubig A., Rodríguez-Cabello J.C., Doyle B., et al. Spatially Heterogeneous Tubular Scaffolds for In Situ Heart Valve Tissue Engineering Using Melt Electrowriting. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202110716. [DOI] [Google Scholar]
- 149.Jafari A., Vahid Niknezhad S., Kaviani M., Saleh W., Wong N., Van Vliet P.P., Moraes C., Ajji A., Kadem L., Azarpira N., et al. Formulation and Evaluation of PVA/Gelatin/Carrageenan Inks for 3D Printing and Development of Tissue-Engineered Heart Valves. Adv. Funct. Mater. 2024;34 doi: 10.1002/adfm.202305188. [DOI] [Google Scholar]
- 150.Tani H., Tohyama S. Human Engineered Heart Tissue Models for Disease Modeling and Drug Discovery. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.855763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weinberger F., Mannhardt I., Eschenhagen T. Engineering Cardiac Muscle Tissue: A Maturating Field of Research. Circ. Res. 2017;120:1487–1500. doi: 10.1161/CIRCRESAHA.117.310738. [DOI] [PubMed] [Google Scholar]
- 152.Tenreiro M.F., Louro A.F., Alves P.M., Serra M. Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. NPJ Regen. Med. 2021;6 doi: 10.1038/s41536-021-00140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tzatzalos E., Abilez O.J., Shukla P., Wu J.C. Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies. Adv. Drug Deliv. Rev. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jang J., Park H.J., Kim S.W., Kim H., Park J.Y., Na S.J., Kim H.J., Park M.N., Choi S.H., Park S.H., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 155.Cho J., Lee H., Rah W., Chang H.J., Yoon Y.S. From engineered heart tissue to cardiac organoid. Theranostics. 2022;12:2758–2772. doi: 10.7150/thno.67661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Budharaju H., Sundaramurthi D., Sethuraman S. Embedded 3D bioprinting – An emerging strategy to fabricate biomimetic & large vascularized tissue constructs. Bioact. Mater. 2024;32:356–384. doi: 10.1016/J.BIOACTMAT.2023.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mohammadi M.H., Okhovatian S., Savoji H., Campbell S.B., Lai B.F.L., Wu J., Pascual-Gil S., Bannerman D., Rafatian N., Li R.K., Radisic M. Toward Hierarchical Assembly of Aligned Cell Sheets into a Conical Cardiac Ventricle Using Microfabricated Elastomers. Adv. Biol. 2022;6 doi: 10.1002/adbi.202101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eom S., Park S.M., Hwang D.G., Kim H.W., Jang J., Kim D.S. Fabrication of an align-random distinct, heterogeneous nanofiber mat endowed with bifunctional properties for engineered 3D cardiac anisotropy. Compos. B Eng. 2021;226 doi: 10.1016/j.compositesb.2021.109336. [DOI] [Google Scholar]
- 159.Kim H., Jang J., Park J., Lee K.P., Lee S., Lee D.M., Kim K.H., Kim H.K., Cho D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab1a8b. [DOI] [PubMed] [Google Scholar]
- 160.Hinton R.B., Yutzey K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao G., Park J.Y., Kim B.S., Jang J., Cho D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional In aVitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthcare Mater. 2018;7:e1801102. doi: 10.1002/adhm.201801102. [DOI] [PubMed] [Google Scholar]
- 162.Gao G., Park W., Kim B.S., Ahn M., Chae S., Cho W.W., Kim J., Lee J.Y., Jang J., Cho D.W. Construction of a Novel In Vitro Atherosclerotic Model from Geometry-Tunable Artery Equivalents Engineered via In-Bath Coaxial Cell Printing. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202008878. [DOI] [Google Scholar]
- 163.Onwuka E., King N., Heuer E., Breuer C. The heart and great vessels. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Devillard C.D., Marquette C.A. Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.721843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park W., Lee J.S., Gao G., Kim B.S., Cho D.W. 3D bioprinted multilayered cerebrovascular conduits to study cancer extravasation mechanism related with vascular geometry. Nat. Commun. 2023;14 doi: 10.1038/s41467-023-43586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tabriz A.G., Hermida M.A., Leslie N.R., Shu W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/4/045012. [DOI] [PubMed] [Google Scholar]
- 167.Liberthson R.R., Dinsmore R.E., Bharati S., Rubenstein J.J., Caulfield J., Wheeler E.O., Harthorne J.W., Lev M. Aberrant Coronary Artery Origin From the Aorta Diagnosis and Clinical Significance. Circulation. 1974;50:774–779. doi: 10.1161/01.CIR.50.4.774. [DOI] [PubMed] [Google Scholar]
- 168.Fleischer S., Shapira A., Feiner R., Dvir T. Modular assembly of thick multifunctional cardiac patches. Proc. Natl. Acad. Sci. USA. 2017;114:1898–1903. doi: 10.1073/pnas.1615728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kolesky D.B., Homan K.A., Skylar-Scott M.A., Lewis J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang B., Montgomery M., Chamberlain M.D., Ogawa S., Korolj A., Pahnke A., Wells L.A., Massé S., Kim J., Reis L., et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016;15:669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Skylar-Scott M.A., Uzel S.G.M., Nam L.L., Ahrens J.H., Truby R.L., Damaraju S., Lewis J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu T., Zhang M., Laurent T., Xie M., Ding S. Concise Review: Chemical Approaches for Modulating Lineage-Specific Stem Cells and Progenitors. Stem Cells Transl. Med. 2013;2:355–361. doi: 10.5966/sctm.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Neri T., Hiriart E., van Vliet P.P., Faure E., Norris R.A., Farhat B., Jagla B., Lefrancois J., Sugi Y., Moore-Morris T., et al. Human pre-valvular endocardial cells derived from pluripotent stem cells recapitulate cardiac pathophysiological valvulogenesis. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019;10:1–22. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wnorowski A., Yang H., Wu J.C. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev. 2019;140:3–11. doi: 10.1016/j.addr.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Watson S.A., Duff J., Bardi I., Zabielska M., Atanur S.S., Jabbour R.J., Simon A., Tomas A., Smolenski R.T., Harding S.E., et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-10175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ho D.L.L., Lee S., Du J., Weiss J.D., Tam T., Sinha S., Klinger D., Devine S., Hamfeldt A., Leng H.T., et al. Large-Scale Production of Wholly Cellular Bioinks via the Optimization of Human Induced Pluripotent Stem Cell Aggregate Culture in Automated Bioreactors. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202201138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Skylar-Scott M.A., Huang J.Y., Lu A., Ng A.H.M., Duenki T., Liu S., Nam L.L., Damaraju S., Church G.M., Lewis J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022;6:449–462. doi: 10.1038/s41551-022-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li R.L., Russ J., Paschalides C., Ferrari G., Waisman H., Kysar J.W., Kalfa D. Mechanical considerations for polymeric heart valve development: Biomechanics, materials, design and manufacturing. Biomaterials. 2019;225 doi: 10.1016/j.biomaterials.2019.119493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Emig R., Zgierski-Johnston C.M., Timmermann V., Taberner A.J., Nash M.P., Kohl P., Peyronnet R. Passive myocardial mechanical properties: meaning, measurement, models. Biophys. Rev. 2021;13:587–610. doi: 10.1007/s12551-021-00838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rodriguez A.G., Han S.J., Regnier M., Sniadecki N.J. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys. J. 2011;101:2455–2464. doi: 10.1016/j.bpj.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Van Belleghem S., Torres L., Santoro M., Mahadik B., Wolfand A., Kofinas P., Fisher J.P. Hybrid 3D Printing of Synthetic and Cell-Laden Bioinks for Shape Retaining Soft Tissue Grafts. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201907145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang M., Li W., Hao J., Gonzales A., Zhao Z., Flores R.S., Kuang X., Mu X., Ching T., Tang G., et al. Molecularly cleavable bioinks facilitate high-performance digital light processing-based bioprinting of functional volumetric soft tissues. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-31002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skylar-Scott M.A., Mueller J., Visser C.W., Lewis J.A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature. 2019;575:330–335. doi: 10.1038/s41586-019-1736-8. [DOI] [PubMed] [Google Scholar]
- 185.Levato R., Lim K.S., Li W., Asua A.U., Peña L.B., Wang M., Falandt M., Bernal P.N., Gawlitta D., Zhang Y.S., et al. High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins. Mater. Today. Bio. 2021;12 doi: 10.1016/j.mtbio.2021.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lian L., Xie M., Luo Z., Zhang Z., Maharjan S., Mu X., Garciamendez-Mijares C.E., Kuang X., Sahoo J.K., Tang G., et al. Rapid Volumetric Bioprinting of Decellularized Extracellular Matrix Bioinks. Adv. Mater. 2024;36 doi: 10.1002/adma.202304846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bernal P.N., Delrot P., Loterie D., Li Y., Malda J., Moser C., Levato R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019;31 doi: 10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 188.Größbacher G., Bartolf-Kopp M., Gergely C., Bernal P.N., Florczak S., de Ruijter M., Rodriguez N.G., Groll J., Malda J., Jungst T., et al. Volumetric Printing Across Melt Electrowritten Scaffolds Fabricates Multi-Material Living Constructs with Tunable Architecture and Mechanics. Adv. Mater. 2023;35 doi: 10.1002/adma.202300756. [DOI] [PubMed] [Google Scholar]
- 189.Jo Y., Hwang D.G., Kim M., Yong U., Jang J. Bioprinting-assisted tissue assembly to generate organ substitutes at scale. Trends Biotechnol. 2023;41:93–105. doi: 10.1016/j.tibtech.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 190.Levato R., Dudaryeva O., Garciamendez-Mijares C.E., Kirkpatrick B.E., Rizzo R., Schimelman J., Anseth K.S., Chen S., Zenobi-Wong M., Zhang Y.S. Light-based vat-polymerization bioprinting. Nat. Rev. Methods Primers. 2023;3 doi: 10.1038/s43586-023-00231-0. [DOI] [Google Scholar]
- 191.Hwang D.G., Choi Y.M., Jang J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in vitro Studies. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.685507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Montgomery M., Zhang B., Radisic M. Cardiac tissue vascularization: From angiogenesis to microfluidic blood vessels. J. Cardiovasc. Pharmacol. Therapeut. 2014;19:382–393. doi: 10.1177/1074248414528576. [DOI] [PubMed] [Google Scholar]
- 193.Jang J. 3D bioprinting and in vitro cardiovascular tissue modeling. Bioengineering. 2017;4 doi: 10.3390/bioengineering4030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brady E.L., Prado O., Johansson F., Mitchell S.N., Martinson A.M., Karbassi E., Reinecke H., Murry C.E., Davis J., Stevens K.R. Engineered tissue vascularization and engraftment depends on host model. Sci. Rep. 2023;13 doi: 10.1038/s41598-022-23895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.O’Connor C., Brady E., Zheng Y., Moore E., Stevens K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022;7:702–716. doi: 10.1038/s41578-022-00447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gratz D., Onal B., Dalic A., Hund T.J. Synchronization of Pacemaking in the Sinoatrial Node: A Mathematical Modeling Study. Front. Physiol. 2018;6 doi: 10.3389/fphy.2018.00063. [DOI] [Google Scholar]
- 197.Hayashi T., Tokihiro T., Kurihara H., Yasuda K. Community effect of cardiomyocytes in beating rhythms is determined by stable cells. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rajendran P.S., Challis R.C., Fowlkes C.C., Hanna P., Tompkins J.D., Jordan M.C., Hiyari S., Gabris-Weber B.A., Greenbaum A., Chan K.Y., et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mandla R., Jung C., Vedantham V. Transcriptional and Epigenetic Landscape of Cardiac Pacemaker Cells: Insights Into Cellular Specialization in the Sinoatrial Node. Front. Physiol. 2021;12 doi: 10.3389/FPHYS.2021.712666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhao M.T., Shao N.Y., Garg V. Subtype-specific cardiomyocytes for precision medicine: Where are we now? Stem Cell. 2020;38:822–833. doi: 10.1002/STEM.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Prodan N., Ershad F., Reyes-Alcaraz A., Li L., Mistretta B., Gonzalez L., Rao Z., Yu C., Gunaratne P.H., Li N., et al. Direct reprogramming of cardiomyocytes into cardiac Purkinje-like cells. iScience. 2022;25 doi: 10.1016/J.ISCI.2022.105402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bootman M.D., Smyrnias I., Thul R., Coombes S., Roderick H.L. Atrial cardiomyocyte calcium signalling. Biochim. Biophys. Acta. 2011;1813:922–934. doi: 10.1016/J.BBAMCR.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 203.Ideker R.E., Kong W., Pogwizd S. Purkinje fibers and arrhythmias. Pacing Clin. Electrophysiol. 2009;32:283–285. doi: 10.1111/j.1540-8159.2008.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Logantha S.J.R.J., Cai X.J., Yanni J., Jones C.B., Stephenson R.S., Stuart L., Quigley G., Monfredi O., Nakao S., Oh I.Y., et al. Remodeling of the Purkinje Network in Congestive Heart Failure in the Rabbit. Circ. Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Winbo A., Ramanan S., Eugster E., Jovinge S., Skinner J.R., Montgomery J.M. Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. Am. J. Physiol. Heart Circ. Physiol. 2020;319:927–937. doi: 10.1152/ajpheart.00546.2020. [DOI] [PubMed] [Google Scholar]
- 206.Oh Y., Cho G.S., Li Z., Hong I., Zhu R., Kim M.-J., Kim Y.J., Tampakakis E., Tung L., Huganir R., et al. Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons. Cell Stem Cell. 2016;19:95–106. doi: 10.1016/j.stem.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Takayama Y., Kushige H., Akagi Y., Suzuki Y., Kumagai Y., Kida Y.S. Selective Induction of Human Autonomic Neurons Enables Precise Control of Cardiomyocyte Beating. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-66303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hansson A., Holm M., Blomström P., Johansson R., Lührs C., Brandt J., Olsson S.B. Right atrial free wall conduction velocity and degree of anisotropy in patients with stable sinus rhythm studied during open heart surgery. Eur. Heart J. 1998;19:293–300. doi: 10.1053/euhj.1997.0742. [DOI] [PubMed] [Google Scholar]
- 209.Ramanathan C., Jia P., Ghanem R., Ryu K., Rudy Y. Activation and repolarization of the normal humanheart under complete physiological conditions. Proc. Natl. Acad. Sci. USA. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kupersmith J., Krongrad E., Waldo A.L. Conduction Intervals and Conduction Velocity in the Human Cardiac Conduction System Studies during Open-Heart Surgery. Circulation. 1973;47:776–785. doi: 10.1161/01.CIR.47.4.776. [DOI] [PubMed] [Google Scholar]
- 211.Mattapally S., Zhu W., Fast V.G., Gao L., Worley C., Kannappan R., Borovjagin A.V., Zhang J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am. J. Physiol. Heart Circ. Physiol. 2018;315:327–339. doi: 10.1152/ajpheart.00688.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bliley J.M., Vermeer M.C.S.C., Duffy R.M., Batalov I., Kramer D., Tashman J.W., Shiwarski D.J., Lee A., Teplenin A.S., Volkers L., et al. Dynamic loading of human engineered heart tissue enhances contractile function and drives a desmosome-linked disease phenotype. Sci. Transl. Med. 2021;13:1817. doi: 10.5281/zenodo.5034507. [DOI] [PubMed] [Google Scholar]
- 213.Jackman C.P., Carlson A.L., Bursac N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials. 2016;111:66–79. doi: 10.1016/j.biomaterials.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Benam K.H., Dauth S., Hassell B., Herland A., Jain A., Jang K.J., Karalis K., Kim H.J., MacQueen L., Mahmoodian R., et al. Engineered in vitro disease models. Annu. Rev. Pathol. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- 215.Yong U., Kim D., Kim H., Hwang D.G., Cho S., Nam H., Kim S., Kim T., Jeong U., Kim K., et al. Biohybrid 3D Printing of a Tissue-Sensor Platform for Wireless, Real-Time, and Continuous Monitoring of Drug-Induced Cardiotoxicity. Adv. Mater. 2023;35 doi: 10.1002/adma.202208983. [DOI] [PubMed] [Google Scholar]
- 216.Ma Y., Deng B., He R., Huang P. Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e24593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14 doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]