Abstract
Background
High-grade serous ovarian cancer (HGSOC) accounts for 70–80% of all ovarian cancer-related deaths. Multiple studies have suggested that the fallopian tube epithelium (FTE) serves as the cell of origin of HGSOC. Phosphatase and tensin homolog (PTEN) is a tumor suppressor and its loss is sufficient to induce numerous tumorigenic changes in FTE, including increased migration, formation of multicellular tumor spheroids (MTSs), and ovarian colonization. In murine oviductal epithelial (MOE) cells (the equivalent of human FTE) loss of PTEN results in the upregulation of transcripts associated with the extracellular matrix, with a specific focus on the elevation of lysyl oxidase-like 2 (LOXL2). Although LOXL2 is known to drive transformation and invasion in solid tumors and is associated with a poor prognosis in ovarian cancer, its specific role in the tumorigenesis of ovarian cancer originating from FTE remains unclear. Therefore, we aim to investigate whether LOXL2 mediates tumorigenesis from the fallopian tube epithelium.
Methods
In this study, we utilized clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (CAS9) technology to delete LOXL2 in PTEN-deficient MOE cells to understand its role in mediating the oncogenic effects of PTEN loss. In addition, CRISPR-CAS9 was used to delete LOXL2 in OVCAR8 ovarian cancer cells. We monitored the changes in tumorigenic properties, such as migration, invasion, and growth of three-dimensional (3D) spheroids, to assess whether the loss of LOXL2 resulted in any changes.
Results
We found that a reduction in LOXL2 expression did not significantly change the migration or invasive capabilities of PTEN-depleted MOE or human ovarian cancer cells. However, we found that a reduction in LOXL2 expression resulted in a significant reduction in 3D MTS formation and survival in both lines.
Conclusions
These results reveal for the first time that PTEN loss in FTE cells increases LOXL2 expression through downregulation of Pax2, and LOXL2 deletion blocks 3D spheroid formation.
Keywords: LOXL2, PTEN, PAX2, Fallopian tube, Ovarian cancer
Graphical abstract

Highlights
-
•
Loss of phosphatase and tensin homolog (PTEN) in the fallopian tube epithelium results in the upregulation of lysyl oxidase (Loxl-like)2.
-
•
Lysyl oxidase-like 2 (Loxl2) plays a role in three-dimensional (3D) spheroid formation and survival.
-
•
Loss of the pair box2 gene (PAX2) downstream of the loss of PTEN is required for Loxl2 transcription increase.
Introduction
High-grade serous ovarian cancer (HGSOC) is the most lethal gynecological malignancy, primarily due to the challenges associated with early detection. Current evidence supports the idea that HGSOC originates in the fallopian tube epithelium (FTE).1 Therefore, investigating early tumorigenesis using FTE may provide valuable insights into the identification of pathways that could be targeted for the development of novel strategies for early detection. Somatic mutations in TP53 are nearly universal in HGSOC cases, resulting in both the loss of its tumor suppressor function and the gain of a new function.2,3 However, despite the prevalence of TP53 alterations in HGSOCs, this mutation alone is insufficient to drive ovarian cancer.4,5 Loss of the paired box2 gene (PAX2) is common in fallopian tube precursor lesions, starting at the secretory cell outgrowth (SCOUT) lesion, where the loss of this transcription factor is linked to secretory cell expansion.6 Phosphatase and tensin homolog (PTEN) downregulates the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT) signaling pathway and plays a crucial role in early HGSOC development.7 In mouse models, the deletion of Tp53 in the FTE was insufficient for in vivo tumor formation but generated tumors when combined with the loss of the phosphatase and tensin homolog (PTEN).4 Notably, PTEN loss alone was sufficient to drive tumorigenesis, while mutated Tp53 only led to increased migration.8, 9, 10 PTEN loss or activation of the PI3K-AKT pathway was observed in approximately 70% of 521 cases of HGSOC, highlighting its significance as an early driver of HGSOC development.11,12 Research from our laboratory has demonstrated that PTEN loss from the fallopian tube results in the downregulation of PAX2. Interestingly, re-expression of PAX2 inhibits tumor growth.13 Furthermore, PTEN loss upregulates cancer stem cell markers, such as WNT4, CD44, ALDH1, and LGR5, and these markers are reversed when PAX2 is re-expressed.14 PTEN also regulates interactions with the extracellular matrix (ECM), a critical factor in tumorigenesis.15 Our previous ribonucleic acid (RNA) sequencing data revealed that PTEN silencing in murine oviductal epithelial (MOE) cells led to the upregulation of ECM-associated genes, including LOXL2, a member of the lysyl oxidase (LOX) family responsible for elastin and collagen crosslinking, making it a key regulator of ECM remodeling.14 Although lysyl oxidase-like 2 (LOXL2) has been implicated in cancer metastasis16 and is a marker of poor prognosis in ovarian cancer,17 its role in early tumorigenesis in the fallopian tube has not yet been elucidated. This study aims to assess whether increased LOXL2 expression in murine and human tumorigenic cells is crucial for growth and survival under anchorage-independent conditions and whether the regulation of LOXL2 depends on the loss of PAX2.
Methods
Cells and media
MOE and OVCAR8 cells were cultured as described previously.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Clones were kept in a 37 °C incubator and passaged after reaching moderate to complete confluence.
Generation of lysyl oxidase-like 2 clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 9 clones
MOE and OVCAR8 cells were used in all experiments. To simulate PTEN loss, MOE cells were transfected with short hairpin RNA (shRNA) to silence PTEN expression, and stable clones were isolated. Validated MOE:PTENshRNA clones9, 10, 11, 12, 13 were used for further studies. CRISPR/Cas9 technology was used to eliminate the LOXL2 gene from MOE:PTENshRNA and OVCAR8 cells. Guide RNA (gRNA) oligos were annealed and phosphorylated in a single reaction using T4 ligase and T4 polynucleotide kinase (PNK). The plasmid pX330 containing the hSpCas9 cassette was digested with the Bbsl restriction enzyme and ligated to the previously annealed and phosphorylated gRNA oligos using T7 ligase. Transformation of the T4 ligation product was performed in DH5 alpha competent bacteria, and several colonies were isolated and amplified using a Qiagen kit for Maxi-prep plasmid purification. The plasmids were sequenced using a US6 primer, and the purest and most positive plasmids were selected. The targeting sequence for MOE:PTENshRNA was CTGAGCCTAGCACAGTACGA, whereas the targeting sequence for OVCAR8 was GCCGGTTGCTGGAAGTACTCG.
Transfection of the lysyl oxidase-like 2 clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 9 plasmid into murine oviductal epithelial:PTENshRNA and OVCAR8 cells
Purified plasmids (with CRISPR targeting murine or human LOXL2) were transfected into MOE:PTENshRNA or OVCAR8 cells using the Mirus TransIT-LT1 (Mirus, #MIR2300) transfection reagent for 24 h. Since the CRISPR plasmids did not exhibit resistance to mammalian antibiotics, they were co-transfected with a pCGN-Neo plasmid containing resistance to neomycin. The day after transfection, the medium was replaced with a medium containing neomycin and incubated for 24 h. Subsequently, the mixed population was trypsinized and seeded at a low density. After a week, several colonies were formed. Twelve clones were isolated using mini-glass cylinders, and clones were plated into a 12-well plate for expansion. The clones were passed two more times to obtain a confluent 10 cm dish for validation.
Validation of lysyl oxidase-like 2 messenger ribonucleic acid loss by quantitative polymerase chain reaction following clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 99
Several clones were screened for reduced mRNA expression by RT-qPCR. Specifically, cells were collected using trypsin/ethylenediaminetetraacetic acid (EDTA), and RNA was extracted using TRIzol (Sigma, 15596018) as the lysis reagent, according to the manufacturer's instructions. qPCR analysis was conducted using the Applied Bioscience Protocol with SYBR Green (Fisher, #A25780) for fluorescence. The following primers were used: human LOXL2 For-5′-CACATAGGTGGTTCCTTCAG-3′ and Rev-5′-CACGCAGGCTTCTTTACT-3′; murine LOXL2 For-5′-AGCCTATAAAACCTTTGCCTCGCGG-3′ and Rev-3′-TAGGCTTTCCGGAACCTTG. Clones that displayed higher LOXL2 Ct values relative to the PTEN control were considered candidate clones for subsequent experiments, as those clones exhibited reduced LOXL2 expression at the mRNA level.
Validation of lysyl oxidase-like 2 protein loss by western blot following clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 9
Protein quantification was performed using a bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay Kit) to calculate equal amounts of proteins in all conditions (30 μg). Cell clones were lysed using radioimmunoprecipitation assay (RIPA) buffer comprising 50 mmol/L Tris pH 7.6, 150 mmol/L NaCl, 1% Triton X-100, and 0.1% sodium dodecyl sulfate (SDS). The lysates were loaded onto an SDS-polyacrylamide-gel electrophoresis (PAGE) gel and subsequently transferred onto a nitrocellulose membrane. The primary LOXL2 antibody (Abclonal, #A14638) was added and incubated overnight at 4 °C. Thereafter, the horseradish peroxidase (HRP)-linked anti-rabbit secondary antibody was used for 30 min at room temperature (Cell Signaling Technologies). Blots were blocked with 5% dry milk in Tris-buffered saline with 0.1% Tween detergent (TSB-T) containing the respective antibodies. Clones that displayed reduced LOXL2 protein expression relative to the PTEN control were considered candidate clones for the subsequent.
Cell migration assay
Cells were collected using trypsin/EDTA at full confluence, and cellular concentrations were determined using a hemocytometer. Cells were plated in a 24-well plate at a concentration of 5 × 103 cells/well to obtain 100% confluence the following day; the experiment was performed in triplicate. A scratch was made through the middle of the monolayer using a 1 mL pipette tip, after which the cells were washed once with phosphate buffered saline (PBS). A photograph was captured of the plate labeled “Time 0.” A second photograph of the plate was captured after 8 h. The scratched area was quantified using ImageJ software.
Boyden chamber invasion assay
Aliquots of Matrigel (Fisher-BD, #356234) were thawed on ice and diluted to a concentration of 300 μg/mL. Approximately 120 μL of the diluted Matrigel was added to the inside of a 0.8 μm-pore transwell insert and incubated in a dry incubator at 37 °C for 1 h. Excess Matrigel was aspirated. Thereafter, 500 μL of complete media was added into each well of a 24-well plate, with the transwell insert being placed into the media. The cells were then collected using trypsin/EDTA, counted, and resuspended in a serum-free medium. Next, 120 μL of the cell suspension was added to the inside of the transwell insert at a concentration of 1 × 104 cells/well, after which they were allowed to incubate for 24 h. Three inserts were processed for each experimental group. After incubation, the excess cells were removed from the top of the inserts using cotton swabs. The inserts were fixed in 4% paraformaldehyde (PFA) for 5 min, permeabilized in 75% methanol for 5 min, and stained with 0.2% crystal violet in 10% ethanol for 10 min. The inserts were rinsed twice with PBS and allowed to dry overnight. Images of each insert were obtained using an inverted microscope (Nikon Eclipse 100). Invading cells were quantified using ImageJ software.
Multicellular tumor spheroid formation
MOE:PTENshRNA, PTENshRNA-LOXL2−/−, OVCAR8PCGN, and OVCAR8-LOXL2−/− cells were collected using trypsin/EDTA, counted, and resuspended in complete media. The cell suspension was diluted to 2 × 104 cells/mL. Thereafter, 100 μL of cells were added to a 96-well round-bottom ultra-low attachment (Corning, #07-201-680) plate. The cells were left for 7 days to facilitate spheroid formation. The resulting spheroids were photographed at a 10× magnification to obtain high-resolution images, and the relative diameters of the spheroids were analyzed using Celigo (Nexcelome).
Spheroid live/dead staining
Spheroids were generated as described previously.12 After 13–20 days the spheroids were subjected to live/dead staining (#R37601; Thermo Scientific). Calcein Am/component A (live green) and ethidium homodimer-1/component B (dead red) were thawed at room temperature. Component A was transferred to component B to create a 2× staining solution. Approximately 100 μL of the staining solution was added directly to the spheroids and incubated for 15 min at 20–25 °C. Images were captured using a Nikon Eclipse TE200 Fluorescence Microscope at 10× magnification. Fluorescent images were quantified using ImageJ software.
Statistical analyses
All data are represented as mean ± standard error. Statistical analyses were performed using GraphPad Prism software (GraphPad, La Jolla, CA, USA). All conditions were tested at least three times. Statistical significance was determined using the Student's t-test when only two populations were compared, one-way analysis of variance (ANOVA) when more than two populations were compared, and two-way ANOVA when more than two populations were divided into groups. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001 were considered significant; ns indicates non-significant.
Results
Loss of phosphatase and tensin homolog by short hairpin RNA increases lysyl oxidase-like 2 expression
In our investigation of PTEN loss in MOE cells, we employed stable PTENshRNA clones that were previously generated using short hairpin RNA targeting PTEN, subjected them to high-throughput RNA sequencing (RNA-seq), and compared them to ScrambleshRNA cell lines.10 Our findings revealed that PTEN silencing elevated the expression of various ECM-related transcripts [Figure 1A and B]. Notably, LOXL2, a critical enzyme involved in cancer progression, emerged as one of the significantly upregulated transcripts with a false rate discovery (FDR)-adjusted P value of <0.05 [Figure 1A]. To validate LOXL2 messenger RNA (mRNA) expression, MOE:PTENshRNA cells were further examined and compared to ScrambleshRNA through quantitative polymerase chain reaction (qPCR) analysis, confirming a statistically significant upregulation of LOXL2 mRNA in MOE:PTENshRNA [Figure 1C]. This finding was further supported by western blot analysis, which demonstrated the increased expression of LOXL2 at the protein level in MOE:PTENshRNA cells [Figure 1D].
Figure 1.
LOXL2 is upregulated upon PTEN loss. (A) Volcano plot showing genes that are significantly upregulated or downregulated following murine oviductal epithelial (MOE):PTENshRNA expression compared to the scrambled control. (B) Heat map showing the regulation of ECM-related genes in MOE:PTENshRNA cells compared to the scrambled control. (C) qPCR confirming that LOXL2 mRNA is increased in MOE:PTENshRNA cells compared to the scrambled control. (D) Western blot confirming that LOXL2 is also increased in MOE:PTENshRNA cells compared to the scrambled control. Three independent experiments were performed, and a Student's t-test was applied. Asterisks (∗∗∗∗) indicate a P value < 0.0001. ECM: Extracellular matrix; LOXL2: Lysyl oxidase-like 2; MOE: Murine oviductal epithelial; PAX2: Pair box2 gene; PTEN: Phosphatase and tensin homolog; PTENshRNA: Short hairpin RNA targeting PTEN qPCR: Quantitative polymerase chain reaction; SCR: Scramble; SCRshRNA: Short hairpin RNA targeting a scrambled sequence.
Loss of lysyl oxidase-like 2 using clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 9 does not impact the invasive or migratory capability of murine oviductal epithelial:PTENshRNA cells
To investigate the role of LOXL2 in mediating the survival and expansion of MOE:PTENshRNA cells, which have previously demonstrated tumor formation in both nude and Friend Virus B (FVB) mice, we used clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) to inactivate the LOXL2 gene. Loss of LOXL2 expression was assessed at both the protein and mRNA levels using western blotting and qPCR, respectively [Figure 2A and B]. Among the LOXL2 CRISPR/Cas9 clones, several exhibited reduced protein and mRNA expression compared to unmodified MOE:PTENshRNA cells, including clones 4, 9, 12, 2B, 3, and 5 [Figure 2A], which were selected for subsequent experiments.
Figure 2.
LOXL2 is required for spheroid formation in murine PTEN-depleted cells. (A) Western blot to detect LOXL2 upon deletion in the different clones. (B) qPCR showing loss of LOXL2 mRNA upon CRISPR deletion. (C) Migration assay of MOE:PTENshRNA cells with LOXL2 CRISPR clones vs. MOE:PTENshRNA. (D) Boyden chamber invasion assay of MOE:PTENshRNA cells with LOXL2 CRISPR #4, 9, and 12 clones vs. MOE:PTENshRNA. (E) Bright field images of spheroids from MOE:PTENshRNA cells with LOXL2 CRISPR clones #4, 9, and 12 vs. MOE:PTENshRNA. Scale bar = 500 μm. (F) Quantification of spheroids from MOE:PTENshRNA cells with LOXL2 CRISPR clones #4, 9, and 12 vs. MOE:PTENshRNA grown in ultra-low attachment (ULA) plate. (G) Bright field images of spheroids from MOE:PTENshRNA cells with LOXL2 CRISPR clones #2B, 3, and 5 vs. MOE:PTENshRNA. Scale bar = 500 μm. (H) Spheroid formation assay in ULA plate containing MOE:PTENshRNA cells with LOXL2 CRISPR clones #2B, 3, and 5 vs. MOE:PTENshRNA. (H–J) Live-dead assay of MOE:PTENshRNA cells with LOXL2 CRISPR clones #2B, 3, and 5 vs. MOE:PTENshRNA. Live cells are stained green and dead cells are stained red. Quantification of green and red fluorescence intensity was performed using ImageJ software. Three independent experiments were performed, and a one-way analysis of variance (ANOVA) was conducted, with asterisks indicating: (∗) P value ≤ 0.05; (∗∗) P value ≤ 0.01; (∗∗∗∗) P value ≤ 0.0001; (ns) P value > 0.05. ANOVA: Analysis of variance; CRISPR: Clustered regularly interspaced short palindromic repeats; LOXL2: Lysyl oxidase-like 2; MOE: Murine oviductal epithelial; ns: Non-specific; PTEN: Phosphatase and tensin homolog; PTENshRNA: Short hairpin RNA targeting PTEN; qPCR: Quantitative polymerase chain reaction; ULA: Ultra-low attachment.
MOE:PTENshRNA cells were previously reported to have an increased invasive capability toward collagen I, which is one of the most abundant components of the ovarian ECM.12 These cells also exhibit enhanced migratory abilities and a tendency to colonize peritoneal organs, based on in vivo experiments in athymic nude mice.9,10 Wound healing assays were conducted to investigate the role of LOXL2 in the migration of MOE:PTENshRNA cells. The removal of LOXL2 did not have a statistically significant impact on cell migration [Figure 2C].
To assess the impact of LOXL2 loss on the invasive ability of MOE:PTENshRNA cells, LOXL2 CRISPR/Cas9 clones were seeded in Boyden chambers for invasion assays using Matrigel-coated transwells. The results indicated that the removal of LOXL2 did not result in a statistically significant change in the number of invaded cells compared to MOE:PTENshRNA cells [Figure 2D]. These experiments suggest that under the conditions of these experiments, LOXL2 may not be a major contributor to the migratory and invasive abilities of MOE:PTENshRNA cells.
Loss of lysyl oxidase-like 2 reduces multicellular tumor spheroid formation in murine oviductal epithelial:PTENshRNA cells
The presence and formation of multicellular tumor spheroid (MTS) structures in HGSOC tissues originating from FTE are considered crucial and are hypothesized to enhance the survival of cells from the fallopian tube after detachment, and therefore, potentially contribute to the colonization of the ovary.9 To investigate this, MOE:PTENshRNA and LOXL2 deleted clones were subjected to low-adhesion conditions for 7 days to induce MTS formation. After 7 days, the LOXL2-deleted clones formed smaller spheroids than the MOE:PTENshRNA cells [Figure 2E and F]. The average diameters of the MTS structures formed by LOXL2 clones 4, 9, and 12 were 53%, 58%, and 65% smaller than the MTS structures formed by MOE:PTENshRNA clones, respectively [Figure 2F]. The reduction in MTS size in each clone was statistically significant (P < 0.05). Further investigations included additional LOXL2-deleted clones (2B, 3, and 5), confirming that reduced LOXL2 expression correlated with decreased spheroid formation ability [Figure 2G and H] and decreased survival as three-dimensional (3D) structures compared with MOE:PTENshRNA cells [Figure 2I and J]. These findings suggest that LOXL2 plays a critical role in MTS formation and survival during tumorigenesis in FTE.
Loss of lysyl oxidase-like 2 in the OVCAR8 human ovarian cancer line also affected the spheroid formation and survival without affecting migration
After evaluating LOXL2 expression in different cell lines, we observed higher LOXL2 protein levels in OVCAR8 high-grade serous cancer cells compared to non-tumorigenic FTE cells (FT33-TAg) [Figure 3A]. Using CRISPR/Cas9, we obtained clones (#2 and #6-2) with reduced LOXL2 protein expression compared to the empty control vector, PCGN [Figure 3B]. The isolation of clones with complete deletions was impossible, possibly because of lethality. Wound healing assays indicated that LOXL2 knockdown did not significantly affect OVCAR8 cells [Figure 3C]. However, consistent with LOXL2 deletion in MOE:PTENshRNA cells, OVCAR8-LOXL2 CRISPR/Cas9 clones formed spheroids with reduced average diameters compared with cells expressing an empty vector [Figure 3D and E]. Furthermore, live/dead staining revealed that the loss of LOXL2 resulted in the reduced survival of MTS structures [Figure 3F and G]. These findings highlight the significance of LOXL2 in spheroid formation and survival in OVCAR8 high-grade serous cancer.
Figure 3.
LOXL2 is required for spheroid survival in OVCAR8 cells. (A) Western blot showing that LOXL2 protein expression is higher in OVCAR8 ovarian cancer cells compared to non-tumorigenic FT33-TAg human fallopian tube epithelial (FTE) cells. (B) Western blot showing reduced LOXL2 protein expression in the OVCAR8 CRISPR clones compared to cells transfected with empty vectors. (C) Migration assay of OVCAR8 LOXL2 clones vs. OVCAR8 empty vector control (PCGN). (D) Bright-field images of spheroids from OVCAR8 LOXL2 CRISPR clones vs. OVCAR8 empty vector control. Scale bar = 500 μm. (E) Spheroid formation assay in ULA plate containing OVCAR8 LOXL2 CRISPR clones vs. OVCAR8 empty vector control. (F and G) Live assay showing live cells in green. The red represents red fluorescent protein (RFP). Quantification of green fluorescence intensity was performed using ImageJ software. Three independent experiments were performed, and one-way ANOVA was conducted, with asterisks indicating: (∗∗) P value < 0.01; (∗∗∗∗) P value < 0.0001; (ns) P value > 0.05. ANOVA: Analysis of variance; CRISPR: Clustered regularly interspaced short palindromic repeats; FTE: Fallopian tube epithelium; FT33-TAg: Fallopian tube cell line 33 SV40-T antigens; LOXL2: Lysyl oxidase-like 2; ns: Non-specific; OVCAR8: Ovarian carcinoma line 8; PCGN: Influenza virus hemagglutinin coding sequence containing plasmid; RFP: Red fluorescent protein; ULA: Ultra-low attachment.
Re-introduction of paired box2 gene reverses phosphatase and tensin homolog-induced extracellular matrix gene expression
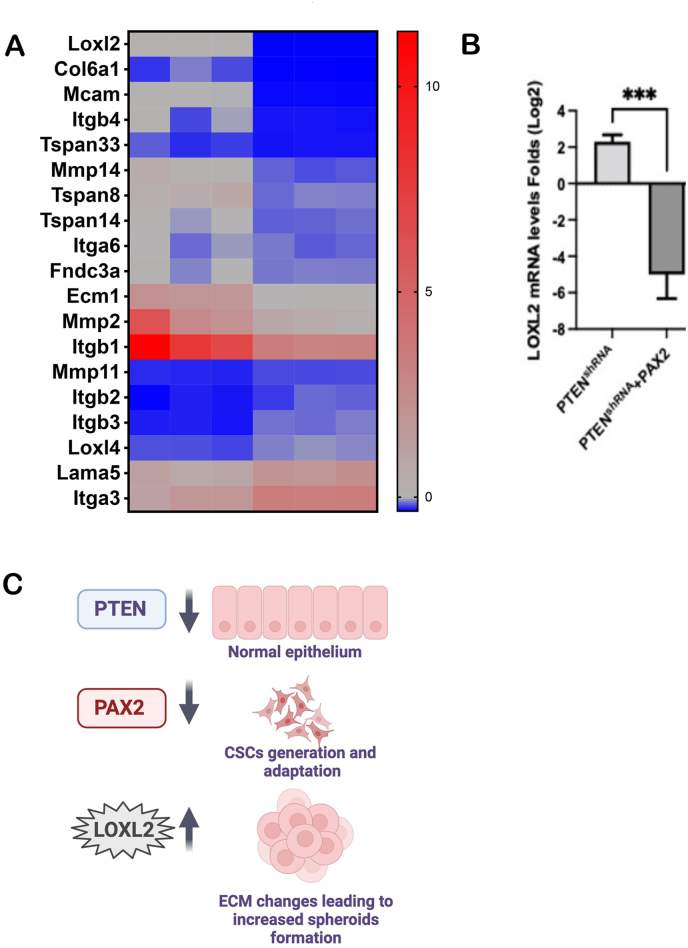
Loss of PTEN in MOE:PTENshRNA cells has been previously associated with a concurrent loss of PAX2. Notably, the restoration of PAX2 in these cells has shown promising outcomes, reducing tumor formation in vivo and diminishing the expression of cancer stem cell markers.13,14 We conducted a comprehensive analysis to explore the potential effects of PAX2 restoration on LOXL2 expression. A comparison of the RNA-seq datasets between MOE:PTENshRNA +PAX2 and MOE:PTENshRNA revealed a notable reduction in ECM-related genes, including LOXL2 [Figure 4A]. This observation was further substantiated by qPCR, which confirmed that the re-introduction of PAX2 into MOE:PTENshRNA cells led to a significant decrease in LOXL2 mRNA levels [Figure 4B]. These data suggest that the loss of PAX2 may be required for LOXL2-mediated effects. In summary, our experiments, using previously validated MOE:PTENshRNA +PAX2,13 provide novel insights, indicating that the loss of PTEN upregulates LOXL2 expression through the downregulation of PAX2.
Figure 4.
PAX2 re-expression in PTEN-depleted cells reduces LOXL2 expression. (A) Heat map showing downregulation of ECM-related genes upon re-expression of PAX2 in MOE:PTENshRNA cells. (B) qPCR confirming that LOXL2 mRNA expression is reduced upon re-expression of PAX2. (C) Schematic of the signaling pathways leading to increased LOXL2 expression. Loss of PTEN leads to PAX2 downregulation, increasing LOXL2 expression and spheroid formation. Three independent experiments were performed, and one-way ANOVA was conducted, with asterisks indicating: (∗∗∗) P ≤ value 0.001. ANOVA: Analysis of variance; CSC: Cancer stem cells; ECM: Extracellular matrix; LOXL2: Lysyl oxidase-like 2; MOE: Murine oviductal epithelial; PAX2: Paired box2 gene; PTEN: Phosphatase and tensin homolog; PTENshRNA: Short hairpin RNA targeting PTEN; qPCR: Quantitative polymerase chain reaction.
Discussion
The progression and metastasis of HGSOC are favored by ECM remodeling and fibrosis,19 and drugs that block fibrosis have been shown to induce chemosensitivity.20 LOXL2 is the primary enzyme that regulates elastin and collagen crosslinking, which is a major hallmark of ECM fibrosis and a predictor of cancer morbidity.16 LOXL2 increases ECM stiffness and promotes the migration and invasion of tumor cells.21 Patients with ovarian cancer have increased LOXL2 serum levels compared to healthy individuals,22 and increased expression of LOXL2 leads to poor prognosis in many cancers, including ovarian cancer,17, 18, 19, 20, 21, 22, 23 suggesting that LOXL2 may play a critical role in HGSOC progression. Loss of PTEN in MOE cells is essential for driving tumor formation in vivo, as well as other tumorigenic behaviors.4, 5, 6, 7, 8, 9, 10, 11, 12 In addition, pathways regulated upon the loss of PTEN, such as PI3K/AKT,24,25 are frequently activated in HGSOC, making this pathway one of the most critical.26 Herein, we demonstrated that the loss of PTEN leads to an increase in ECM-associated transcripts, one of which is LOXL2, which is overexpressed in numerous cancers.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 We also demonstrated that the loss of LOXL2 in MOE:PTENshRNA and OVCAR8 cells reduced the growth and survival in anchorage-independent. The loss of PTEN alone in a tissue-specific model of tumorigenesis from FTE was sufficient to drive tumorigenesis and primary metastasis to the ovary. Increased LOXL2 expression in PTEN-deficient cells increased the growth and survival of 3D structures. Previous research has reported the role of these spheroids in the fallopian tubes and suggested that these structures may mediate primary metastasis to the ovaries.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 This suggests that increased LOXL2 expression due to the loss of PTEN may be one of the mechanisms by which metastases are promoted.
PTEN knockdown in MOE cells triggers the loss of the PAX2, a transcription factor that is expressed in normal FTE,13 and is lost in SCOUTs, which is one of the early alterations leading to HGSOC development.29 We previously revealed that PAX2 rescue in PTEN knockdown MOE cells reduced the tumor burden and cancer stem cell marker expression.13,14 Here, we found that the re-expression of PAX2 blocked the PTEN-induced upregulation of LOXL2. Interestingly, LOXL2 has been suggested to be involved in the activation of AKT30; therefore, it was relevant to investigate whether the deletion of LOXL2 could reduce AKT activation in MOE:PTENshRNA cells.13
Preclinical studies using selective LOXL2 inhibitors did not reduce tumors by themselves, but increased the chemosensitivity of breast23 and ovarian cancer cells.31 Our findings suggest that LOXL2 inhibitors can be used in combination with small molecules to increase PAX2 expression13 and affect tumor development and progression. In addition, molecules that increase PAX2 expression, such as the flavonoid luteolin, which has been used in clinical trials for inflammatory diseases and cancer,32 should be studied in preclinical models of ovarian cancer to determine whether they affect LOXL2 expression and reduce the risk of ovarian cancer. In conclusion, in this study, the loss of PTEN and PAX2 were associated, for the first time, with increased LOXL2 transcription and acquisition of survival under anchorage-independent conditions through the possible regulation of ECM remodeling. Further studies will be necessary to address whether increased LOXL2 upon PTEN deletion affects collagen distribution and increases stiffness.
We have previously shown that the loss of PTEN leads to increased tumorigenesis. However, we have not yet performed in vivo studies of PTEN and LOXL2 depletion to assess whether the loss of LOXL2 reduces tumor growth and metastasis. These experiments will potentially justify testing the role of LOXL2 inhibitors in vivo. A highly selective inhibitor of LOXL2, PAT-1251, made it through phase I clinical trial33 and it is now in phase II (NCI-2019-03143) to treat thrombocytosis myelofibrosis and could be a candidate small molecule to test in ovarian cancer.
In conclusion, PTEN, PAX2, and LOXL2 appear to be a part of a regulatory axis that influences MTS formation and survival [Figure 4C]. PAX2 has been shown to be crucial for the expression of cancer stem cells (CSCs) markers such as CD44, WNT4, and ALDH,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 however, whether this is mediated by LOXL2 expression is unknown. Interestingly, LOXL2 expression has also been associated with increased CSC phenotype35 and increased EMT by preventing degradation of SNAIL36
Authors contribution
Conceptualization: Angela Russo and Joanna E. Burdette; methodology: Angela Russo, Junlone Moy, Manead Khin, and Alfredo-Carrero Lopez; writing: Angela Russo; supervision and funding acquisition: Joanna E. Burdette. All the authors have read and approved the final version of the manuscript.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Illinois in Chicago (IRB# 2017-0574) for studies involving humans. The animal study protocol was approved by the Institutional Review Board (Ethics Committee) of the University of Illinois, Chicago (protocol code 22-009 and date of approval) for studies involving animals. Informed consent was obtained from all participants involved in the study, allowing their tissues to be used for research.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that generative artificial intelligence (AI) and AI assisted technologies were not used in the writing process or any other process during the preparation of this manuscript.
Funding
This research was supported by the National Institutes of Health (NIH) funding programs R01 (Nos: CA240301 and CA240423) and the Rivkin Pilot Study Award (No. 572533), which constituted a crucial resource for conducting our study.
Data availability statement
The datasets used in the current study are available from the corresponding author on reasonable request.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to acknowledge Biorender, which we used to generate the schematics.
Managing Editor: Peng Lyu
References
- 1.Shih I.M., Wang Y., Wang T.L. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191:26–39. doi: 10.1016/j.ajpath.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Collie S.V., Fang J.Y., Xu J. Gain of function of mutant p53: R282W on the peak? Oncogenesis. 2016;5:e196. doi: 10.1038/oncsis.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He C., Li L., Guan X., Xiong L., Miao X. Mutant p53 gain of function and chemoresistance: the role of mutant p53 in response to clinical chemotherapy. Chemotherapy. 2017;62:43–53. doi: 10.1159/000446361. [DOI] [PubMed] [Google Scholar]
- 4.Perets R., Wyant G.A., Muto K.W., et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quartuccio S.M., Lantvit D.D., Bosland M.C., Burdette J.E. Conditional inactivation of p53 in mouse ovarian surface epithelium does not alter MIS driven Smad2-dominant negative epithelium-lined inclusion cysts or teratomas. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning G., Bijron J.G., Yamamoto Y., et al. The PAX2-null immunophenotype defines multiple lineages with common expression signatures in benign and neoplastic oviductal epithelium. J Pathol. 2014;234:478–487. doi: 10.1002/path.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgescu M.M. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quartuccio S.M., Karthikeyan S., Eddie S.L., et al. Mutant p53 expression in fallopian tube epithelium drives cell migration. Int J Cancer. 2015;137:1528–15238. doi: 10.1002/ijc.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddie S.L., Quartuccio S.M., Moyle-Heyrman G., et al. Tumorigenesis and peritoneal colonization from fallopian tube epithelium. Oncotarget. 2015;6:20500–20512. doi: 10.18632/oncotarget.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo A., Czarnecki A.A., Dean M., et al. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene. 2018;37:1976–1990. doi: 10.1038/s41388-017-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasparri M.L., Bardhi E., Ruscito I., et al. PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe Frauenheilkd. 2017;77:1095–1103. doi: 10.1055/s-0043-118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean M., Jin V., Bergsten T.M., et al. Loss of PTEN in fallopian tube epithelium results in multicellular tumor spheroid formation and metastasis to the ovary. Cancers (Basel) 2019;11:884. doi: 10.3390/cancers11060884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modi D.A., Tagare R.D., Karthikeyan S., et al. PAX2 function, regulation and targeting in fallopian tube-derived high-grade serous ovarian cancer. Oncogene. 2017;36:3015–3024. doi: 10.1038/onc.2016.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo A., Colina J.A., Moy J., et al. Silencing PTEN in the fallopian tube promotes enrichment of cancer stem cell-like function through loss of PAX2. Cell Death Dis. 2021;12:375. doi: 10.1038/s41419-021-03663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C.E., Hammer A.M., Cho Y.J., et al. Stromal PTEN regulates extracellular matrix organization in the mammary gland. Neoplasia. 2019;21:132–145. doi: 10.1016/j.neo.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen B., Xu L.Y., Li E.M. LOXL2 in cancer: regulation, downstream effectors and novel roles. Biochim Biophys Acta Rev Cancer. 2020;1874:188435. doi: 10.1016/j.bbcan.2020.188435. [DOI] [PubMed] [Google Scholar]
- 17.Ye M., Zhou J., Gao Y., Pan S., Zhu X. The prognostic value of the lysyl oxidase family in ovarian cancer. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karthikeyan S., Russo A., Dean M., Lantvit D.D., Endsley M., Burdette J.E. Prolactin signaling drives tumorigenesis in human high grade serous ovarian cancer cells and in a spontaneous fallopian tube derived model. Cancer Lett. 2018;433:221–231. doi: 10.1016/j.canlet.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho A., Howell V.M., Colvin E.K. The extracellular matrix in epithelial ovarian cancer - a piece of a puzzle. Front Oncol. 2015;5:245. doi: 10.3389/fonc.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung T.L., Leung S.L., Yip K.P., et al. Anticancer immunotherapy by MFAP5 blockade inhibits fibrosis and enhances chemosensitivity in ovarian and pancreatic cancer. Clin Cancer Res. 2019;25:6417–6428. doi: 10.1158/1078-0432.CCR-19-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radic J., Kozic B., Nikolic I., et al. Multiple roles of LOXL2 in the progression of hepatocellular carcinoma and its potential for therapeutic targeting. Int J Mol Sci. 2023;24:11745. doi: 10.3390/ijms241411745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeming D.J., Willumsen N., Sand J.M.B., et al. A serological marker of the N-terminal neoepitope generated during LOXL2 maturation is elevated in patients with cancer or idiopathic pulmonary fibrosis. Biochem Biophys Rep. 2019;17:38–43. doi: 10.1016/j.bbrep.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J., Lucas M.C., Leonte L.E., et al. Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget. 2017;8:26066–26078. doi: 10.18632/oncotarget.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinne N., Christie E.L., Ardasheva A., et al. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021;4:573–595. doi: 10.20517/cdr.2021.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghoneum A., Said N. PI3K-AKT-mTOR and NFkappaB pathways in ovarian cancer: implications for targeted therapeutics. Cancers (Basel) 2019;11:949. doi: 10.3390/cancers11070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Wu L., Zhu Y. The function and mechanisms of action of LOXL2 in cancer (Review) Int J Mol Med. 2015;36:1200–1204. doi: 10.3892/ijmm.2015.2337. [DOI] [PubMed] [Google Scholar]
- 28.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–10564. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meserve E.E.K., Brouwer J., Crum C.P. Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol. 2017;30:710–721. doi: 10.1038/modpathol.2016.238. [DOI] [PubMed] [Google Scholar]
- 30.Fan Z., Liu Y., Xinya L., et al. Phosphorylation of AKT by lysyl oxidase-like 2 activates the PI3K/AKT signaling pathway to promote proliferation, invasion and metastasis in esophageal squamous carcinoma. Clin Transl Oncol. 2023;25:2487–2498. doi: 10.1007/s12094-023-03133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaffryar-Eilot S., Marshall D., Voloshin T., et al. Lysyl oxidase-like-2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis. 2013;34:2370–2379. doi: 10.1093/carcin/bgt241. [DOI] [PubMed] [Google Scholar]
- 32.Shi M., Chen Z., Gong H., et al. Luteolin, a flavone ingredient: anticancer mechanisms, combined medication strategy, pharmacokinetics, clinical trials, and pharmaceutical researches. Phytother Res. 2024;38:880–911. doi: 10.1002/ptr.8066. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira S., Saraiva N., Rijo P., Fernandes A.S., et al. LOXL2 inhibitors and breast cancer progression. Antioxidants (Basel) 2021;10:312. doi: 10.3390/antiox10020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alwosaibai K., Abedini A., Al-Hujaily E.M., et al. PAX2 maintains the differentiation of mouse oviductal epithelium and inhibits the transition to a stem cell-like state. Oncotarget. 2017;8:76881–76897. doi: 10.18632/oncotarget.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidenfeld K., Schif-Zuck S., Abu-Tayeh H., et al. Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth. Oncotarget. 2016;7:71362–71377. doi: 10.18632/oncotarget.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano A., Santamaria P.G., Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8:1095–10108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.