Fifty years ago, scientists from the Botany and Biochemistry departments at the University of Wisconsin announced the isolation, crystallization, characterization, and synthesis of 6-furfurylaminopurine (Miller et al., 1955a, 1955b, 1956), a plant hormone in a class now referred to as cytokinins (Skoog et al., 1965). They proposed the trivial name kinetin for this substance, and described its ability to promote cell division in test tissues from tobacco (Nicotiana tabacum). The discovery of kinetin marked the culmination of several years of research on a variety of projects conducted by many people in the two departments.
A description of the little victories, the interesting twists, and the roles of persistence, patience, and good luck in this discovery has never been presented in print. Hopefully, this account will serve as an illustration of how scientific advancement can arise from fruitful collaboration and the utilization of many odd bits of information often obtained rather fortuitously.
In the early 1950s, Professor Folke Skoog and his group in the Botany Department were continuing their work on the chemical control of new shoot formation in excised pieces of tobacco stems grown in culture (Skoog fondly referred to these de novo formed shoots as “buddies”). This research effort was hindered by the fact that the stem pieces, which were obtained from plants grown in the greenhouse, were difficult to obtain in a sterile and completely healthy condition. The results obtained with the stem segments were quite variable, probably because of the harsh sterilization protocol (mercuric chloride was employed for sterilization), varying light intensities and temperatures at different times of year, as well as greenhouse management procedures. To avoid the difficulties and variability of using greenhouse-grown stem pieces, Skoog sought an alternative tissue to use in these studies. By the 1950s, there had been advances in the art of plant cell culture; in particular, several laboratories had reported that certain complex materials added to an otherwise chemically defined culture medium (consisting of minerals, a carbon source such as Suc, and often the plant hormone auxin) could result in the sustained growth of plant tissues or cells. These materials included coconut (Cocos nucifera) milk, which had first been used by van Overbeek to stimulate the growth of young Datura stramonium embryos in culture (van Overbeek et al., 1941). Skoog explored whether his group could continuously culture tobacco tissue on coconut milk-containing medium, and thereby produce “standardized” samples of tissue for their studies of shoot formation. Skoog's group found that although coconut milk sometimes promoted the proliferation of tobacco tissue, the effect was quite variable; in fact, some batches of coconut milk inhibited growth. They were using coconuts purchased at a local grocery store, and recognized that some of this variability might be due to inhibitors present in certain batches of coconuts.
Skoog therefore sought advice from Professor F.C. Steward at Cornell University. Steward's laboratory was attempting to purify and identify materials in coconut milk that promote the growth of carrot (Daucus carota) cells in culture. Skoog hoped to obtain some details of Steward's coconut milk purification procedure, not with the intent to compete with Steward on the identification of the active factor(s) but rather to partially purify the coconut milk factors to an extent that would enable his group to prepare consistently healthy cell cultures for their studies of new shoot formation.
Steward replied to this request by sending a letter to Skoog stating that it would be best to leave such matters to the Cornell investigators, and Steward also included a few newspaper clippings that praised his own work but provided no useful details of the purification methods. Skoog, who competed in the 1,500-meter race for Sweden in the 1932 Olympics, was not the type of person who would let such a rebuff go unchallenged. Within minutes of receiving Steward's reply, he contacted Professor Frank Strong, a natural products expert in the Department of Biochemistry at the University of Wisconsin, to initiate a collaboration to compete with Steward's group on the identification of the coconut milk factor(s).
The early stages of the project moved rather quickly. Graduate students Jack Mauney (Botany), Rod Clayton (Biochemistry), and Bill Hillman (Botany) did the tough laboratory work of developing methods to purify the active factor(s) and developing a bioassay to test each step of the purification. At first, their assay employed serially cultured tobacco tissue, but later they used callus tissue derived from carrot root pieces. As a starting material for purification of the active factor(s), they shifted to an extract of ground coconut meat rather than the milk because their assays indicated the meat was a richer source of activity. In late 1952, their work was published in Physiologia Plantarum (Mauney et al., 1952). They described the preparation of a fraction that was over 4,000 times as active on a fresh weight basis as the crude coconut meat extract. The active substance(s) in this fraction was heat stable, acid and alkali labile, nonvolatile, water soluble, and organic. It was present in a Neuberg mercuric precipitate and was bound quite strongly by activated charcoal. The factor moved toward the cathode during electrodialysis and appeared to be amphoteric. The paper also reported that ion-exchangers such as Dowex 50 “held the activity so tenaciously that successful elution of the factor was never accomplished” (p. 495). Progress slowed as graduate students completed their theses or shifted emphasis, and this paper was the only publication from Wisconsin on the activity from coconut.
In 1951, Carlos Miller, who had recently received a Ph.D. from Ohio State University, began postdoctoral work in Skoog's group. Two of the projects that Miller initiated were to converge, prompting a new line of investigation in the search for growth promoting activity. One project continued the investigation of ways to manipulate organ formation from tobacco stem pieces in culture. As discussed above, results with greenhouse-grown stem segments were quite variable. Miller thought that enriching the culture medium might mitigate this variability, for example, stem segments from less vigorous plants might respond better if a range of metabolites (that might otherwise be limiting) were supplied to the culture medium. To test this, he added yeast (Saccharomyces cerevisiae) extract to a medium that was known to promote root formation because it contained a certain level of the auxin indoleacetic acid. To his surprise, the yeast extract did not enhance root formation but instead caused a massive amount of undifferentiated growth and cell division! In a separate project, Miller was searching for a source of growth-promoting material that was more reliable than coconut extract in the carrot tissue assay. He found that yeast extract was quite active in promoting growth of the carrot tissue when used directly in the culture medium. In the Physiologia Plantarum paper (Mauney et al., 1952), which primarily reported on the coconut project, it was noted that a sample of yeast extract was inactive when used directly in the carrot tissue assay (p. 490), but it was also noted that some activity could be obtained from yeast extract after partial purification (p. 496). However, Miller had fortuitously chosen a particular bottle containing a sample of yeast extract that had relatively high activity when used directly in the culture medium.
Miller set out to purify the active material(s) from yeast extract. There was a dwindling supply of yeast extract in the particular bottle that had relatively high activity, so several large containers of different Anheuser-Busch brewer yeasts were ordered in the hope of finding a plentiful source of factor(s). Unfortunately, none of these batches exhibited activity. Yet Miller made progress working with the limited supply from the small bottle of yeast extract that had activity. The chemical techniques available at that time for compound identification from a crude mixture were limited; one common technique was precipitation with metal ions. As previously found in the coconut work, considerable activity was present in a mercuric precipitate, and some activity was adsorbed onto activated charcoal. Every step of the purification had to be evaluated using the bioassay of cell proliferation in culture. The carrot culture assay was giving variable results and so, in May of 1953, Miller started using callus tissue from tobacco stem pieces. When tobacco stem pieces are placed on a simple medium without yeast extract, there is typically an initial burst of growth and cell division from the basal end of the stem piece (this burst of growth formed what was sometimes called “wound callus”). However, in Miller's assays there was no further proliferation (even if the tissue was transferred to fresh medium) unless the activity from yeast extract was present in the medium.
In June of 1953, Miller found that the active material present in yeast extract was precipitated by silver nitrate under slightly acid conditions (Fig. 1). This finding was extremely important to what ensued. The silver nitrate method was more selective than the mercuric precipitation procedure. Miller recognized that purine and pyrimidines are one class of compounds precipitated by silver nitrate, so he tested individual purine or pyrimidines, such as adenine, cytosine, guanine, thymine, uracil, etc., in the tobacco assay. Although none of the tested compounds had any substantial growth promotion activity, the notion that a purine derivative might be involved remained very appealing to him. Skoog's group had previously shown (beginning with the work of Cheng Tsui) that addition of the purine adenine to the culture medium of tobacco stem segments would enhance shoot formation (Skoog and Tsui, 1948, 1951; Miller and Skoog, 1953) and, in the presence of auxin, would cause some cell division (Skoog and Tsui, 1951). Some weak cell division activity from adenine had also been noted in the Physiologia Plantarum paper on coconut milk (Mauney et al., 1952).
Figure 1.
Growth of tobacco cells on control medium containing auxin (left) and on the same medium with addition of the silver nitrate precipitable material. This image is from the original work of Miller in 1953.
Miller continued to pursue his hunch that a purine derivative might be involved. He sampled bottles of nucleic acids from the laboratory shelf for activity in the bioassay because he thought they might contain additional purine derivatives. RNA did not promote growth, but, almost unbelievably, very good growth occurred when as little as 50 mg/L of herring sperm DNA was added to the culture medium. Moreover, as had been found with yeast extract, the active material could be precipitated from a solution of herring sperm DNA with silver nitrate. That a concentrated source of activity was commercially available was cause for great celebration! To ensure a large supply of active material for further studies, Skoog ordered a keg of herring sperm DNA that had been prepared by the same company using the same procedures by which the small active sample that Miller had first tested had been prepared. However, the new batch of herring sperm DNA did not possess any trace of growth-promoting activity. The excitement abated. Miller had serendipitously chosen samples of both yeast extract and herring sperm DNA with relatively high activity, but no activity was present in any newly purchased batches.
For the next several months, work continued with the dwindling supply of active samples of yeast extract and herring sperm DNA. Miller achieved a partial purification of the activity by preparing ethanolic extracts of both sources, followed by silver precipitation, partition into diethyl ether or n-butanol, and paper chromatography. This yielded preparations that were active in the tobacco stem callus test at less than 1 mg/L. Clearly, the active materials were quite potent. The absorption spectra of the ether-soluble factors from both yeast extract and herring sperm DNA were very similar in several solvents and exhibited a similar shift in alkaline solution. In addition, both activities migrated almost identically on paper chromatograms. Thus, each of the two sources seemed to have yielded the same or closely related active substances. Moreover, if the ether-soluble fraction was subjected to chromatography on filter paper using only water as the developing solvent, the activity could be visualized as a dark quenching spot under shortwave UV light at about Rf 0.5. Miller and Skoog submitted a manuscript describing these findings to the Proceedings of the Society for Experimental Biology and Medicine. It was rejected. Part of the reviewers' concerns had to do with the rather poor quantitative nature of the assay for growth-promoting activity, but Miller and Skoog considered many other points raised by the reviewers to lack validity. They knew the project was gaining momentum, and they decided not to address the reviewers' comments or to make any additional effort to publish their progress. The rejection increased their resolve to identify the active compound(s) and caused Skoog to terminate his membership in the Society for Experimental Biology and Medicine (this latter point is from Skoog, 1994).
By this time, Miller was becoming increasingly confident that the active substance was an adenine derivative. Some of this confidence came from a chemical test that he had performed earlier for the presence of indoleacetic acid and other indole compounds in the active fraction. At that time, many growth phenomena were regarded as being influenced or even caused by auxin. Therefore, Miller had tested the active material for the presence of indoles by performing paper chromatography of the material with water as the solvent and then spraying with Salkowski reagent (a mixture of sulfuric acid and ferric chloride). No color appeared after several minutes, i.e. the test for indoles was negative. However, to evaluate whether the sample might contain bound or conjugated indoles that could not react with the Salkowski reagent, Miller held the chromatogram over a hot plate. As the paper began to char, a pink spot appeared at the position of the active material. Miller then found that a spray of sulfuric acid alone was even more effective at producing the color, indicating that the color was not due to the reaction of a bound indole compound. His search of the literature revealed that a mixture of sulfuric acid with Cys (known as the Dische reagent) would form a pink product when reacted with deoxyribonucleosides. He found that the Dische reagent was quite effective at reacting with the active material to form a pink compound. As discussed below, this would turn out to provide an important clue for the determination of the structure of kinetin. In addition, when some of the purified active factor was treated with the Dische reagent (or with sulfuric acid alone) and then subjected to chromatography, a new quenching compound (a compound with a different Rf value) was detected. The new compound appeared to be adenine because of its position on the chromatogram as well as other chemical properties, i.e. acid treatment appeared to liberate adenine from the active material; thus, there was growing evidence that the factor might be a substituted adenine!
Many further attempts were made to obtain active substances from the newer but inactive batch of herring sperm DNA, such as hydrolysis of the nucleic acid, but these attempts were not successful. However, the new batch of DNA, which had been stored at room temperature for many months, was repeatedly assayed and very gradually began to show activity. Some change was occurring in the DNA sample during storage! In fact, this gradual acquisition of activity had been noted in the rejected manuscript. Miller collected samples of DNA from laboratories across the Wisconsin campus with the idea to test the samples for activity and, if any additional samples tested positive, to explore whether the active samples had anything in common. Seven DNA samples were tested for activity in the tobacco stem callus assay. Three of them had activity. Samples that had been stored at room temperature for relatively long times had activity, whereas newly purchased samples or old bottles of DNA kept desiccated and refrigerated were inactive.
With these observations in mind, Miller tried a simple method to “age” a new sample of inactive DNA: he scooped some DNA into water in a 1-L flask and autoclaved it for thirty minutes. After cooling, he extracted the darkened, autoclaved slurry of DNA with n-butanol and examined the butanol fraction for a compound with the same chromatographic properties (UV quenching and Dische reagent reactivity at the same Rf value) and spectroscopic characteristics of the active substance from the original samples. A compound with the right properties was present! Bioassays confirmed that autoclaving the DNA produced an enormous amount of a cell division-promoting activity. A procedure for activating the DNA had been found! Further tests, conducted in early November of 1954, indicated that an acidic condition during autoclaving was necessary for the activation; in the initial test, the DNA sample itself had provided sufficient acidity for activity to be generated. Miller then prepared a large batch of the active material. The autoclaved slurry was extracted with n-butanol, and the butanol was driven off by distillation, leaving an aqueous preparation from which insolubles were removed by filtration. The remaining solution was adjusted to pH 6.8 and then passed through a large column of the cation-exchanger Dowex 50 (H+). The exchanger bound the activity very strongly; recall that activity from the coconut extract had been held by, but not recovered from, Dowex 50. Therefore, Miller tried more severe elution conditions than those previously used in the coconut work. He found that the active substance could be gradually eluted from the Dowex resin by strong acid (1.5 n HCl) or more rapidly eluted by 1 n ammonium hydroxide. The elution by strong acid provided a significant purification: several inactive compounds eluted in the early fractions as the column was washed with acid, whereas the activity came off in much later fractions. Miller combined the later-eluting fractions, and to remove the 1.5 n acid without risking destruction of the active factor, he simply diluted the combined fractions to about 0.5 n HCl and ran them through Dowex 50 again (the activity bound to the Dowex at this lower level of acid). He washed the Dowex column thoroughly with water and then added 1 n ammonium hydroxide to elute the activity. On the evening of December 16, 1954, Miller saw a swirling white band of crystals moving down the column with the ammonium hydroxide front against the dark brown background of the exchange resin! He collected the crystals as they exited the column, decanted the excess ammonium hydroxide solution, and washed the crystals with water before drying them.
To determine if the crystals might represent the purified factor, Miller dissolved a small portion in ethanol and performed paper chromatography as described above. All of the properties of the crystalline material were identical to those of the active substance obtained from the old bottle of DNA: quenching material moved to the expected position on a paper chromatogram, the region of the chromatogram with the active substance turned pink when sprayed with the Dische reagent, and the material had the expected absorption peak. The next morning, Miller showed the crystals to Skoog, confidently declaring them to be the cell-division factor. On December 21, 1954, after recrystallization to provide a further purification, Miller set up a series of tobacco stem callus bioassays using several concentrations of the factor. He then went to Ohio to spend the holiday break with his family. During the break, Skoog sent a letter to Ohio excitedly reporting that the factor was giving positive results.
The next phase of the research was an intense effort to learn the structure of the compound. Frank Strong and his group in the Biochemistry Department, which included Malcom von Saltza, generated much critical data in an incredibly short time. Strong's group found that the crystalline compound had an equivalent weight of 215.2 and, by elemental analysis, fit an empirical formula of C10H9N5O. They also determined the infrared spectrum, melting point, and sublimation temperature, and noted two pKa values (indicating that the compound was amphoteric). Another key finding was that the compound could not be acetylated. In January of 1955, approximately 1 month after the crystals first appeared in the Dowex column, a paper was submitted to the Journal of the American Chemical Society reporting their progress on defining the chemical properties of the active compound and suggesting that it be named kinetin. This paper appeared in the March 5 issue (Miller et al., 1955a).
A very important question remained unanswered: What was the structure of kinetin? Assuming that kinetin was a substituted adenine and that the equivalent and molecular weights were the same, the substituting group (which presumably replaced a hydrogen) must consist of C5H5O. That kinetin was precipitated by silver nitrate indicated the substituting group was not on the 9-position of adenine. Acetylated derivatives are typically produced on free amino groups, and the failure to obtain acetylated derivatives raised the possibility that the substituting group was on the amino group of adenine. But what was the configuration of the substituting group?
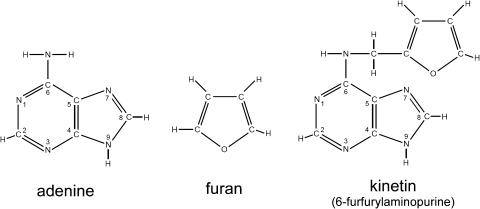
One hint came from an absorption band at 8.01 μ in the infrared spectrum. Miller's after-dinner perusals of chemical journals revealed that compounds having C–O–C ether bonds absorbed sharply at this wavelength. Furthermore, his prior tinkering with the Salkowski test for the presence of indoles had led to the discovery of the reactivity of the compound with the Dische reagent. Reading the chemical literature, Miller learned that the route of deoxyribonucleosides to a pink product in the Dische reagent was likely to involve the conversion of deoxy-ribose into a furan-containing compound. He thought that a related reaction might have contributed to production of the active factor from DNA. Miller thus proposed that the active material was 6-furfurylaminopurine (Fig. 2), a compound with a furan derivative attached to the amino group of adenine at the 6 position of the purine ring.
Figure 2.
The structures of kinetin, adenine, and a furan ring.
Acting on Miller's proposal, Frank Strong and Francis Okumura rapidly synthesized 6-furfurylaminopurine by refluxing 6-methylmercaptopurine in furfurylamine overnight. As was typical for work from Strong's lab, the initial attempt was successful—the protocol gave a 60% yield of kinetin. All of the chemical and biological properties of the synthesized compound were identical to those of the factor obtained from DNA, confirming the proposed structure! The luck of testing old bottles of DNA and yeast extract, and the blending of the particular talents, knowledge, and insights of the groups in the Biochemistry and Botany Departments, had led to the discovery of the first highly active member of the cytokinin class of plant hormones. In March of 1955, the paper describing the structure and properties of kinetin was submitted to the Journal of the American Chemical Society, and it appeared in the May 5 issue of the journal (Miller et al., 1955b; a more complete description with substantiating data appeared later: Miller et al., 1956).
EPILOGUE
This discovery received much attention. There were press conferences and photography sessions (Fig. 3). The research had been supported in part by the American Cancer Society, and, based on the ability of kinetin to control cell proliferation, speculation that the discovery would lead to strategies to combat cancer appeared in the popular press. Cancer patients sought advice from the discoverers. Representatives of drug and chemical companies visited the Wisconsin laboratories to obtain additional information. The Wisconsin Alumni Research Foundation patented the discovery.
Figure 3.
The authors of Miller et al. (1955b). Carlos Miller is on the left. On the right, front to back, are Frank Strong, Folke Skoog, Francis Okumura, and Malcom von Saltza.
Despite all the attention, laboratory work continued. In Biochemistry, new purine derivatives related to kinetin were synthesized, and their effects on plant cells were tested in Botany. 6-Benzyladenine, now a commonly used cytokinin, was the first of several highly active cytokinins to be discovered by Skoog and Strong and their co-workers quite soon after the discovery of kinetin. The effect of kinetin on developmental processes that were also affected by red light, such as leaf expansion (Miller, 1956) and seed germination (Miller, 1958), was discovered. Other groups quickly discovered other developmental processes that could be influenced by cytokinin, such as leaf senescence (Richmond and Lang, 1957).
In the same year that the identification of kinetin was published, Steward's group published a paper reporting that diphenylurea was a compound from coconut milk active in promoting cell division (Shantz and Steward, 1955). In their studies, Steward and his group had collected a large batch of coconuts that had become available after a storm in Florida (Jacobs, 1979). To prepare this large batch, commercial equipment from a DuPont plant was used (Jacobs, 1979; P. Davies, personal communication). Steward's group did not know that the equipment had been previously used for preparing phenylurea herbicides. It was unfortunate that they diluted residual diphenylurea with coconut milk only to repurify it as an active factor, but fortunately this mishap led to the discovery that di-substituted ureas also have cytokinin activity. Recently, it has been shown that a di-substituted urea (thidiazuron) can activate a putative cytokinin receptor similar to adenine-type cytokinins (Inoue et al., 2001; Yamada et al., 2001). Therefore, active di-substituted ureas should be considered as true cytokinins. It should be noted, however, that there is also evidence that thidiazuron can act to prevent the metabolism of adenine-type cytokinins by inhibiting cytokinin oxidase (Laloue and Fox, 1989).
In 1956, the term kinin was proposed for substances that promote cytokinesis (i.e. the partition of a cell into new cells) and therefore “permit continuous growth of various plant tissues in vitro” (Miller et al., 1956; p. 1376). By this time, the ability of diphenylurea to promote cell division had been published, and, thus, kinetin and diphenylurea were the first kinins to be reported. In their 1956 paper, Miller and Skoog and co-workers question “whether kinetin exists as such and functions as a kinin in nature” (p. 1377)—i.e. perhaps it was not naturally occurring (Miller et al., 1956). Indeed, kinetin can be formed by simply autoclaving adenine and deoxy-ribose at a moderately low pH (Hall and deRopp, 1955). The deoxy-ribose apparently can rearrange to a furfuryl group and become joined to the 6 amino group of adenine. However, as noted by Skoog (1994), it cannot be ruled out that some kinetin could be formed in plants under certain conditions, such as those in wounded tissue.
Although kinetin was the first highly active member of the cytokinin class of plant hormones to be discovered, whether kinetin was the first example of a chemically defined cytokinin is arguable. As noted in Miller (1968), Skoog and Tsui had earlier reported, in their studies of growth and bud formation, an “array of cytokinin effects” (quote from Miller, 1968; p. 44) when adenine was added to the culture medium of tobacco stem segments (Skoog and Tsui, 1948, 1951). Adenine promoted new shoot formation without auxin or in the presence of low concentrations of auxin, whereas with higher concentrations of auxin, adenine promoted some cell proliferation. The weak cell division-promoting effects of adenine had been mentioned again (without presenting supporting data) in the Physiologia Plantarum paper on coconut milk (Mauney et al., 1952). In a later study, the ability of adenine to promote the growth of both soybean (Glycine max) and tobacco tissues through repeated subcultures was carefully examined (Miller, 1968). Adenine was effective in a very narrow range of concentrations, and the promotion required relatively high concentrations of adenine (greater than 0.1 mm), whereas kinetin was effective at much lower concentrations (less than 0.0001 mm; Miller, 1968). That the effect was truly due to adenine and not a contaminant was shown by testing several different commercial samples, recrystallizing a sample of adenine, and following the activity in paper chromatography (Miller, 1968). Further support that adenine was a weak cytokinin came from the study of the cell division-promoting activity of a series of 6-alkyl-aminopurines (e.g. n-butyl, n-propyl, ethyl, and methyl). As the length of the alkyl substituting group decreased, so did the activity, and adenine fit nicely into this series as the weakest cytokinin (Miller, 1968). As noted in the paper, however, other explanations cannot be ruled out, for example, that adding a relatively high level of adenine to the culture medium may permit some cytokinins to be formed.
As discussed above, several years before the advent of kinetin, Skoog had recognized and first published with Tsui (Skoog and Tsui, 1948, 1951) the existence of a relationship between adenine and auxin in relation to organ formation in culture. The quantitative nature of this relationship, however, was not easily detected because the biologically effective range of adenine concentrations was rather narrow. After the discovery of kinetin, Miller performed experiments that showed a similar relationship between kinetin and auxin in relation to organ formation; in these studies, auxin and kinetin had interacting effects over a broad concentration range of both hormones (Fig. 4). Skoog realized and emphasized the importance of the concentration ratio of auxin and kinetin in the classic 1957 paper on this topic (Skoog and Miller, 1957).
Figure 4.
Plate 4 from Skoog and Miller (1957) showing the effect of the auxin to cytokinin ratio on the pattern of development.
After moving to Indiana University, Miller pursued the identity of naturally occurring cytokinins. In 1961, he reported the partial purification from maize (Zea mays) kernels of a cytokinin that was a purine derivative distinct from kinetin (Miller, 1961). From Miller's work and that of David Letham and his group came zeatin—the first example of a naturally occurring adenine-based cytokinin (Letham and Miller, 1965). The currently used term, cytokinin, for this class of plant hormones was proposed in 1965 to avoid confusion with the kinins referred to in studies of animal physiology (Skoog et al., 1965).
Acknowledgments
This account of the discovery of kinetin was constructed from published papers, the laboratory notebooks of Carlos Miller, and on his recollections of this exciting time. I think this is an interesting story, and one that nicely illustrates the varied paths to scientific discovery. On many occasions over many years, I have asked Carlos Miller about the details of the discovery of kinetin. I thank him for patiently discussing this history with me.
References
- Hall RH, deRopp RS (1955) Formation of 6-furfurylaminopurine from DNA breakdown products. J Am Chem Soc 77: 6400 [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063 [DOI] [PubMed] [Google Scholar]
- Jacobs WP (1979) Plant Hormones and Plant Development. Cambridge University Press, Cambridge, UK
- Laloue M, Fox JE (1989) Cytokinin oxidase from wheat. Partial purification and general properties. Plant Physiol 90: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letham DS, Miller CO (1965) Identity of kinetin-like factors from Zea mays. Plant Cell Physiol 6: 355–359 [Google Scholar]
- Mauney JR, Hillman WS, Miller CO, Skoog F, Clayton RA, Strong FM (1952) Bioassay, purification and properties of a growth factor from coconut. Physiol Plant 5: 485–497 [Google Scholar]
- Miller C, Skoog F (1953) Chemical control of bud formation in tobacco stem segments. Am J Bot 40: 768–773 [Google Scholar]
- Miller CO (1956) Similarity of some kinetin and red light effects. Plant Physiol 31: 318–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CO (1958) The relationship of the kinetin and red-light effects promotions of lettuce seed germination. Plant Physiol 33: 115–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CO (1961) A kinetin-like compound in maize. Proc Natl Acad Sci USA 47: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CO (1968) Naturally-occurring cytokinins. In F Wightman, G Setterfield, eds, Biochemistry and Physiology of Plant Growth Substances. Runge Press, Ottawa, pp 33–45
- Miller CO, Skoog F, Okumura FS, von Saltza MH, Strong FM (1955. b) Structure and synthesis of kinetin. J Am Chem Soc 78: 2662–2663 [Google Scholar]
- Miller CO, Skoog F, Okumura FS, von Saltza MH, Strong FM (1956) Isolation, structure and synthesis of kinetin, a substance promoting cell division. J Am Chem Soc 78: 1375–1380 [Google Scholar]
- Miller CO, Skoog F, Von Saltza MH, Strong F (1955. a) Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc 77: 1392 [Google Scholar]
- Richmond AE, Lang A (1957) Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125: 650–65113421662 [Google Scholar]
- Shantz EM, Steward FC (1955) The identification of compound A from coconut milk as 1,3-diphenylurea. J Am Chem Soc 77: 6351–6353 [Google Scholar]
- Skoog F (1994) A personal history of cytokinin and plant hormone research. In DWS Mok, MC Mok, eds, Cytokinins: Chemistry, Activity, and Function. CRC Press, Boca Raton, FL, pp 1–14
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol XI: 118–131 [PubMed] [Google Scholar]
- Skoog F, Strong FM, Miller CO (1965) Cytokinins. Science 148: 532–533 [DOI] [PubMed] [Google Scholar]
- Skoog F, Tsui C (1948) Chemical control of growth and bud formation in tobacco stem segments and callus cultured in vitro. Am J Bot 35: 782–787 [Google Scholar]
- Skoog F, Tsui C (1951) Growth substances and the formation of buds in plant tissues. In F Skoog, ed, Plant Growth Substances. University of Wisconsin Press, Madison, WI, pp 263–285
- van Overbeek J, Conklin ME, Blakeslee AF (1941) Factors in coconut milk essential for growth and development of Datura embryos. Science 94: 350. [DOI] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol 42: 1017–1023 [DOI] [PubMed] [Google Scholar]