Abstract
Aminoglycosides are concentration-dependent antibiotics exerting a bactericidal effect when concentrations at the site of infection are equal to or greater than 5 times the minimum inhibitory concentrations (MIC). When administered intravenously, they exhibit poor lung penetration and high systemic renal and ototoxicity, imposing to restrict their administration to 5 days. Experimental studies conducted in anesthetized and mechanically ventilated sheep and pigs provide evidence that high doses of nebulized aminoglycosides induce a rapid and potent bacterial killing in the infected lung parenchyma. They also confirm that the alveolar-capillary membrane, either normal or injured by the infectious process, restricts the penetration of intravenous aminoglycosides in the infected lung parenchyma, precluding a bactericidal effect at the site of infection. However, injury of the alveolar-capillary membrane promotes the systemic diffusion of nebulized aminoglycosides. Based on experimental data obtained in animals with inoculation pneumonia, it challenges the classical belief that nebulization protects against systemic toxicity. Loss of lung aeration decreases the lung penetration of nebulized aminoglycosides. Nevertheless, lung tissue concentrations measured in non-aerated lung regions with severe and extended pneumonia are most often greater than 5 times the MICs, resulting in a bactericidal effect followed by a progressive pulmonary reaeration. It is likely that the penetration into the consolidated lung, results from the bronchial diffusion of nebulized aminoglycosides toward adjacent non-aerated infected alveolar spaces and their penetration into mechanical ventilation-induced intraparenchymal pseudocysts and distended bronchioles. In animals receiving nebulized aminoglycosides, epithelial lining fluid concentrations grossly overestimate lung interstitial fluid concentrations because of the bronchial contamination of the distal tip of the bronchoscope during the bronchoalveolar procedures. Lung microdialysis is the only technique able to accurately assess lung pharmacokinetics in animals with inoculation pneumonia treated by nebulized aminoglycosides. In 2024, the European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia (ENAVAP) called for the creation of an international research network for Lung Microdialysis applied to Nebulized Antibiotics (LUMINA) to promote multicentered, experimental, randomized, and controlled studies addressing lung pharmacokinetics of intravenous vs. nebulized antibiotics, using different dosing and ventilator settings.
Keywords: Aminoglycosides, Ventilator-associated pneumonia, Nebulized amikacin, Nebulized tobramycin, Lung microdialysis, Experimental intensive care unit
Introduction
Discovered 80 years ago, aminoglycosides hold a distinctive position among antibiotics, and remain extensively used worldwide due to their potential for a rapid and bactericidal efficacy resulting from irreversible binding to ribosomes and mistranslation of proteins.[1] Three “classical” molecules, gentamicin, tobramycin, and amikacin released between 1963 and 1972, are commonly used in critically ill patients: plazomicin, the latest generation released in 2010, is active against most strains resistant to gentamicin, tobramycin, and amikacin.[2] The different aminoglycosides have similar pharmacokinetic profiles and differ in their microbiological spectrum of activity. Tobramycin and amikacin are the most effective on Pseudomonas species with the lowest resistance rate. Gentamicin is slightly more effective than amikacin on Serratia marcescens, whereas tobramycin is ineffective.[3] Amikacin is the only aminoglycoside active on Providencia species. Plazomicin is effective against many bacteria-producing carbapenemases or other specific hydrolases. Aminoglycosides are inactive against Stenotrophomonas maltophilia and anaerobes.
The bactericidal activity on Gram-negative bacteria is concentration dependent and is achieved at the site of infection when the ratio of tissue concentration over the minimum inhibitory concentration (MIC) is greater than 5. Bacterial killing is maximum at the peak tissue concentration. It continues when tissue concentrations decrease, even when they become less than MICs. This postantibiotic effect lasts 3–7 hours, is exposure-dependent, and is related to the time needed to synthesize new ribosomes.[4] This is the reason why a single daily 30-min infusion provides a potent bactericidal activity.[5] Protein binding is low, approximately 20%, and the elimination half-life is limited to 2–3 h due to rapid renal elimination. Following their glomerular filtration, aminoglycosides are sequestered by the epithelial cells of the proximal tubules and accumulate in the cortical area of the kidney, causing renal toxicity. The process is reversible and depends on the regenerative capacity of the tubular epithelial cells. Aminoglycosides also produce direct oxidative damage to the vestibular organ, cochlea, hairy cells, and respective cranial nerves. Ototoxicity is irreversible, permanent, and must be prevented. Toxicity depends on trough rather than peak plasma concentrations, restricting the administration of aminoglycoside to 3–5 day periods.
After intravenous administration, aminoglycosides exhibit a very limited penetration in many tissues. Composed of amino sugars and aminocyclic alcohols, aminoglycosides are small hydrophobic cationic molecules and their intracellular penetration is weak and volume of distribution limited. Diffusion in the central nervous system, the bronchial secretions, and the pulmonary parenchyma are limited, preventing it from reaching bactericidal concentrations at the site of infection. The lung interstitial fluid (ISF) penetration is low, and due to mucin and surfactant binding, the free epithelial lining fluid (ELF) aminoglycoside concentration is much lower than the total concentration. As a consequence, less than 10% of peak plasma concentrations can be expected in the alveoli.[6] In other words, intravenous aminoglycosides cannot be considered an effective treatment of hospital-acquired and ventilator-associated pneumonia (VAP).[7] By bypassing the capillary alveolar membrane, the nebulization of aminoglycosides results in very high lung tissue concentrations ensuring a rapid and potent bacterial killing.[8,9]
The aim of this review is to examine the experimental literature supporting the use of nebulized aminoglycosides for treating VAP. The clinical literature supporting their use will be examined in a forthcoming article.
Methodological Issues
Animal models
Experimental studies conducted in rodents present challenges in extrapolating results to humans with VAP due to differences in body weight, tracheobronchial dimensions, lung segmentation, and physiological respiratory parameters (Table 1).[10,11] In addition, pathogenic mechanisms of VAP such as aspiration of oropharyngeal pathogens, long-term mechanical ventilation, prolonged sedation and paralysis, gastric colonization, and body position are difficult to replicate.
Table 1.
Airway dimensions in rats and humans.
| Parameter | Human | Rat | Human/Rat |
|---|---|---|---|
| Body weight (kg) | 70.0 | 0.3 | 233.3 |
| Lung weight (g) | 1.00 × 103 | 1.48 | 675.68 |
| Airways volume (mL) | 3.2 × 103 | 6.5 | 492.31 |
| Generation number (n) | 23 | 25–28 | ∼1 |
| Mean alveolar diameter (mm) | 0.200 | 0.086 | 2.325 |
| Terminal bronchioles (n) | 26–32 × 103 | 2487 | 10–13 |
| Alveolar macrophages (n) | 7 × 109 | 26 × 106 | 269 |
| Tidal volume (mL) | 5.00 × 102 | 2.74 | 182.48 |
| Respiratory frequency (min-1) | 14 | 98 | 14 × 10-2 |
| Minute ventilation (mL/min) | 7000 | 268 | 26 |
Conversely, studies in large animals such as piglets, pigs, and ewes more closely resemble human pathophysiological conditions characterizing VAP.[12] Pigs’ and Ewe's pulmonary anatomy and physiology are close to human lung anatomy and physiology.[[13], [14], [15]] Airway dimensions, bronchial segmentation, and nebulized particle tracking within the bronchial tree are similar in both species, inducing comparable lung deposition. As a consequence, the results obtained in such models can be reasonably extrapolated to humans with VAP.[15] The “large animal” model for assessing pharmacokinetics and bactericidal efficacy of nebulized antibiotics in VAP is quite demanding as it implies four methodological conditions: an experimental intensive care unit for ensuring mechanical ventilation under general anesthesia for periods longer than 24 h (Figure 1); an experimental model of bacterial pneumonia; a reliable tool for assessing antibiotic interstitial lung tissue concentrations; a bacteriological method for assessing lung bacterial burden.
Figure 1.
Experimental laboratories for studying nebulized aminoglycosides. A and B: The Experimental Intensive Care Unit of the University of Lille, France (Department Hospitalo-Universitaire de Recherche Expérimentale). The equipment includes mechanical ventilators, cardiorespiratory monitoring, strip-chart recorder, electrical infusors, material for endotracheal suctioning and thoracic drainage, fiberscope, material for antibiotic nebulization (mesh nebulizers), and surgical material for postmortem lung biopsies. Physicians and technicians are permanently present throughout experiments on a 24-hour period shift. In the experimental model of inoculation pneumonia, anesthetized piglets are mechanically ventilated in the prone position for periods ranging between 2 days and 3 days (reproduced from Ref. [12] with permission from the publisher). C and D: The Experimental Intensive Care Unit of the University of Barcelona, Spain (Hospital Clinic of Barcelona – Institut d'Investigacions Biomèdiques August Pi i Sunyer). The laboratory has the same facilities as the experimental intensive care unit of Lille. For the aspiration of the oropharyngeal secretions model, pigs are placed in the anti-Trendelenburg position equivalent to the semirecumbent position in humans. The bed is covered by an anti-slip surface and the rear legs are secured to the bed through cohesive bandage (reproduced from Ref. [30] with permission from the authors by A. Soler-Comas). E and F: The Experimental Intensive Care Unit of the University of Queensland, Brisbane, Australia (Centre for Clinical Research). The laboratory is equipped for performing experiments using lung microdialysis: expertise and equipment for performing thoracotomy and inserting microdialysis probes in different pulmonary lobes of anesthetized ewes; microdialysis probes (0.6 mm × 50 mm) with an intercostal catheter connected to the microdialysis probes; microdialysis pumps for saline administration at a flow rate of 0.1–10 µL/min; liquid chromatography-tandem mass spectrometry for analyzing the collected microdialysate containing the antibiotic (reproduced from Ref. [34] with permission from the publisher).
Inoculation pneumonia is the common model of experimental pneumonia:[12] using bronchoscopy, 40 mL of a suspension containing 106 colony-forming units per milliliter of different bacterial species—Escherichia coli,[16,17] Pseudomonas aeruginosa,[13,[18], [19], [20], [21], [22]] Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus,[23] Streptococcus pneumonia[24]—can be directly instilled in different parts of the respiratory tract. Superimposed lung infection by Pasteurella multocida and Streptococcus suis is frequently observed, resulting from prolonged mechanical ventilation.[12] The inoculation pneumonia model is aimed at reproducing the heterogeneous distribution of lung infection characterizing VAP[25] and the aggravating role of mechanical ventilation-induced volutraumatism.[26] It differs from VAP by the massive bacterial inoculum-producing lung infection. It may prolong the aminoglycosides’ postantibiotic effect, which is inoculum-dependent.[4]
Aspiration of oropharyngeal secretions is another model of experimental pneumonia. Its implementation requires a 4-day period of mechanical ventilation. The model was initially developed in mechanically ventilated Landrace--white piglets in whom tracheal stenosis was created.[27] The model was further expanded to animals with healthy lungs: after 4 days of mechanical ventilation in the prone position, disseminated foci of bronchopneumonia are observed in dependent lung segments. The anti-Trendelenburg position, equivalent to the semirecumbent position in the critically ill, is indispensable to generate pneumonia.[28,29] Pasteurella multocida and Streptococcus suis, two pathogens colonizing the porcine oropharynx, are the predominant causative organisms. The experimental model of VAP was further refined by adding a bacterial pharyngeal inoculation with microorganisms that cause VAP in critically ill patients.[30] Following the bacterial challenge, VAP develops in 48 h in animals lying in the anti-Trendelenburg position (Figure 1C and 1D). The model reproduces the primary pathogenic mechanisms of human VAP but requires 4 to 5 days experiments in an experimental intensive care unit.
Measurement of lung tissue aminoglycoside concentrations
During the nebulization of aminoglycosides, the deposition of nebulized particles on bronchial walls skews the ELF concentrations.[31] Bronchial contamination contributes to the high ELF concentrations observed during nebulization, overestimating true lung tissue concentrations. In anesthetized and spontaneously breathing rats with healthy lungs receiving an intratracheal dose of 3 mg/kg tobramycin, the ELF antibiotic concentrations measured in the first 60 min following nebulization ranged between 200 mg/mL and 12,000 mg/mL.[32] In anesthetized and ventilated pigs with inoculation pneumonia caused by multidrug-resistant Pseudomonas aeruginosa, median ELF amikacin concentrations measured 120 min after the nebulization of 400 mg were 150 mg/L, a concentration far greater than the lung tissue concentration measured in post-mortem.[33] In a recent experimental study conducted in healthy, anesthetized, and ventilated ewes receiving a 400 mg aerosol dose of tobramycin, the ELF concentrations were 100-fold higher than the ISF concentrations measured using lung microdialysis.[34] In critically ill patients with VAP receiving low-dose nebulized amikacin (5 mg/kg/day for 3 days), the median amikacin ELF concentration measured at the end of the treatment was 976 mg/L, with lower and upper values of 136 mg/L and 16,128 mg/L.[35] To collect ELF samples, the fiberscope is advanced as far as possible in the bronchial tree and contaminated by the nebulized particles containing aminoglycoside deposited on the bronchial walls. When the fiberscope cannot be further advanced, 3 aliquots of 20 mL saline are instilled and the aspirate from the third aliquot is used for measuring ELF concentrations. Because the aspirate also retrieves bronchial secretions containing aminoglycosides, ELF concentrations largely overestimate lung interstitial concentrations and cannot be considered as reflecting lung tissue concentrations. Further experimental studies performed in ventilated animals receiving nebulized aminoglycosides should be performed to compare antibiotic concentrations measured by direct sampling in the proximal bronchi, distal bronchioles, and ELF with ISF concentrations measured by lung microdialysis [36].
In experimental studies, there are two reference methods for assessing aminoglycoside lung interstitial concentrations: (1) post-mortem lung tissue biopsies that are cryofixed in nitrogen, weighed, and homogenized in buffer solution[37] for measuring aminoglycoside concentrations using high-performance liquid chromatography;[12] (2) lung microdialysis.[36] Tissue homogenates provide a single measurement of aminoglycoside concentrations in distal bronchiole and alveolar space[16,17] and do not allow for longitudinal pharmacokinetic analyses. Lung microdialysis has several advantages over lung biopsies: measurement of unbound fraction, continuous sampling allowing lung pharmacokinetics, regional assessment of aminoglycoside lung concentrations, and no risk of bronchial contamination.[36] However, it is invasive and cannot be routinely used in patients. It is costly and necessitates specialist techniques involving large animals undergoing prolonged mechanical ventilation.
In clinical studies, there is no alternative to the ELF concentration assay. It is therefore essential to be aware of its limitations and properly interpret aminoglycoside ELF concentrations to avoid wrong conclusions. As an example, finding high ELF concentrations after the nebulization of low doses of aminoglycosides does not indicate bactericidal efficacy at the lung tissue level. Determining the right nebulized dose for patients should rely on experimental studies performed in large animals whose respiratory system anatomy is close to human anatomy and where lung tissue aminoglycoside concentrations are measured either on post-mortem lung biopsies or using in vivo lung microdialysis.
Assessment of bactericidal efficacy of nebulized aminoglycosides
Methodological restrictions concerning the interpretation of ELF aminoglycoside concentrations also apply to the interpretation of quantitative bacteriology of bronchoalveolar lavage in patients or animals receiving nebulized aminoglycosides. The per-procedure contamination of the distal tip of the bronchoscope by nebulized particles deposited on the bronchial walls skews the quantitative bacterial analysis of the distal sample. It artificially decreases the bacterial count and is more of a bacterial analysis of the distal bronchioles than the infected lung parenchyma. As there is no alternative to bronchoalveolar lavage techniques, a gap of 24 h should be maintained between the last nebulization and the quantitative bacterial analysis of distal pulmonary samples retrieved from the bronchoalveolar lavage.
Experimental Studies Supporting the Nebulization of Aminoglycosides for Treating VAP
Animal studies based on post-mortem lung biopsies
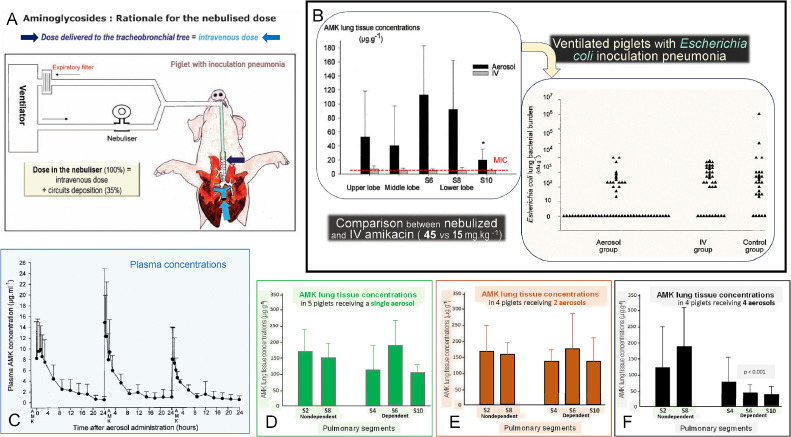
The reference experimental studies demonstrating the antibacterial efficiency of nebulized aminoglycosides were published in 2002.[16,17] Twenty-four hours after massive intrabronchial inoculation of Sensitive Escherichia coli (MIC= 4 mg/L), 36 anesthetized and ventilated piglets were treated either by nebulized (n=21) or intravenous (n=15) amikacin. In order to fairly compare both routes of administration, a dose equivalent to the intravenous dose (15 mg/kg) was delivered by nebulization to the distal tip of the endotracheal tube (Figure 2A). Aerosol deposition in respiratory circuits (including the nebulizer and the endotracheal tube), was measured in 18 piglets with healthy lungs.[37] Extrapulmonary deposition represented 60% of the dose inserted into the nebulizer chamber, and therefore amikacin 45 mg/kg was nebulized in a single daily dose 24 h and 48 h following bacterial inoculation. One hour following the second nebulization, animals were sacrificed and lung specimens were sampled from the upper, middle, and lower lobes where aeration and severity of bronchopneumonia were quantified.[17] Quantitative bacterial analysis and assessment of amikacin tissue concentration on each lung specimen were performed by investigators blinded to the pulmonary lobe, to the mode of amikacin administration, and the degree of lung aeration. Following intravenous administration, peak amikacin lung tissue concentrations were found in the range of MICs. After nebulization, peak amikacin lung tissue concentrations were 3–30 folds higher than after intravenous administration and significantly above MIC (4 mg/L). After two doses, 71% of lung segments were sterile, whereas cultures of lung segments were comparable in control/untreated (n=6) and intravenously treated animals (n=8) (Figure 2B), confirming previous experimental studies demonstrating the lack of bactericidal effect of intravenous amikacin.[33,38] In piglets with healthy lungs receiving a single daily nebulization of amikacin 40 mg/kg, neither systemic nor lung accumulation was observed (Figure 2C-F).[39]
Figure 2.
Experimental data that demonstrate the superiority of nebulized over intravenous amikacin for treating Escherichia coli inoculation pneumonia in anesthetized and mechanically ventilated piglets lying in the prone position (physiologic position). A: The rationale for comparing intravenous and inhalation routes was to administer the same dose at the entrance to the respiratory system (distal tip of the endotracheal tube) and at the entrance to the pulmonary circulation (main pulmonary artery). As a consequence 45 mg/kg of nebulized AMK was compared to 15 mg/kg of intravenous AMK (Reproduced from Ref. [42] with permission of the publisher). B: Following AMK nebulization, lung tissue concentrations were much higher than the MIC (4 mg/L) in the upper, middle, and lower lobes. In segment 10, the most dependent and consolidated lung region, AMK lung tissue concentrations were lower than in other lung segments but still equal to 5 times the MIC. Following intravenous AMK, lung tissue concentrations were in the range of MIC. Bactericidal activity (each triangle represents the Escherichia coli lung tissue concentration of a given pulmonary segment) was exclusively observed after nebulization (aerosol group). In animals treated by intravenous AMK, Escherichia coli lung tissue concentrations were not different from those in non-treated animals (Reproduced from Ref. [16] with permission of the publisher). C: AMK serum concentrations following three consecutive 40 mg/kg/day nebulizations in nine anesthetized ventilated piglets with healthy lungs. There is no evidence of any AMK systemic accumulation. D–F: AMK lung tissue concentrations following 1 (n=5), 2 (n=4), and 4 (n=9) 40 mg/kg/day nebulizations in 18 anesthetized ventilated piglets with healthy lungs. There is no evidence of AMK lung tissue accumulation. Reproduced from Ref. [39] with permission of the publisher.
AMK: Amikacin; MIC: Minimum inhibitory concentrations; IV: Intravenous.
Lung aeration loss had opposite effects on amikacin lung penetration according to the route of administration. Amikacin tissue concentrations increased in the intravenous group and decreased in the aerosol group (Figure 3A). The severity of lung infection (stage of bronchopneumonia) decreased tissue concentrations in piglets receiving nebulized amikacin and had no effect in animals receiving intravenous amikacin (Figure 3B). Lung aeration was quantified on postmortem histologic slices using specifically designed software (Figure 3C).[17] In several experimental studies, histologic grade of pneumonia was classified on postmortem histologic slices as mild or severe (Figure 3D and E).[13,14,16,17] Confluent bronchopneumonia increases the alveolar-capillary barrier permeability, which fosters penetration of intravenous amikacin into the lung parenchyma. It also generates obstruction of distal bronchioles by purulent secretions which impair alveolar deposition of aerosolized aminoglycosides. However, in the most severely infected lung areas characterized by extensive consolidation and markedly reduced lung aeration, lung tissue concentrations are frequently found above MICs. In a majority of pulmonary segments infected by Escherichia coli with lung aeration <30%, amikacin concentrations ranged between 2 and 50 times the MICs, providing a potent bactericidal effect (Figure 4A). Similar results were found following the nebulization of colistimethate sodium and ceftazidime in animals with Pseudomonas aeruginosa inoculation pneumonia (Figure 4B and C).
Figure 3.
Impact of lung aeration loss and bronchopneumonia severity on amikacin lung tissue concentration and bactericidal efficacy. The effects of lung aeration loss (A) and bronchopneumonia severity (B) were assessed in a series of anesthetized piglets with Escherichia coli inoculation pneumonia treated either by intravenous amikacin 15 mg/kg or by nebulized amikacin 45 mg/kg. C: The method used to quantify lung aeration. An image analyzer computerized system was coupled to a high-resolution color camera, and an optical microscope objective to quantify lung aeration on histologic slices. An interactive software program using a specifically designed computerized program served for the detection of air space structures. Each histologic section was analyzed on a screen of a personal computer connected to the optical microscope and the color camera. Each optical field was analyzed as an automatically delineated rectangular elementary unit with an area of 2.289 mm2. Within the elementary unit, aerated lung structures were automatically identified by a color encoding system (yellow for bronchi, red for alveoli). Air-like structures, such as pulmonary vessels and interlobular septa, were visually detected and manually deselected to include lung aeration alveolar and bronchial air-filled structures only. Lung aeration of the elementary unit expressed as a percentage was computed as the area of alveolar and bronchial air-filled structures divided by the difference between 2.289 mm2 and the area of air-like structures.[40] D: Mild bronchopneumonia characterized by foci of pneumonia centered by an infected bronchiole. Surrounding alveoli are inflammatory but still aerated. E: Severe bronchopneumonia characterized by infection extending to adjacent alveolar spaces without any more aeration. Reproduced from Ref. [17] with permission of the publisher.
AMK: Amikacin; BPN: Bronchopneumonia.
Figure 4.
Effect of lung aeration loss and bronchopneumonia severity on lung tissue concentration of nebulized antibiotics in anesthetized and ventilated piglets with inoculation pneumonia. The method used to quantify lung aeration is shown in Figure 3. A: Eleven animals with Escherichia coli inoculation pneumonia (MIC=2 mg/L), received two nebulizations of 40 mg/kg amikacin at 24-h intervals. One hour following the second administration, animals were killed, and 55 lung specimens were sampled for assessing amikacin pulmonary concentrations and quantifying lung aeration on histologic sections. In lung segments with lung aeration of 30% or less, mean pulmonary concentrations of amikacin were (18±7) µg/g, ie. 9 times the MIC (MIC, red dashed line). Mean amikacin pulmonary concentrations increased to 20 times the MIC in lung segments whose aeration ranged between 30% and 50% and to 33 times the MIC in lung segments whose aeration was greater than 50%. Reproduced from Ref. [17] with permission of the publisher. B: Six animals with Pseudomonas aeruginosa inoculation pneumonia (MIC=2 mg/L), received three nebulizations of 100,000 IU/kg (8 mg/kg) of colistimethate sodium diluted every 12 h. One hour following the third administration, animals were killed, and 30 lung specimens were sampled for assessing colistin pulmonary concentrations and quantifying pneumonia severity on histologic sections. Severe pneumonia was defined by confluent and purulent pneumonia reducing lung aeration to 30% or less. Mild pneumonia was defined by bronchiolitis, interstitial pneumonia, and foci of bronchopneumonia maintaining lung aeration above 30%. In 7 among the 13 lung segments with severe pneumonia, mean pulmonary concentrations of colistin ranged between 2 and 4 times the MIC, providing a bactericidal effect. In six segments, mean colistin concentrations were equal to or less than MIC, precluding any bactericidal effect. Reproduced from reference Ref. [13] with permission of the publisher. C: Six animals with Peudomonas aeruginosa inoculation pneumonia (MIC=16 mg/L), received ceftazidime nebulizations of 25 mg/kg every 3 h. Three hours following the eighth nebulization, animals were killed, and 30 lung specimens were sampled for assessing ceftazidime pulmonary concentrations and quantifying pneumonia severity on histologic sections. In 3 among the 10 lung segments with severe pneumonia, mean pulmonary concentrations of ceftazidime ranged between 2 and 3 times the MIC, providing a bactericidal effect. In 20 segments, mean ceftazidime concentrations were equal to or less than MIC, precluding any bactericidal effect. Reproduced from Ref. [14] with permission of the publisher.
MIC: Minimum inhibitory concentration; IU: International Units.
Several routes of penetration may account for the diffusion of nebulized aminoglycosides into adjacent nonaerated infected alveoli. Aerated bronchioles are frequently observed within consolidated lung areas, allowing the bronchial diffusion of nebulized aminoglycosides in adjacent infected alveoli. Intrapulmonary deposition may also occur through intraparenchymal pseudocysts and distended bronchioles that are commonly observed in the lung parenchyma of ventilated piglets with inoculation pneumonia,[26,40] These experimental data clearly support the administration of nebulized antibiotics at early stages of VAP,[41,42] There is however clinical and radiological evidence that nebulized amikacin at a dose of 45 mg/kg can cure severe Pseudomonas aeruginosa VAP characterized by consolidated and non-aerated lower lobes (Figure 5).[43]
Figure 5.
Computerized tomographic assessment of lung reaeration resulting from 8-day nebulization of ceftazidime and amikacin 25 mg/kg/day in a 53-year-old patient with VAP caused by susceptible Pseudomonas aeruginosa. VAP developed in the postoperative period after the surgical repair of a major thoracoabdominal aneurysm. On the left, six computerized tomographic sections representative of both lungs and acquired before starting nebulized antibiotics are shown with a color-encoding system showing normally aerated lung regions in dark gray, interstitial pneumonia and/or edema in light gray (mild loss of lung aeration), and confluent pneumonia in red (non-aerated lung region). On the right, the same six computerized tomographic sections acquired after 8-day nebulization of ceftazidime and amikacin are shown. Nebulization of antibiotics induced a 1000-mL lung reaeration, predominating in regions of confluent pneumonia associated with a regression of clinical signs of infection and normalization of quantitative bacteriology of protected mini-bronchoalveolar lavage. The patient was successfully extubated. Reproduced from Ref. [43] with permission of the publisher.
VAP: Ventilator-associated pneumonia.
Intact alveolar-capillary barrier and airway epithelium reduce lung penetration of intravenous antibiotics and systemic diffusion of nebulized antibiotics (Figure 6A).[44] Lung infection as any type of lung injury, markedly increases the permeability of the alveolar-capillary barrier[45] and promotes the diffusion of nebulized aminoglycosides into the pulmonary circulation. In animals with severe pneumonia, amikacin trough plasma concentrations were found in the same range after nebulization and intravenous administration (Figure 6B). In other words, lung infection-induced injury of the alveolar-capillary barrier increases the systemic diffusion of nebulized aminoglycosides (Figure 6C) and thereby, the toxicity risk. Pharmacokinetics data question the common belief that nebulization reduces aminoglycosides’ renal and ototoxicity in patients with VAP. They also challenge another classical belief: the combination of intravenous and nebulization of aminoglycosides provides benefits in the treatment of pneumonia. In fact, the penetration of intravenous aminoglycosides into the infected lung parenchyma is limited whereas the penetration of nebulized aminoglycosides into the systemic compartment is high. Therefore, combining both modes of administration likely results in an increased risk of toxicity without increasing antibacterial efficiency.
Figure 6.
Impact of anatomy of airways and alveoli on systemic diffusion of nebulized amikacin. A: Representation of anatomy and histology of airways and alveoli. Airway epithelium and alveolar-capillary membrane offer resistance to lung penetration of nebulized aminoglycosides Reproduced from Ref. [44] with permission of the publisher. B: AMK serum concentrations after 2 AMK nebulizations of 45 mg/kg (yellow circles) and 2 AMK intravenous administration of 15 mg/kg (blue circles) in 18 anesthetized and ventilated piglets with Escherichia coli inoculation pneumonia. Peak concentrations are higher after the intravenous route whereas trough concentrations are higher after nebulization. Reproduced from Ref. [37] with permission of the publisher. C: AMK serum concentrations after 2 AMK nebulizations of 45 mg/kg in 18 anesthetized and ventilated piglets with healthy lungs (green circles) and 10 anesthetized and ventilated piglets with Escherichia coli inoculation pneumonia (black circles). The infectious process injures the airway and alveolar epitheliums and facilitates systemic diffusion of nebulized aminoglycosides. Reproduced from Refs. [[16], [37]] with permission of the publisher.
AMK: amikacin.
Animal studies based on lung microdialysis
Pharmacokinetic studies based on lung microdialysis and performed in mechanically ventilated animals with inoculation pneumonia receiving nebulized aminoglycosides are lacking. Two studies have reported lung pharmacokinetics of nebulized tobramycin in ventilated sheep with healthy lungs.[34,46]
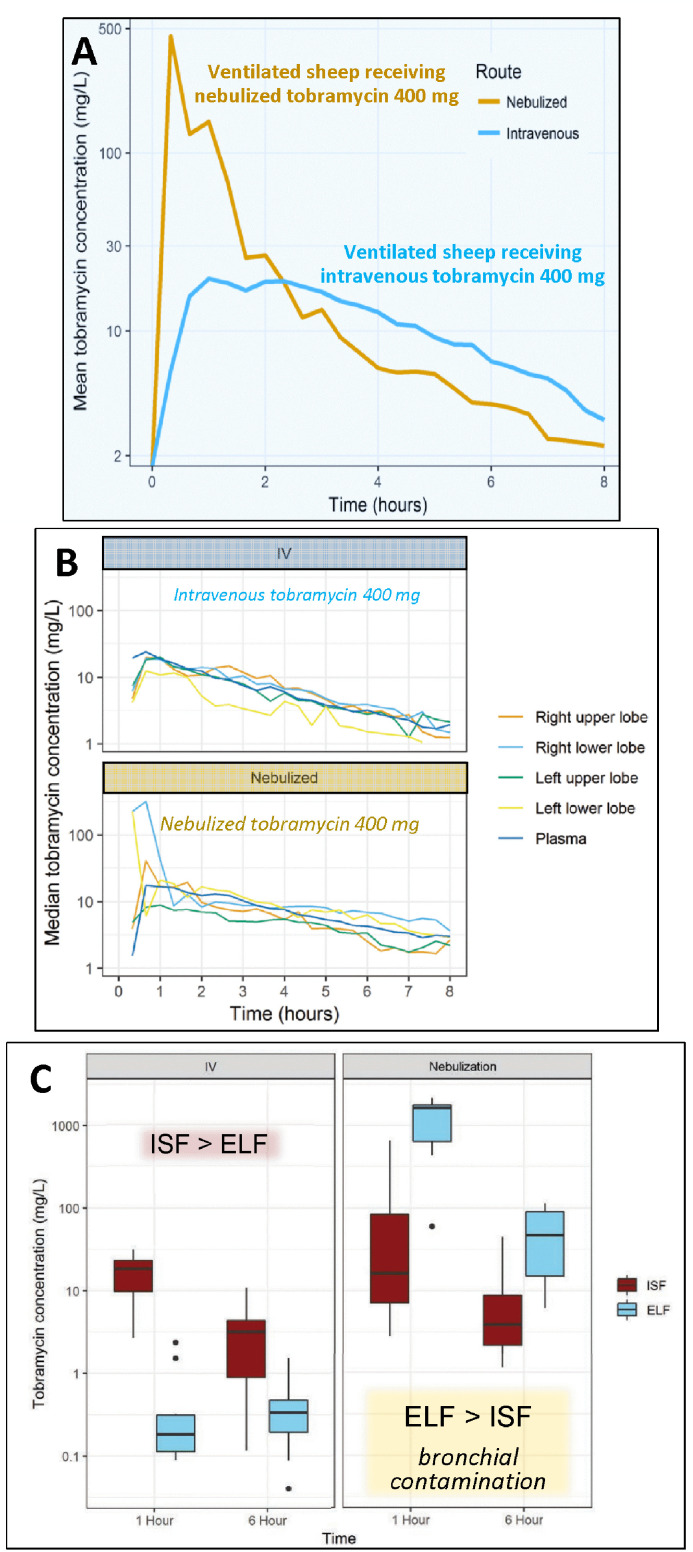
In the first study, 400 mg of tobramycin was administered either intravenously or by nebulization to two mechanically ventilated Merino sheep. Lung ISF concentrations were measured by lung microdialysis. Lung distribution of Technetium-99m-labeled tobramycin was assessed by pulmonary scintigraphy in two additional animals.[46] Peak lung ISF concentrations were 20-fold higher in the sheep receiving nebulized tobramycin than in the sheep receiving intravenous tobramycin (Figure 7A). Intrapulmonary tobramycin distribution was more homogenous after nebulization than after intravenous administration.
Figure 7.
Lung ISF concentrations assessed with lung microdialysis in sheep, which received either nebulized or intravenous tobramycin. A: Lung ISF concentrations measured in an anesthetized and ventilated sheep, which received nebulized tobramycin 400 mg (yellow curve) and in another sheep, which received intravenous tobramycin 400 mg (blue curve). Reproduced from Ref. [46] with permission of the publisher. B: Individual lung ISF concentrations measured in anesthetized and ventilated ewes after receiving intravenous tobramycin 400 mg (n=5) or nebulized tobramycin 400 mg (n=5). C: Comparison of median lung interstitial and ELF concentrations in 10 anesthetized and ventilated ewes after receiving 400 mg Tobramycin either by the intravenous route (n=5) or by nebulization (n=5). B and C are reproduced from Ref. [34]. with permission of the publisher.
ELF: Epithelial lining fluid; ISF: Interstitial fluid.
In the second study, lung ISF concentrations were measured by lung microdialysis in mechanically ventilated ewes receiving 400 mg of tobramycin, either intravenously (n=5) or by nebulization (n=5).[34] Median peak lung ISF concentrations, which is the by pharmacokinetics parameter determining bactericidal effectiveness, were two-fold higher after nebulization than after intravenous administration. Multiple probes were inserted in each animal to assess lung ISF concentrations in the upper and lower lobes (Figure 1F and 7B). As expected, lung ISF concentrations were much higher than ELF concentrations in the five animals who received intravenous tobramycin (Figure 7C). In contrast, ELF concentrations were much higher than lung ISF concentrations in the five animals who received nebulized tobramycin, suggesting bronchial contamination of the bronchoscope during the bronchoalveolar procedure.
Recently, addressing the high costs and complexity of lung microdialysis, the European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia (ENAVAP) decided the creation of an international research network for Lung Microdialysis applied to Nebulised Antibiotics (LUMINA). The main purpose of the LUMINA network will be to promote multicentered, experimental, randomized, controlled studies addressing lung pharmacokinetics/pharmacodynamics of intravenous vs. nebulized antibiotics.[36]
Conclusions
Experimental studies performed in anesthetized and mechanically ventilated animals provide evidence that high doses of nebulized aminoglycosides exert a rapid and potent bacterial killing in the infected lung parenchyma. They also confirm that the limited pulmonary penetration of intravenous aminoglycosides precludes any bactericidal effect at the site of infection. The injury of the alveolar-capillary membrane promotes the systemic diffusion of nebulized aminoglycosides, and the classical belief that nebulization protects against systemic toxicity is not confirmed by experimental data. Lung aeration loss decreases the lung penetration of nebulized aminoglycosides. Nevertheless, lung tissue concentrations measured in nonaerated infected lung regions remain largely above MICs, allowing a bactericidal effect. In animals receiving nebulized aminoglycosides, epithelial fluid concentrations grossly overestimate lung ISF concentrations because of the bronchial contamination of the bronchoscope. Lung microdialysis is the only technique able to accurately assess lung pharmacokinetics in animals with inoculation pneumonia treated by nebulized aminoglycosides. The ENAVAP calls for the creation of an international research network for LUMINA to promote multicentered, experimental, randomized, and studies addressing lung pharmacokinetics/pharmacodynamic of intravenous vs. nebulized antibiotics, using different dosing and different ventilator settings.
CRediT authorship contribution statement
Jean-Jacques Rouby: Writing – original draft, Supervision, Conceptualization. Jing Xia: Writing – review & editing, Conceptualization. Jayesh Dhanani: Writing – review & editing, Validation. Gianluigi Li Bassi: Writing – review & editing. Antoine Monsel: Writing – review & editing, Validation. Antoni Torres: Writing – review & editing, Validation.
Acknowledgments
Acknowledgments
Each following member of the European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia contributed to the final article, approved its content, and is a co-author of the article (listed by alphabetic order).
Kostoula Arvaniti arvanitik@hotmail.com, Papageorgiou Hospital of Thessaloniki, Intensive Care Unit Department, Thessaloniki, Greece; Mona Assefi mona.assefi@aphp.fr Medicine Sorbonne University, Multidisciplinary Intensive Care Unit, Department of Anaesthesiology and Critical Care, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; Matteo Bassetti matteo.bassetti@asuiud.sanita.fvg.it, Infectious Diseases Clinic, Department of Health Sciences, University of Genoa, Genoa and Hospital Policlinico San Martino – IRCCS, Genoa, Italy; Stijn Blot stijn.blot@UGent.be, Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Matthieu Boisson matthieu.boisson@chu-poitiers.fr, University of Poitiers, Anaesthesiology and Intensive Care Department, University hospital of Poitiers, Poitiers, France; Adrien Bouglé adrien.bougle@aphp.fr Medicine Sorbonne University, Anaesthesiology and Critical Care, Cardiology Institute, Department of Anaesthesiology and Critical Care, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; Jean-Michel Constantin jean-michel.constantin@aphp.fr, Medicine Sorbonne University, Multidisciplinary Intensive Care Unit, Department of Anaesthesiology and Critical Care, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; George Dimopoulos gdimop@med.uoa.gr, Department of Critical Care Medicine, Attikon University Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece; Jonathan Dugernier jonathan.dugernier@rhne.ch, Department of Physiotherapy, Neuchâtel hospital, Neuchâtel, Switzerland; Pauline Dureau pauline.dureau@aphp.fr Medicine Sorbonne University, Anaesthesiology and Critical Care, Cardiology Institute, Department of Anaesthesiology and Critical Care, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; Timothy Felton timothy.felton@manchester.ac.uk, The University of Manchester and Manchester University NHS Foundation Trust, Manchester, United Kingdom; Marin Kollef kollefm@wustl.edu, Division of Pulmonary and Critical Care Medicine, Washington University School of Medicine, St. Louis, Missouri, USA; Antonia Koutsoukou koutsoukou@yahoo.gr, Intensive Care Unit, First Department of Respiratory Medicine, School of Medicine, Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece; Anna Kyriakoudi Annkyr@gmail.com, Intensive Care Unit, First Department of Respiratory Medicine, School of Medicine, Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece; Pierre-François Laterre pierre-francois.laterre@uclouvain.be, St. Luc Clinical Coordinating Center, Department of Critical Care Medicine, St Luc University Hospital, Université Catholique de Louvain, Brussels, Belgium; Marc Leone marc.leone@ap-hm.fr, University Aix-Marseille, Department of Anaesthesiology and Critical Care, North Hospital, Marseille, France; Victoria Lepère, victoria.lepere@aphp.fr, Medicine Sorbonne University, Anaesthesiology and Critical Care, Cardiology Institute, Department of Anaesthesiology and Critical Care, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; Xuelian Liao xuelianliao@hotmail.com, Department of Critical Care Medicine, West China Hospital of Sichuan University, Chengdu, China; Olivier Mimoz o.mimoz@chu-poitiers.fr, University of Poitiers, Anaesthesiology and Intensive Care Department, University hospital of Poitiers, Poitiers, France; Girish B Nair Girish.Nair@beaumont.org Interstitial Lung Disease Program Director Pulmonary Research OUWB School of Medicine, Royal Oak, MI, USA; Michael Niederman msn9004@med.cornell Pulmonary and Critical Care, New York Presbyterian/Weill Cornell Medical Center, Weill Cornell Medical College, New York, USA; Lucy B Palmer lucy.b.palmer@stonybrook.edu Stony Brook University Medical Center Pulmonary, Critical Care and Sleep Division, SUNY at Stony Brook, HSC T17–040, Stony Brook, New York, USA; Jose Manuel Pereira jmcrpereira@yahoo.com, Emergency and Intensive Care Department, Centro Hospitalar São João EPE, Faculdade de Medicina da Universidade do Porto, Porto, Portugal; Konstantinos Pontikis kostis_pontikis@yahoo.gr, Intensive Care Unit, First Department of Respiratory Medicine, School of Medicine, Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece; Garyphalia Poulakou gpoulakou@gmail.com, Third Department of Medicine, School of Medicine, Sotiria General Hospital, National and Kapodistrian University of Athens, Athens, Greece; Jérôme Pugin jerome.pugin@unige.ch, Intensive Care Division, University Hospitals of Geneva, Geneva, Switzerland; Chuanyun Qian, qianchuanyun@126.com, Emergency department, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China; Jie-ming Qu jmqu0906@163.com, Department of Pulmonary and Critical Care Medicine, Rui-jin Hospital, Shanghai Jiao-tong University School of Medicine, Shanghai, China; Institute of Respiratory Disease, Shanghai Jiao-tong University School of Medicine, Shanghai, China; Jordi Rello jrello@crips.es, Centro de Investigación Biomédica en Red (CIBERES), Instituto de Salud Carlos III, Madrid, Spain, Clinical Research & Innovation in Pneumonia & Sepsis, Vall d'Hebron Institute of Research (VHIR), Barcelona, Spain and Clinical Research, CHU Nîmes, Université Montpellier-Nîmes, Nîmes, France; Jason Roberts j.roberts2@uq.edu.au, (1) University of Queensland Centre for Clinical Research, Faculty of Medicine & Centre for Translational Anti-infective Pharmacodynamics, School of Pharmacy, The University of Queensland, Brisbane, Australia (2) Departments of Pharmacy and Intensive Care Medicine, Royal Brisbane and Women's Hospital, Brisbane, Australia (3) Division of Anaesthesiology Critical Care Emergency and Pain Medicine, Nîmes University Hospital, University of Montpellier, Nîmes France; Christina Routsi chroutsi@hotmail.com, First Department of Intensive Care, School of Medicine, Evangelismos Hospital, National and Kapodistrian University of Athens, Athens, Greece; Gerald C. Smaldone gerald.smaldone@stonybrook.edu Stony Brook University Medical Center Health Sciences Center Stony Brook New York, NY USA; Melda Türkoğlu meldaturkoglu@yahoo.com.tr, Subdivision of Critical Care, Internal Medicine Intensive Care Unit, Department of Internal Medicine, Gazi University Faculty of Medicine, Ankara, Turkey; Tobias Welte welte.tobias@mh-hannover.de, University of Hannover, School of Medicine, Hannover, Germany; Michel Wolff m.wolff@ghu-paris.fr, Service de Neuro-Réanimation, Groupe Hospitalo-Universitaire Paris Psychiatrie & Neurosciences, Hôpital Ste Anne Paris France; Ting Yang, 459803440@qq.com, Emergency department, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China; Ying-gang Zhu robinzyg@gmail.com, Department of Pulmonary and Critical Care Medicine, Hua-dong Hospital, Fudan University, Shanghai, China.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Statement
Not applicable.
Conflict of Interest
Antoni Torres and Gianluigi Li Bassi received an unrestrictive grant from Bayer AG (Berlin, Germany) through their affiliated institution. Bayer AG provided funds to support research on nebulized amikacin. Bayer AG had no role in the writing and conception of the present manuscript.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Managing Editor: Jingling Bao/Zhiyu Wang
Contributor Information
Jean-Jacques Rouby, Email: jjrouby@invivo.edu.
European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia (ENAVAP):
Kostoula Arvaniti, Mona Assefi, Matteo Bassetti, Stijn Blot, Matthieu Boisson, Adrien Bouglé, Jean-Michel Constantin, Jayesh Dhanani, George Dimopoulos, Jonathan Dugernier, Pauline Dureau, Stephan Ehrmann, Timothy Felton, Marin Kollef, Antonia Koutsoukou, Anna Kyriakoudi, Pierre-François Laterre, Marc Leone, Victoria Lepère, Gianluigi Li Bassi, Xuelian Liao, Shakti Bedanta Mishra, Olivier Mimoz, Antoine Monsel, Girish B Nair, Michael Niederman, Lucy B Palmer, Jose Manuel Pereira, Konstantinos Pontikis, Garyphalia Poulakou, Jérôme Pugin, Chuanyun Qian, Jie-ming Qu, Jordi Rello, Jason Roberts, Jean-Jacques Rouby, Christina Routsi, Gerald C. Smaldone, Antoni Torres, Melda Türkoğlu, Tobias Welte, Michel Wolff, Xia Jing, Li Yang, Ting Yang, and Ying-gang Zhu
References
- 1.Thy M., Timsit J.F., de Montmollin E. Aminoglycosides for the treatment of severe infection due to resistant gram-negative pathogens. Antibiotics (Basel) 2023;12:860. doi: 10.3390/antibiotics12050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfieri A., Di Franco S., Donatiello V., Maffei V., Fittipaldi C., Fiore M., et al. Plazomicin against multidrug-resistant bacteria: a scoping review. Life (Basel) 2022;12:1949. doi: 10.3390/life12121949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zivkovic Zaric R., Zaric M., Sekulic M., Zornic N., Nesic J., Rosic V., et al. Antimicrobial treatment of Serratia marcescens invasive infections: systematic review. Antibiotics (Basel) 2023;12:367. doi: 10.3390/antibiotics12020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilanus A., Drusano G. Inoculum-based dosing: a novel concept for combining time with concentration-dependent antibiotics to optimize clinical and microbiological outcomes in severe gram negative sepsis. Antibiotics (Basel) 2023;12:1581. doi: 10.3390/antibiotics12111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimzadeh I., Abdollahpour-Alitappeh M., Ghaffari S., Mahi-Birjand M., Barkhordari A., Alemzadeh E. Aminoglycosides: single- or multiple-daily dosing? An updated qualitative systematic review of randomized trials on toxicity and efficacy. Curr Mol Med. 2024;24:1358–1373. doi: 10.2174/1566524023666230801160452. [DOI] [PubMed] [Google Scholar]
- 6.Heffernan A.J., Sime F.B., Lipman J., Dhanani J., Andrews K., Ellwood D., et al. Intrapulmonary pharmacokinetics of antibiotics used to treat nosocomial pneumonia caused by Gram-negative bacilli: a systematic review. Int J Antimicrob Agents. 2019;53:234–245. doi: 10.1016/j.ijantimicag.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree T.D., Pelletier S.J., Gleason T.G., Pruett T.L., Sawyer R.G. Analysis of aminoglycosides in the treatment of gram-negative infections in surgical patients. Arch Surg. 1999;134:1293–1298. doi: 10.1001/archsurg.134.12.1293. [DOI] [PubMed] [Google Scholar]
- 8.Rouby J.J., Sole-Lleonart C., Rello J., European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia Ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: understanding nebulization of aminoglycosides and colistin. Intensive Care Med. 2020;46:766–770. doi: 10.1007/s00134-019-05890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rello J., Bouglé A., Rouby J.J. Aerosolised antibiotics in critical care. Intensive Care Med. 2023;49:848–852. doi: 10.1007/s00134-023-07036-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang H. 2015. Thèse de Doctorat. [Google Scholar]
- 11.Wang H., Sebrié C., Ruaud J.P., Guillot G., Bouazizi-Verdier K., Willoquet G., et al. Aerosol deposition in the lungs of spontaneously breathing rats using Gd-DOTA-based contrast agents and ultra-short echo time MRI at 1.5 Tesla. Magn Reson Med. 2016;75:594–605. doi: 10.1002/mrm.25617. [DOI] [PubMed] [Google Scholar]
- 12.Rouby J.J., Bouhemad B., Monsel A., Brisson H., Arbelot C., Lu Q., et al. Aerosolized antibiotics for ventilator-associated pneumonia: lessons from experimental studies. Anesthesiology. 2012;117:1364–1380. doi: 10.1097/ALN.0b013e3182755d7a. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q., Girardi C., Zhang M., Bouhemad B., Louchahi K., Petitjean O., et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 2010;36:1147–1155. doi: 10.1007/s00134-010-1879-4. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari F., Lu Q., Girardi C., Petitjean O., Marquette C.H., Wallet F., et al. Nebulized ceftazidime in experimental pneumonia caused by partially resistant Pseudomonas aeruginosa. Intensive Care Med. 2009;35:1792–1800. doi: 10.1007/s00134-009-1605-2. [DOI] [PubMed] [Google Scholar]
- 15.Judge E.P., Lynne Hughes J.M., Egan J.J., Maguire M., Molloy E.L., O'Dea S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol. 2014;51:334–343. doi: 10.1165/rcmb.2013-0453TR. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein I., Wallet F., Nicolas-Robin A., Ferrari F., Marquette C.H., Rouby J.J. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002;166:1375–1381. doi: 10.1164/rccm.200204-363OC. [DOI] [PubMed] [Google Scholar]
- 17.Elman M., Goldstein I., Marquette C.H., Wallet F., Lenaour G., Rouby J.J., et al. Influence of lung aeration on pulmonary concentrations of nebulized and intravenous amikacin in ventilated piglets with severe bronchopneumonia. Anesthesiology. 2002;97:199–206. doi: 10.1097/00000542-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Li Bassi G., Comaru T., Martí D., Xiol E.A., Chiurazzi C., Travierso C., et al. Recruitment manoeuvres dislodge mucus towards the distal airways in an experimental model of severe pneumonia. Br J Anaesth. 2019;122:269–276. doi: 10.1016/j.bja.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Luna C.M., Baquero S., Gando S., Patrón J.R., Morato J.G., Sibila O., et al. Experimental severe Pseudomonas aeruginosa pneumonia and antibiotic therapy in piglets receiving mechanical ventilation. Chest. 2007;132(2):523–531. doi: 10.1378/chest.07-0185. [DOI] [PubMed] [Google Scholar]
- 20.Li Bassi G., Marti J.D., Xiol E.A., Comaru T., De Rosa F., Rigol M., et al. The effects of direct hemoperfusion using a polymyxin B-immobilized column in a pig model of severe Pseudomonas aeruginosa pneumonia. Ann Intensive Care. 2016;6:58. doi: 10.1186/s13613-016-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Bassi G., Martí J.D., Comaru T., Aguilera-Xiol E., Rigol M., Ntoumenopoulos G., et al. Short term appraisal of the effects and safety of manual versus ventilator hyperinflation in an animal model of severe pneumonia. Respir Care. 2019;64:760–770. doi: 10.4187/respcare.06487. [DOI] [PubMed] [Google Scholar]
- 22.Meli A., Barbeta Viñas E., Battaglini D., Li Bassi G., Yang H., Yang M., et al. Lateral position during severe mono-lateral pneumonia: an experimental study. Sci Rep. 2020;9(10):19372. doi: 10.1038/s41598-020-76216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battaglini D., Motos A., Li Bassi G., Yang H., Pagliara F., Yang M., et al. Efficacy of telavancin in comparison to linezolid in a porcine model of severe methicillin-resistant Staphylococcus aureus pneumonia. Antimicrob Agents Chemother. 2021;65:e1009–e1020. doi: 10.1128/AAC.01009-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaro R., Li Bassi G., Motos A., Fernandez-Barat L., Aguilera Xiol E., Rigol M., et al. Development and characterization of a new swine model of invasive pneumococcal pneumonia. Lab Anim (NY) 2021;50:327–335. doi: 10.1038/s41684-021-00876-y. [DOI] [PubMed] [Google Scholar]
- 25.Rouby J.J., Martin De Lassale E., Poete P., Nicolas M.H., Bodin L., Jarlier V., et al. Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. Am Rev Respir Dis. 1992;146:1059–1066. doi: 10.1164/ajrccm/146.4.1059. [DOI] [PubMed] [Google Scholar]
- 26.Sartorius A., Lu Q., Vieira S., Tonnellier M., Lenaour G., Goldstein I., et al. Mechanical ventilation and lung infection in the genesis of air-space enlargement. Crit Care. 2007;11:R14. doi: 10.1186/cc5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquette C.H., Wermert D., Wallet F., Copin M.C., Tonnel A.B. Characterization of an animal model of ventilator-acquired pneumonia. Chest. 1999;115:200–209. doi: 10.1378/chest.115.1.200. [DOI] [PubMed] [Google Scholar]
- 28.Li Bassi G., Zanella A., Cressoni M., Stylianou M., Kolobow T. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med. 2008;36:518–525. doi: 10.1097/01.CCM.0000299741.32078.E9. [DOI] [PubMed] [Google Scholar]
- 29.Zanella A., Cressoni M., Epp M., Hoffmann V., Stylianou M., Kolobow T. Effects of tracheal orientation on development of ventilator-associated pneumonia: an experimental study. Intensive Care Med. 2012;38:677–685. doi: 10.1007/s00134-012-2495-2. [DOI] [PubMed] [Google Scholar]
- 30.Li Bassi G., Rigol M., Marti J.D., Saucedo L., Ranzani O.T., Roca I., et al. A novel porcine model of ventilator-associated pneumonia caused by oropharyngeal challenge with Pseudomonas aeruginosa. Anesthesiology. 2014;120:1205–1215. doi: 10.1097/ALN.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 31.Rouby J.J., Monsel A. Nebulized antibiotics: epithelial lining fluid concentrations overestimate lung tissue concentrations. Anesthesiology. 2019;131:229–232. doi: 10.1097/ALN.0000000000002824. [DOI] [PubMed] [Google Scholar]
- 32.Marchand S., Gregoire N., Brillault J., Lamarche I., Gobin P., Couet W. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 3. Tobramycin. Antimicrob Agents Chemother. 2015;59:6646–6647. doi: 10.1128/AAC.01647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motos A., Yang H., Li Bassi G., Yang M., Meli A., Battaglini D., et al. Inhaled amikacin for pneumonia treatment and dissemination prevention: an experimental model of severe monolateral Pseudomonas aeruginosa pneumonia. Crit Care. 2023;27:60. doi: 10.1186/s13054-023-04331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhanani J.A., Diab S., Chaudhary J., Cohen J., Parker S.L., Wallis S.C., et al. Lung pharmacokinetics of tobramycin by intravenous and nebulized dosing in a mechanically ventilated healthy ovine model. Anesthesiology. 2019;131:344–355. doi: 10.1097/ALN.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 35.Luyt C.E., Clavel M., Guntupalli K., Johannigman J., Kennedy J.I., Wood C., et al. Pharmacokinetics and lung delivery of PDDS aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care. 2009;13:R200. doi: 10.1186/cc8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhanani J., Roberts J.A., Monsel A., Antoni Torres A., Kollef M., Rouby J.J., et al. Understanding the nebulization of antibiotics: the key role of lung microdialysis studies. Crit Care. 2024;28:49. doi: 10.1186/s13054-024-04828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein I., Wallet F., Robert J., Becquemin M.H., Marquette C.H., Rouby J.J., et al. Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med. 2002;165:171–175. doi: 10.1164/ajrccm.165.2.2107025. [DOI] [PubMed] [Google Scholar]
- 38.Li Bassi G, Motos A., Fernandez-Barat L., Aguilera Xiol E., Chiurazzi C., Senussi T., et al. Nebulized Amikacin and Fosfomycin for Severe Pseudomonas aeruginosa pneumonia: an Experimental Study. Crit Care Med. 2019;47:e470–e477. doi: 10.1097/CCM.0000000000003724. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari F., Goldstein I., Nieszkowszka A., Elman M., Marquette C.H., Rouby J.J., et al. Study Group. Lack of lung tissue and systemic accumulation after consecutive daily aerosols of amikacin in ventilated piglets with healthy lungs. Anesthesiology. 2003;98:1016–1019. doi: 10.1097/00000542-200304000-00033. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein I., Bughalo M.T., Marquette C.H., Lenaour G., Lu Q., Rouby J.J., et al. Mechanical ventilation- induced air-space enlargement during experimental pneumonia in piglets. Am J Respir Crit Care Med. 2001;163:958–964. doi: 10.1164/ajrccm.163.4.2006072. [DOI] [PubMed] [Google Scholar]
- 41.Torres A., Motos A., Battaglini D., Li Bassi G. Inhaled amikacin for severe Gram-negative pulmonary infections in the intensive care unit: current status and future prospects. Crit Care. 2018;22:343. doi: 10.1186/s13054-018-1958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monsel A., Torres A., Zhu Y., Pugin J., Rello J., Rouby J.J., et al. Nebulized antibiotics for ventilator-associated pneumonia: methodological framework for future multicenter randomized controlled trials. Curr Opin Infect Dis. 2021;34:156–168. doi: 10.1097/QCO.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 43.Lu Q., Yang J., Liu Z., Gutierrez C., Aymard G., Rouby J.J., et al. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;184:106–115. doi: 10.1164/rccm.201011-1894OC. [DOI] [PubMed] [Google Scholar]
- 44.Eedara B.B., Bastola R., Das S.C. Dissolution and absorption of inhaled drug particles in the lungs. Pharmaceutics. 2022;14:2667. doi: 10.3390/pharmaceutics14122667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthay M.A. Function of the alveolar epithelial barrier under pathologic conditions. Chest. 1994;105(3 Suppl) doi: 10.1378/chest.105.3_supplement.67s. 67S–74S. [DOI] [PubMed] [Google Scholar]
- 46.Dhanani J.A., Cohen J., Parker S.L., Chan H.K., Tang P., Ahern B.J., et al. A research pathway for the study of the delivery and disposition of nebulised antibiotics: an incremental approach from in vitro to large animal models. Intensive Care Med Exp. 2018;6:17. doi: 10.1186/s40635-018-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.