Abstract
Perhaps the most obvious phenotypes associated with chemical signaling between plants are manifested by parasitic species of Orobanchaceae. The development of haustoria, invasive root structures that allow hemiparasitic plants to transition from autotrophic to heterotrophic growth, is rapid, highly synchronous, and readily observed in vitro. Haustorium development is initiated in aseptic roots of the facultative parasite Triphysaria versicolor when exposed to phenolic molecules associated with host root exudates and rhizosphere bioactivity. Morphological features of early haustorium ontogeny include rapid cessation of root elongation, expansion, and differentiation of epidermal cells into haustorial hairs, and cortical cell expansion. These developmental processes were stimulated in aseptic T. versicolor seedlings by the application of exogenous phytohormones and inhibited by the application of hormone antagonists. Surgically dissected root tips formed haustoria if the root was exposed to haustorial-inducing factors prior to dissection. In contrast, root tips that were dissected prior to inducing-factor treatment were unable to form haustoria unless supplemented with indole-3-acetic acid. A transient transformation assay demonstrated that auxin and ethylene-responsive promoters were up-regulated when T. versicolor was exposed to either exogenous hormones or purified haustoria-inducing factors. These experiments demonstrate that localized auxin and ethylene accumulation are early events in haustorium development and that parasitic plants recruit established plant developmental mechanisms to realize parasite-specific functions.
It has been estimated that over 4,000 species of angiosperms are able to directly invade and parasitize other plants (Nickrent, 2003). Parasitic plant species have widely different hosts and habits, ranging from mistletoes that grow on the tops of conifer trees to root parasites that live most of their lives underground (Press and Graves, 1995). A single feature common to all parasitic plants is the ability to develop invasive structures called haustoria (Riopel and Timko, 1995). After invasion, parasitic plant haustoria function as physiological bridges through which the parasite robs host plants of water and nutrients. The competence to form haustoria is the defining characteristic of parasitic plants, distinguishing them from epiphytic and mycoheterotrophic plants that either use host plants for physical support or associate via mycorrhizal intermediates (Kuijt, 1969; Leake, 1994).
It is clear from several lines of evidence that parasitic plants evolved from nonparasitic autotrophs (Kuijt, 1969). There are two general models for the evolutionary origins of the haustoria that define plant parasitism. One model proposes that genes encoding haustorium development are foreign to plants and were introduced into parasitic species via an endosymbiotic or horizontal gene transfer event, perhaps from a haustorial-producing fungus or bacteria (Atsatt, 1973). An alterative hypothesis proposes that genes for haustorium development are present in nonparasitic plants where they fulfill functions unrelated to parasitism. In this model, homeotic mutations in one or more developmental pathways results in the development of parasite-specific organs. It should be possible to distinguish these models by identifying critical pathways in haustorium development and determining their occurrence in other organisms.
Parasitic plants in the Orobanchaceae initiate haustorium development in response to specific phenolic derivatives present in host root exudates. The purification and identification of haustorial-inducing factors (HIFs) exploited earlier observations that haustorium development can be visually monitored by applying host root factors to aseptic parasite seedlings in vitro (Atsatt et al., 1978; Riopel and Musselman, 1979). The quinone 2,6-dimethoxybenzoquinone (DMBQ) was identified as an HIF from sorghum root washes (Chang and Lynn, 1986). Subsequently a number of purified phenolic molecules, including phenolic acids, quinines, and flavonoids, have been characterized as HIFs (Steffens et al., 1982; Riopel and Timko, 1995; Albrecht et al., 1999). It is likely that there is a redundancy of molecules in host root exudates that initiate haustorium formation (Jamison and Yoder, 2001).
The early ontogeny of haustorium development has been described for several root parasites including Striga asiatica, Agalinis purpurea, and Triphysaria versicolor (Atsatt and Musselman, 1977; Nickrent et al., 1979; Riopel and Musselman, 1979; Baird and Riopel, 1984; Heide-Jorgensen and Kuijt, 1995; Matvienko et al., 2001). A similar sequence of events occurs in each organism, though the actual timing of events may differ. Time lapse animations of early haustorium development in the hemiparasite T. versicolor can be viewed at http://pscroph.ucdavis.edu.
The first phenotype observed upon exposure of parasite roots to HIFs is the almost immediate cessation of root tip elongation. Shortly thereafter, epidermal cells localized in a ring just behind the root tip begin to develop into hairs that upon maturation exude adhesive molecules that bind the haustoria to the host root (Baird and Riopel, 1985). Approximately concurrent with hair proliferation, cortical cells underlying the proliferating haustorial hairs begin an isodiametric expansion that leads to a noticeable bulge near the root tip. The swelling and hair elongation continues for about 12 h, at which time the haustorium is competent to attach to a host. Epidermal cells near the apex of the bulge and the cortical cells comprising the swollen area begin to divide after the bulk of the haustorium is formed.
Subsequent developmental processes differ between obligate and facultative parasites. In facultative hemiparasites, haustorium development initiates proximal to the tip meristem. After about 12 h of elongation arrest, the root tip reinitiates its normal developmental program, resulting in one or more haustoria being positioned laterally along the maturing root. In contrast, haustorium development in Striga and Orobanche results in the irreversible differentiation of tip meristem into a primary haustorium that terminates further root growth (Okonkwo and Nwoke, 1978; Riopel and Baird, 1987). These species later develop lateral haustoria comparable to those produced by hemiparasites once the primary haustorium has successfully parasitized the host.
It has been recently postulated that polar accumulation of auxin is a universal feature of early plant organogenesis (Benková et al., 2003). Localized auxin and ethylene accumulation is a common theme in root morphogenesis, notably gravitropism, lateral root initiation, and root hair development (Aeschbacher et al., 1994; Schneider et al., 1997; Casson and Lindsey, 2003; Friml, 2003). These hormone systems have been repeatedly recruited for regulatory roles in mediating interactions between roots and other organisms in the rhizosphere (Hirsch et al., 1989; Goverse et al., 2000; Ferguson and Mathesius, 2003). The experiments described in this manuscript explore their role in mediating subterranean interactions between parasite and host plant roots. The overall question being addressed is to what level parasitic plants recruit existing plant regulatory mechanisms for parasite-specific purposes.
RESULTS
Exogenous Phytohormones and Early Haustorium Development
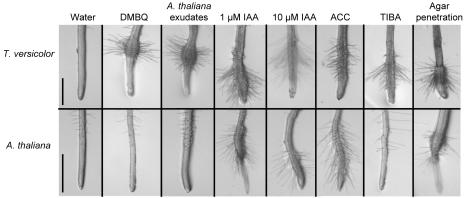
Early morphogenic changes in parasite roots exposed to HIFs suggested a role for endogenous plant hormones in haustorium initiation and development. Figure 1 is a composite photograph showing root phenotypes in seedlings of the parasite T. versicolor and Arabidopsis (Arabidopsis thaliana) exposed to HIFs or hormones. When treated with active HIFs, such as Arabidopsis root exudates or DMBQ, T. versicolor roots formed large, well-defined haustoria that are seen as hairy, swollen bulges approximately 0.5 mm in length and 0.3 mm in width. Similar phenotypes were not observed when Arabidopsis roots were treated with HIFs.
Figure 1.
T. versicolor and Arabidopsis roots exposed to different plant morphogens. Root tips of aseptic T. versicolor or Arabidopsis seedlings were exposed to water, 10 μm DMBQ, Arabidopsis root exudates, 1 μm IAA, 10 μm IAA, 100 μm ACC, or 30 μm TIBA for 24 h and then photographed. Untreated roots (rightmost photos) can form haustorium-like phenotypes when they penetrate the agar surface (1.0% [w/v] Phytagar [GibcoBRL Life Technologies, Rockville, MD]). Bars represent 0.5 mm.
Exposure of either T. versicolor or Arabidopsis roots to indole-3-acetic acid (IAA) or the ethylene precursor aminocyclopropanecarboxylic acid (ACC) resulted in some epidermal hair development and/or swelling, though not as localized or defined as obtained with HIFs (Fig. 1). As previously noted (Wolf and Timko, 1991), there was an apparent thigmotropic component to haustorium initiation. T. versicolor roots, like those of S. asiatica, typically require contact with a solid support, such as agar, to develop normal haustoria. Roots of T. versicolor and to a lesser extent Arabidopsis occasionally formed root hairs at the point of agar penetration in the absence of inducer (Fig. 1, rightmost section). The touch responses were enhanced in both species when auxin was included in the media. HIF-induced haustoria were distinguished from thigmotropic and hormone-induced pseudo-haustoria by the degree and size of swelling and haustorial hairs, by their precise and synchronous location with respect to the root tip, and by the higher number obtained.
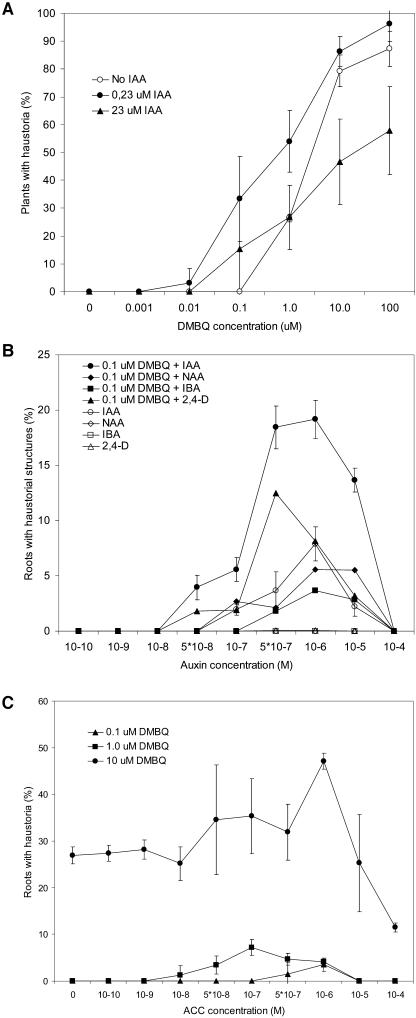
Previous studies showed that T. versicolor root cultures maintained in the presence of IAA formed haustoria more readily than in its absence (Tomilov et al., 2004). To further investigate this observation, we monitored haustorium development in roots of aseptic T. versicolor seedlings in the presence of exogenous IAA and limiting concentrations of DMBQ. As seen in Figure 2A, approximately one-third of T. versicolor roots formed haustoria when exposed to 0.1 μm DMBQ in the presence of 0.23 μm IAA while no haustoria formed in its absence. In the presence of IAA, the concentration of DMBQ needed to initiate haustoria in 50% of the plants was about ten times lower than in hormone-free media.
Figure 2.
Exogenous hormones and early haustorium development. A, Graph shows the percentage of T. versicolor plants that form haustoria as a function of DMBQ concentration in the presence of two concentrations of IAA or water. Results are the average and sds of three experiments with 10 plants each. B, Graph shows percentage of T. versicolor roots forming haustorial structures in the presence of 0.1 μm DMBQ and varying concentrations of IAA; 2,4-D; NAA; or IBA. No haustoria formed with 0.1 μm DMBQ alone. The IAA data averages three experiments with 10 plants each, data for 2,4-D; NAA; IBA points were taken once. Bars represent sd. C, Haustorium formation in the presence of exogenous ACC and three concentrations of DMBQ. The most dramatic enhancement is seen with 0.1 μm DMBQ. Graph shows an average of three experiments with 10 plants each (40–70 total root tips per treatment). Bars represent sd.
Other synthetic auxins, 2,4-dichlorophenoxyacetic acid (2,4-D), 1-naphthalene acetic acid (NAA), and indole-3-butyric acid (IBA), also stimulated the development of haustorial structures in the presence of low concentrations of DMBQ, though not to the same levels as IAA (Fig. 2B). The auxins were generally most effective at concentrations between 0.1 and 1.0 μm with higher auxin concentrations reducing haustoria formation. Exposure to these auxins in the absence of DMBQ also resulted in some root hair development and tip swelling, though not of the size, definition, or regularity of haustoria produced with DMBQ (Fig. 1). Commercial IAA was equally active to that purified in the lab by HPLC arguing against an active contaminant in the preparation (data not shown; LeClere et al., 2002). Similar enhancement was obtained in light and dark conditions.
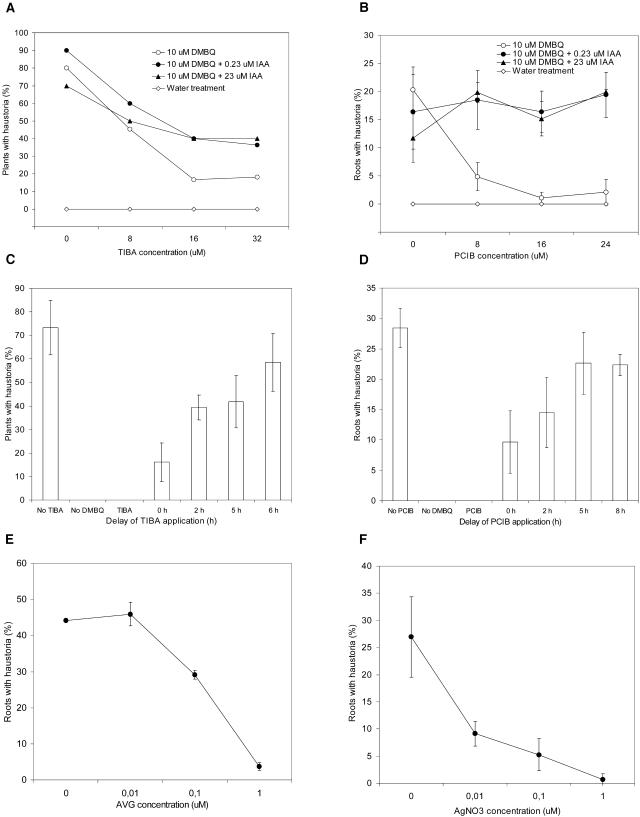
2,3,5-Triiodobenzoic acid (TIBA) is a competitive auxin efflux inhibitor and p-chlorophenoxyisobutyric acid (PCIB) is a competitive auxin antagonist (Geldner et al., 2001; Oono et al., 2003). Both TIBA and PCIB reduced haustorium formation when applied to T. versicolor roots with DMBQ (Fig. 3, A and B). For both inhibitors the normal haustorium activity was restored with the addition of exogenous IAA, indicating that the reduction in haustorium development was associated with IAA inhibition.
Figure 3.
Auxin and ethylene inhibitors. Pharmacological inhibitors of auxin transport, auxin action, and ethylene biosynthesis were added to T. versicolor roots simultaneously exposed to DMBQ. Exogenous IAA was also included in some cases. Results are an average of three experiments with 10 plants (40–70 root tips). Bars represent sd. A, Haustorium development in the presence of TIBA and 10 μm DMBQ B, Haustorium development in the presence of PCIB and 10 μm DMBQ C, TIBA (32 μm) was added to T. versicolor roots at different times following DMBQ exposure. D, PCIB (24 μm) was added to T. versicolor roots at different times after exposure to DMBQ. E, Haustorium development in the presence of AVG and 30 μm DMBQ. F, Haustorium development in the presence of AgNO3 and 30 μm DMBQ.
Time course experiments showed that TIBA and PCIB were most inhibitory when applied to T. versicolor roots together with DMBQ (Fig. 3, C and D). Application of TIBA 2 h after DMBQ resulted in a doubling in the number of roots with haustoria compared to the simultaneous treatment. There was no apparent inhibition when TIBA was added 6 h after DMBQ. This is consistent with auxin transport and action being important within a few hours of DMBQ exposure.
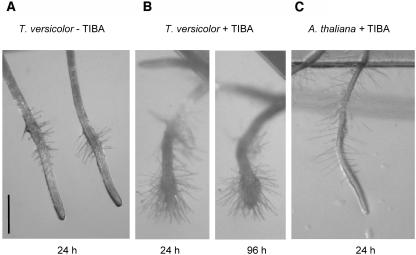
A different observation was made when TIBA was applied specifically to root tips by growing the roots from agar without TIBA into agar containing TIBA. When TIBA was applied at the root tip in this manner, hair proliferation and cortical swelling occurred at the tip, reminiscent of terminal-primary haustoria of Striga. Arabidopsis seedlings similarly treated did not have this phenotype (Fig. 4). This suggested that confining auxin transport to the tip triggers morphogenic events associated with haustorium development.
Figure 4.
Local application of TIBA. A, Haustorium-like structures formed by local tip application of TIBA to T. versicolor roots. T. versicolor seedlings were placed on agar without TIBA solidified on a microscope slide. The slides were then placed on the surface of agar containing TIBA and oriented such that the roots grew down along the surface of the agar, over the edge of the slide, and into the TIBA media (see “Materials and Methods”). Picture was taken 24 and 96 h after root tip contact with TIBA. B, T. versicolor roots in same experimental setup without TIBA in the media. C, Arabidopsis root tips after localized TIBA application. Size bars represent 0.5 mm.
Pharmacological experiments also correlated haustorium development with ethylene production. The effect of enhanced ethylene production was evaluated by applying ACC to T. versicolor roots at the time of DMBQ induction. Figure 2C shows that ACC at concentrations between 0.01 and 1.0 μm enhanced haustorium formation in the presence of 1.0 μm DMBQ. Reciprocally, the ethylene synthesis inhibitor l-α-(2-aminoethoxyvinyl) Gly (AVG) and the ethylene action inhibitor AgNO3 reduced haustorium formation almost completely (Fig. 3, E and F).
Auxin Derived from Root Material Proximal to Haustorial Initials Is Required for Early Haustorium Development
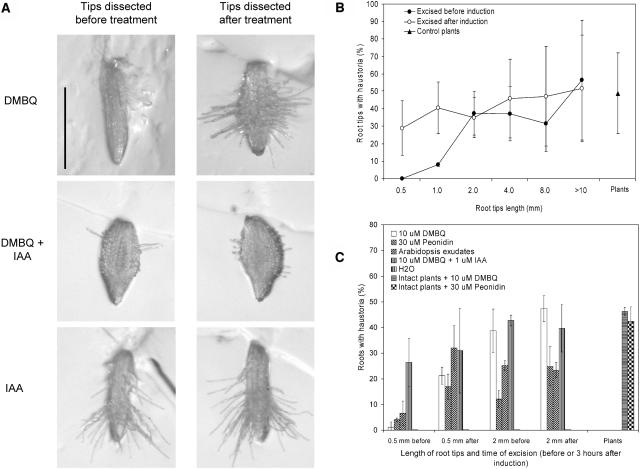
Early studies showed that root cultures lacking all above ground parts are competent to form haustoria (Tomilov et al., 2004). Figure 5 shows early haustorium development in root tips dissected from T. versicolor. Figure 5B shows that the frequency of formation was dependent upon the length of tip dissected. If the dissected root tip is 2 mm in length or longer, the frequency of haustorium development is similar to that obtained using intact seedlings. However when sections less than 2 mm were exposed to DMBQ the frequency of haustoria formation was dramatically reduced (Fig. 5B).
Figure 5.
Haustorium formation in dissected root tips of T. versicolor. A, 0.5-mm root tips were dissected before or after exposure to 10 μm DMBQ, DMBQ + 1 μm IAA, or IAA alone. Haustoria formed if root tips were dissected after, but not before, treatment with DMBQ. The inclusion of IAA allowed root tips dissected before treatment to make haustoria. Size bars represent 0.5 mm. B, Root tips of different sizes were excised from T. versicolor before or after treatment with DMBQ. The graph shows that tips 1 mm or smaller develop haustoria if excised after, but not before, DMBQ treatment. Control plants are not dissected. C, Root tips either 0.5 mm or 2 mm in length were excised from T. versicolor before or after treatment with DMBQ, peonidin, Arabidopsis root exudates, DMBQ plus IAA, or water. Control plants were treated with DMBQ or peonidin without dissection.
Interestingly, root tips smaller than 2 mm in length were competent to form haustoria when DMBQ was applied 3 h before, but not after, dissection. When 0.5-mm root tips were dissected from T. versicolor roots after exposure to DMBQ, 30% to 50% of the tips became swollen and covered with haustorial hairs (Fig. 5, A and B). Similar results were obtained when haustoria was induced using Arabidopsis root exudates or the flavonoid peonidin (Fig. 5C). This suggested that a factor(s) required for early cell swelling in response to DMBQ was present in root tissues proximal to the haustoria initiation site.
Dissected root tips did form haustoria when IAA was added together with DMBQ. As seen in Figure 5C, the inclusion of IAA allowed 0.5-mm root tips to form haustoria at similar frequencies as nondissected tips. These experiments were consistent with auxin present in root tissues proximal to the haustorium initiation site being necessary for early haustorium development.
Spatial and Temporal Accumulation of Endogenous Hormones
The spatial and temporal accumulation of auxin and ethylene in T. versicolor roots was monitored using two reporter gene constructs regulated by hormone-responsive promoters. The IAA2∷GUS reporter gene contains the −424 to +1 AUX1 promoter sequence cloned into the β-glucuronidase (GUS) expression vector pBI121 GUS (Jefferson et al., 1987; Swarup et al., 2001). This promoter has been used to monitor auxin distribution in Arabidopsis roots (Marchant et al., 1999). The 5×EBS∷GUS reporter construction contains the ethylene-responsive element from the EDF1 gene promoter fused to GUS (Alonso et al., 2003; A. Stepanova and J. Ecker, unpublished data).
Transient transformation protocols were optimized using pCAMBIA 1305.2, a cauliflower mosaic virus 35S-GUS construction containing a catalase intron in the GUS gene to eliminate expression in Agrobacterium tumefaciens (Roberts et al., 2004). A. tumefaciens bearing the GUS reporter were introduced into T. versicolor seedlings via vacuum infiltration and GUS expression examined 2 d later. As seen in Figure 6, seedlings infiltrated with the 35S-GUS fusion expressed GUS in roots, hypocotyls, and young leaves. The efficiency of transformation varied, but in good experiments all of the treated plants showed some GUS staining in at least one plant part, 80% to 90% of the time in roots.
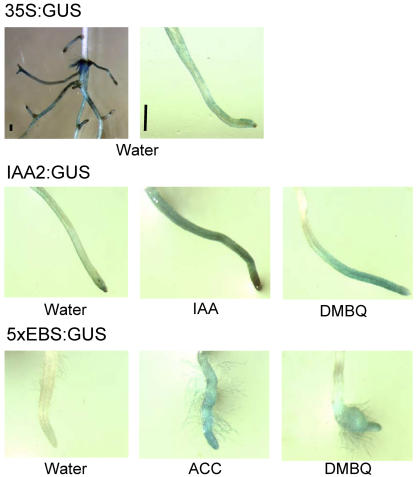
Figure 6.
Transient reporter assays in T. versicolor. T. versicolor seedlings were vacuum infiltrated with A. tumefaciens bearing GUS reporter genes regulated by hormone-responsive promoters. Two days after infiltration, seedlings were divided into subgroups and their roots treated with water, IAA, ACC, or DMBQ, and GUS activity stained at the times indicated below. Bar = 0.5 mm. A, Transient expression of 35S:GUS in whole roots and root tips of T. versicolor. B, Transient expression of the auxin-responsive plasmid IAA2:GUS in T. versicolor roots after 3 h exposure to water, 100 μm IAA, or 100 μm DMBQ. GUS staining after exposure to DMBQ is localized more at the tip than after exposure to IAA. C, Expression of the ethylene-responsive reporter 5 × EBS:GUS in T. versicolor 16 h after treatment with water, 100 μm ACC, or 100 μm DMBQ.
The optimized transformation protocol was then used to introduce the auxin (IAA2∷GUS) and ethylene (5×EBS∷GUS) reporters into T. versicolor. Two days after vacuum infiltration, roots were divided into groups and exposed to DMBQ, IAA, ACC, or water. GUS activity was monitored visually and spectroscopically after staining.
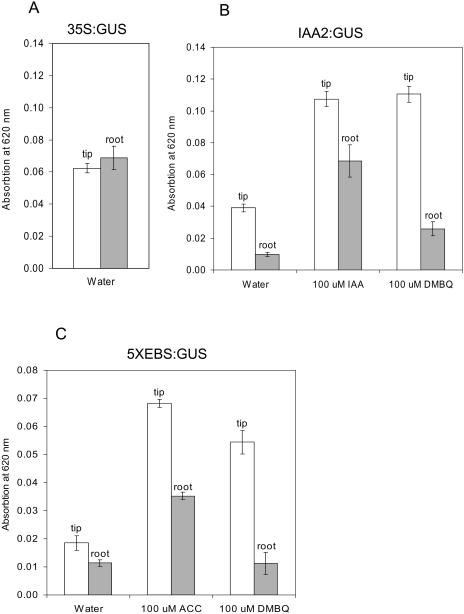
Seedlings infiltrated with the IAA2∷GUS reporter and exposed to water showed relatively more GUS staining in root tips when compared to whole roots (Figs. 6 and 7B). When transformed roots were exposed to exogenous IAA, there was roughly a 3-fold increase in GUS activity in both root tips and whole roots. DMBQ treatments lead to similar levels of GUS expression in root tips, but there was significantly less expression in whole roots than with IAA. This suggested that auxin accumulation in response to DMBQ was preferentially localized to root tips.
Figure 7.
Graphs of hormone-responsive promoters in T. versicolor roots. The GUS precipitate was extracted from root tips (2 mm) or whole roots (8 mm) following transformation and exposure to the hormone or HIF and measured in a spectrophotometer. Graphs show the means and sd from three measurements of 110 roots each. Absorbance values are normalized for the amount of tissue. A, GUS levels in roots and root tips of T. versicolor transformed with the 35S:GUS construction. B, GUS levels in roots and tips of IAA2:GUS transformants after treatment with water, IAA, or DMBQ. C, GUS in roots and shoots of 5 × EBS:GUS transformants after treatment with water, ACC, or DMBQ.
Roots transiently expressing the ethylene-responsive reporter 5×EBS∷GUS weakly expressed GUS in both whole roots and root tips after water treatment (Figs. 6 and 7C). Treatment with ACC resulted in increased GUS expression throughout the root and tip. When transformed roots were exposed to DMBQ, GUS expression was enhanced in root tips relative to the rest of the root (Fig. 7C). This was similar to what was observed with the IAA2:GUS construction.
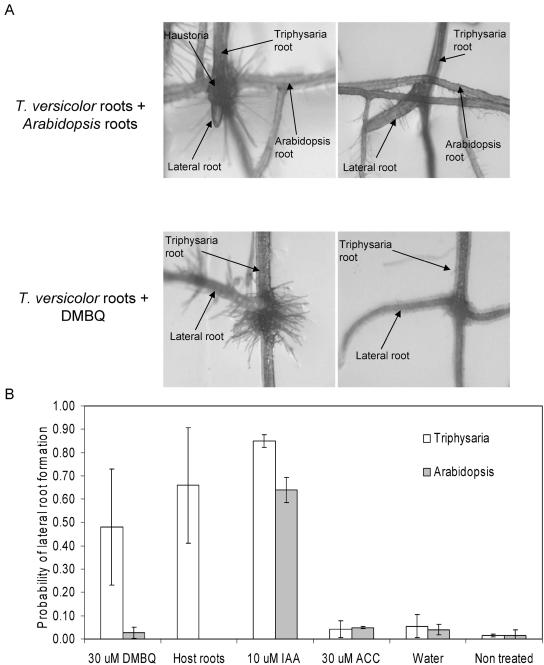
The localized accumulation of auxin at haustorium initiation sites is consistent with the observation that lateral roots frequently emerged from these sites. As seen in Figure 8, lateral roots were frequently observed originating at haustoria initiation sites. Lateral roots were induced at haustoria initiation sites by both DMBQ as well as Arabidopsis (host) roots. Laterals emerged at the point of contact with Arabidopsis even without invasion of the host root (top right photo). This was T. versicolor specific, and under the same conditions Arabidopsis did not develop lateral roots.
Figure 8.
Lateral root formation at haustoria initiation sites. A, T. versicolor seedlings growing on agar plates were exposed to Arabidopsis roots or DMBQ. The top photos show lateral roots developing in T. versicolor at the site of haustorium initiation. Lateral roots formed from sites of fully mature (top left) as well as unattached haustoria (top right). Lateral roots also emerged at the site of haustoria induced by DMBQ (bottom left and right). B, T. versicolor or Arabidopsis roots were exposed to DMBQ, host (Arabidopsis) roots, IAA, ACC, or water. The frequency of lateral roots emerging at haustoria initiation sites (or the corresponding position on Arabidopsis roots) was determined. Values are means and sd from three experiments.
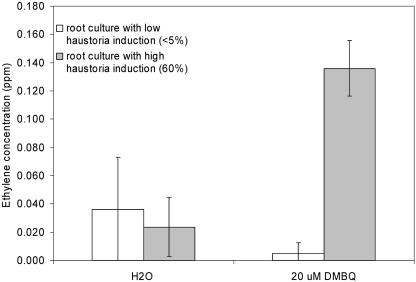
Ethylene Production Is Associated with Early Haustorium Development
Ethylene production in T. versicolor root cultures that were exposed to DMBQ or water was directly measured in the void space of the cultures. Two root cultures were compared; one was recently subcultured and formed haustoria at a high frequency (approximately 60% of tips form haustoria). The second root culture was older and had little competence to form haustoria (<5% of tips form haustoria). Both root cultures produced roughly equal amounts of ethylene over the time course of the experiment when maintained in water (Fig. 9). However when exposed to DMBQ, the concentration of ethylene increased in the haustorium-competent root cultures about 6-fold compared to the water treatment. In contrast, when DMBQ was added to the haustorium nonforming culture, ethylene levels did not increase. This experiment associates ethylene production with early haustorium development.
Figure 9.
Ethylene production during early haustorium formation. Root tips from haustorium forming and nonforming root cultures were treated with either 20 μm DMBQ or water and ethylene measured after 5 d. Values are means with sd from three experiments.
DISCUSSION
The localized accumulation of auxin is a necessary component of probably all organogenic events in plants, including the establishment of cellular polarity and asymmetric cell divisions (Benková et al., 2003; Friml et al., 2003). In roots, auxin and ethylene function together as key regulators of root morphogenesis (Casson and Lindsey, 2003). We demonstrated here that haustorium organogenesis in parasitic plants is closely associated with the accumulation of auxin and ethylene at the parasite root tip soon after exposure to haustoria-inducing factors.
Inhibition of auxin transport or ethylene biosynthesis reduces haustorium development, and these deficiencies are overcome by exogenous application of the appropriate hormone. Transcriptional regulation of auxin and ethylene-responsive promoters by exogenous DMBQ suggested that DMBQ triggered the accumulation and/or production of these hormones in T. versicolor root tips.
Exogenous auxin enhances haustorium development. Enhancement with 0.2 μm IAA was observed at low DMBQ concentrations when the proportion of root tips forming haustoria was suboptimal. Higher IAA concentrations inhibited haustorium initiation (Fig. 2A). This might explain the observation that NAA and IAA inhibit haustorium induction in Striga (Keyes et al., 2000). Alternatively, the primary haustoria of Striga may be differentially sensitive to auxin compared to the lateral haustoria produced by T. versicolor.
Dissection experiments demonstrated that the auxin that accumulates at the haustorium initiation site is derived at least in part from proximal root tissues. T. versicolor root tips dissected 3 h after HIF exposure were competent to form haustoria while those dissected before HIF exposure were not. This shows that haustorium development requires a plant factor that is transported to the haustoria initiation site within 3 h of exposure. Exogenous IAA compensated for the factor transported to the tips, suggesting that the factor missing from the dissected root tips was auxin. Auxin is known to be actively transported to root tips from growing shoots (Lomax et al., 1995). We propose that the auxin accumulates at haustorium initials as a result of a block in polar auxin transport.
Hormone fluxes alter root morphologies in response to rhizosphere challenges ( Hirsch et al., 1989; Dubrovsky et al., 2000; Goverse et al., 2000; Himanen et al., 2002). Haustoria are root organs that develop in response to the challenge of finding appropriate host roots. Preattachment haustoria look morphologically similar to determinant nodules induced by rhizobium but their developmental ontogenies are distinct. The initiation of cell division in the root cortex is a primary event in early nodule organogenesis and nodule primordia are comprised of actively dividing cells. Cell enlargement contributes little to the bulk of the mature nodule (Hirsch, 1992). In contrast, the primary mechanism for haustorial swelling is the isodiametric expansion of root cells. In Agalinis cellular division was not detected until 12 to 18 h after HIF exposure, well past when the swollen haustorium was capable of host attachment (Riopel and Musselman, 1979; Riopel and Timko, 1995). Haustorium development is also distinct from lateral root development since there is no rupture of the parasite epidermis. Anatomical studies show that distinct files of epidermal and subepidermal cells can be followed around the full haustorium (Heide-Jorgensen and Kuijt, 1995; Dubrovsky et al., 2000).
It has been known for some time that auxin levels are enhanced in rhizobium-induced nodules and that the application of auxin transport inhibitors induces nodulation or pseudonodulation (Badenoch-Jones et al., 1984; Ridge et al., 1992; Hirsch et al., 1997). Auxin-responsive GUS sensors showed an increase in auxin between 24 and 48 h after rhizobia inoculation (Mathesius et al., 1998). In our experiments described here, GUS sensors detected auxin accumulation in T. versicolor by 3 h after DMBQ induction. In nodulation, the accumulating auxin is proposed to first stimulate and later inhibit cell division, depending on the resultant auxin/cytokinin ratio (Ferguson and Mathesius, 2003). A reverse cycle seems to occur in the parasites; first cell division is inhibited and later stimulated.
We hypothesize that in root parasites, HIF exposure leads to a local block of acropetal auxin efflux that subsequently arrests cell division and promotes ethylene biosynthesis and cell expansion. Some flavonoids are known to block auxin efflux (Brown et al., 2001) and HIFs taken up by the parasite roots might directly act as auxin transport inhibitors. Alternative hypotheses for auxin accumulation include its release from inactive auxin conjugates or localized biosynthesis, though these models are not supported by the observation that blocking efflux of terminally localized auxin by local application of TIBA to root tips formed haustoria-like structures. In any case, auxin action induces ethylene biosynthesis, and the two hormones in cooperation initiate epidermal hair proliferation and radial expansion (Takahashi et al., 2003). Localized auxin and ethylene at haustorium initials also stimulates lateral root development, resulting in the frequent emergence of lateral roots from haustoria sites.
These experiments show that T. versicolor uses existing plant regulatory mechanisms for realizing early haustorium development. Auxin- and ethylene-regulatory pathways have repeatedly been recruited in the association between roots and other organisms; this has now been extended to at least one association between roots of different plants. The genetic determinants that distinguish parasitic from nonparasitic plants have yet to be identified but presumably function at a stage prior to hormone action.
MATERIALS AND METHODS
Chemicals and Media
The auxins IAA; 2,4-D; NAA; and IBA were purchased from Sigma (St. Louis). The IAA was further purified for some experiments by HPLC chromatography using C18 column and methanol/acetic acid gradient. The polar auxin transport inhibitor TIBA, the auxin action inhibitor PCIB, ethylene precursor ACC, and ethylene biosynthesis inhibitor AVG-Gly hydrochloride (AVG-HCl) was obtained from Aldrich (Milwaukee, WI). N,N-dimethylformamide was obtained from Sigma (Steinheim, Germany). DMBQ was purchased from Pfaltz and Bauer (Waterbury, CT) and X-Glu (cyclohexylammonium salt) from Gold Biotechnology (St. Louis).
Plasmids
The T-DNA vector pCAMBIA 1305.2 was obtained from CAMBIA (Canberra, Australia). This T-DNA vector contains a hygromycin resistance gene as well as the Staphylococcus GUSPlus reporter gene driven by a cauliflower mosaic virus 35S promoter. A catalase intron interrupts the GUS-coding sequence to ensure expression only after transfer into the plant cell (Roberts et al., 2004). The plasmid harboring IAA2:GUS fusion is described (Swarup et al., 2001). The ethylene-responsive reporter 5 × EBS:GUS-A was generously obtained from Prof. Joseph R. Ecker (The Salk Institute, San Diego).
Plants and Seeds
Triphysaria is a broad host range, facultative parasite that grows as an annual wildflower in coastal and grassland stands along the Pacific Coast (Hickman, 1993). Triphysaria versicolor seeds were collected from mature plants growing in a grassland cow pasture near Napa, California. T. versicolor seeds were surface sterilized with 50% bleach before germinating at 16°C on agar plates containing one-fourth strength Hoagland medium (Jamison and Yoder, 2001).
Arabidopsis (Arabidopsis thaliana ecotype Columbia) seeds were obtained from Lehle Seeds (Round Rock, TX).
Root Cultures
T. versicolor root cultures were derived from decapitated seedlings incubated in Hoagland medium supplemented with 2% (w/v) Glc under an auxin step-down protocol (Tomilov et al., 2004). The protocol consists of incubating roots in media containing 23 μm IAA for 2 to 3 d followed by culture in auxin-free media for a month. T. versicolor root cultures remain healthy and vigorous for at least 1 year, during which time they are able to form functional haustoria that are capable of invading host roots (Tomilov et al., 2004).
Root cultures are most primed to form haustoria within 2 weeks of transfer into hormone-free media. After about a month in hormone-free media, root cultures essentially loose haustoria competence. These were used as low-haustoria-responding roots in experiments described in this manuscript.
Haustorium Assays
The assay of haustorium development has been described in detail elsewhere (Jamison and Yoder, 2001). In brief, sterile T. versicolor seedlings were grown along the surface of an agar plate positioned at a near-vertical angle. Haustorial inducers or inhibitors were added to the roots and the plates kept at a horizontal position for 2 h. Plates were returned to the vertical and haustoria were scored with a dissecting microscope 24 h later. The number of plants with haustoria or the number of roots with haustoria was scored.
Root tips were locally exposed to different molecules by growing the root from basal media into media containing the test factor. To do this, T. versicolor and Arabidopsis were germinated on agar medium and 1- to 2-week-old seedlings placed on agar solidified on a microscope slide. These slides were then placed on the surface of agar medium containing the test factors and oriented such that the roots grew down along the surface of the agar. Root tips were locally exposed to test factor when the roots crossed into the test agar.
Transient Expression in T. versicolor Roots
Agrobacterium tumefaciens C58C1 bearing T-DNA derived plasmids were vacuum infiltrated into T. versicolor seedlings as described below. The A. tumefaciens contained one of the three plasmids: pCAMBIA 1305.2 (CAMBIA), IAA2:GUS fusion in pBI121 (Swarup et al., 2001), or 5×EBS:GUSA fusion in pCAMBIA (A. Stepanova and J. Ecker, unpublished data). The bacterial marker in all cases was kanamycin resistance.
Agrobacteria were grown 48 h in liquid culture at 28°C with agitation at 350 rpm in media supplemented with kanamycin (50 mg/L) and tetracycline (5 mg/L). The bacteria were pelleted by centrifugation and suspended in Murashige and Skoog media at an OD600 of 0.5. The cultures were returned to the shaker for an additional hour before infiltration.
T. versicolor seeds were germinated and seedlings maintained in the dark for 3 months at 2°C. One gram of sterile, etiolated seedlings (approximately 1,000 seedlings) were placed into a 10-mL syringe together with 2 mL of A. tumefaciens suspension. A vacuum was applied by hand after which the suspension was incubated at room temperature for 1 h with gentle agitation (Tadeusz Wroblewski, University of California, Davis, personal communication). T. versicolor explants were then transferred into fresh Murashige and Skoog media for 2 d. The etiolated seedlings were less susceptible to exogenous factors so higher concentrations (100 μm) of inducers and hormones were applied.
GUS activity was histochemically stained and visualized (Jefferson et al., 1987). The GUS precipitate was also quantified spectrophotometrically. Two-millimeter root tips were excised from transformed plants and assayed separately from the rest of the root (8 mm). Root and root tip tissues were dehydrated with ethanol and then air dried. The blue colored precipitate was extracted from the tissues three times with 300 μL N-dimethylformamide. The A620 was measured on a Beckman DU Series 600 Spectrophotometer (Beckman Instruments, Fullerton, CA). The extracts were stable for 1 week.
Ethylene Measurements
Ethylene was measured by gas chromatography as described by Grossmann and Kwiatkowski (1995) with the following modifications: T. versicolor root cultures in Hoagland media were treated with DMBQ and sealed in a 5-cm petri dish at 25°C for 5 d in the dark. After incubation, 1 mL was removed and injected into a Carle Series 100 gas chromatograph (AGC, Tulsa, OK) equipped with a 60/80 mesh aluminum column and a flame ionization detector for the quantitative determination of ethylene. The instrument's oven temperature and the injector/detector temperatures were at a constant 80°C. Flow rates of N2 were 90 mL min−1 and of H2 75 mL min−1. Injection of 1 mL of a 1.1 μL L−1 ethylene in air standard was used to calibrate the gas chromatograph.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. Denneal Jamison (California State University, Sacramento) and Russell Reagan (University of California, Davis) for helpful discussion and comments. We also thank Drs. Lars W.J. Anderson and Doreen Gee (U.S. Department of Agriculture-Agricultural Research Service Exotic and Invasive Weed Research, Davis, CA) for help with HPLC purification of TIBA and auxins, Tadeusz Wroblewski (University of California, Davis) for consultations with transient transformation of seedlings, and Prof. Joseph R. Ecker (The Salk Institute) for the 5×EBS:GUS-A fusion plasmid.
This work was supported by the National Science Foundation (grant no. 0236545).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057836.
References
- Aeschbacher RR, Schiefelbein JW, Benfey PN (1994) The genetic and molecular basis of root development. Annu Rev Plant Physiol Plant Mol Biol 45: 25–45 [Google Scholar]
- Albrecht H, Yoder JI, Phillips DA (1999) Flavonoids promote haustoria formation in the root parasite Triphysaria. Plant Physiol 119: 585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Atsatt PR (1973) Parasitic flowering plants: How did they evolve? Am Nat 107: 502–510 [Google Scholar]
- Atsatt PR, Hearn TF, Nelson RL, Heineman RT (1978) Chemical induction and repression of haustoria in Orthocarpus purpurascens (Scophulariaceae). Ann Bot 42: 1177–1184 [Google Scholar]
- Atsatt PR, Musselman L (1977) Surface characteristics of roots and haustoria of Orthocarpus purpurascens. Beitr Biol Pflanz 53: 359–370 [Google Scholar]
- Badenoch-Jones J, Summons R, Rolfe B, Letham D (1984) Phytohormones, Rhizobium mutants, and nodulation in legumes: auxin metabolites in pea root nodules. J Plant Growth Regul 3: 23–39 [Google Scholar]
- Baird WV, Riopel JL (1984) Experimental studies of haustorium initiation and early development in Agalinis purpurea (L.) Raf. (Scrophulariaceae). Am J Bot 71: 803–814 [Google Scholar]
- Baird WV, Riopel JL (1985) Surface characteristics of root haustorial hairs of parasitic Scrophulariaceae. Bot Gaz 146: 63–69 [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Lindsey K (2003) Genes and signaling in root development. New Phytol 158: 11–38 [Google Scholar]
- Chang M, Lynn DG (1986) The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol 12: 561–579 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colon-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Mathesius U (2003) Signaling interactions during nodule development. J Plant Growth Regul 22: 47–72 [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Juergens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Juergens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact 13: 1121–1129 [DOI] [PubMed] [Google Scholar]
- Grossmann K, Kwiatkowski J (1995) Evidence of a causative role of cyanide, derived from ethylene biosynthesis, in the herbicidal mode of action of quinclorac in barnyard grass. Pestic Biochem Physiol 51: 150–160 [Google Scholar]
- Heide-Jorgensen HS, Kuijt J (1995) The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am J Bot 82: 782–797 [Google Scholar]
- Hickman JC (1993) The Jepson Manual: Higher Plants of California. University of California Press, Berkeley, CA
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inze D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122: 211–237 [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari TV, Torrey JG, Bisseling T (1989) Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA 86: 1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, Fang Y, Asad S, Kapulnik Y (1997) The role of phytohormones in plant-microbe symbioses. Plant Soil 194: 171–184 [Google Scholar]
- Jamison DS, Yoder JI (2001) Heritable variation in quinone-induced haustorium development in the parasitic plant Triphysaria. Plant Physiol 125: 1870–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and verstile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes WJ, O'Malley RC, Kim D, Lynn DG (2000) Signaling organogenesis in parasitic angiosperms: xenognosin generation, perception, and response. J Plant Growth Regul V19: 217–231 [DOI] [PubMed] [Google Scholar]
- Kuijt J (1969) The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA
- Leake JR (1994) The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol 127: 171–216 [DOI] [PubMed] [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277: 20446–20452 [DOI] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509–530
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Bayliss C, Weinman JJ, Schlaman HRM, Spaink HP, Rolfe BG, McCully ME, Djordjevic MA (1998) Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol Plant Microbe Interact 11: 1223–1232 [Google Scholar]
- Matvienko M, Torres MJ, Yoder JI (2001) Transcriptional responses in the hemiparasitic plant Triphysaria versicolor to host plant signals. Plant Physiol 127: 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickrent D (2003) Parasitic Plant Connection. Southern Illinois University at Carbondale. http://www.science.siu.edu/parasitic-plants/DanNick.html (June 1, 2004)
- Nickrent DL, Musselman LJ, Riopel JL, Eplee RE (1979) Haustorial initiation and non-host penetration in witchweed (Striga asiatica). Ann Bot 43: 233–236 [Google Scholar]
- Okonkwo SNC, Nwoke FIO (1978) Initiation, development and structure of the primary haustorium in Striga gesnerioides (Scrophulariaceae). Ann Bot 42: 455–463 [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria E, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 133: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press MC, Graves JD, editors (1995) Parasitic Plants. Chapman and Hall, London
- Ridge R, Bender G, Rolfe B (1992) Nodule like structures induced on roots of wheat seedlings by addition of the synthetic auxin 2,4-dichlorophenoxyacetic acid and the effects of microorganisms. Aust J Plant Physiol 19: 481–492 [Google Scholar]
- Riopel J, Musselman L (1979) Experimental initiation of haustoria in Agalinis purpurea. Am J Bot 66: 570–575 [Google Scholar]
- Riopel JL, Baird WV (1987) Morphogenesis of the early development of primary haustoria in Striga asiatica. In LJ Musselman, ed, Parasitic Weeds in Agriculture, Vol 1. CRC Press, Boca Raton, FL, pp 107–125
- Riopel JL, Timko MP (1995) Haustorial initiation and differentiation. In MC Press, JD Graves, eds, Parasitic Plants. Chapman and Hall, London, pp 39–79
- Roberts C, Rajagopal S, Smith L, Nguyen T, Yang W, Nugroho S, Ravi K, Vijayachandra K, Harcourt R, Dransfield L, et al (2004) A comprehensive set of modular vectors for advanced manipulations and efficient transformation of plants by both Agrobacterium and direct DNA uptake methods. CAMBIA, Canberra, ACT, Australia. http://www.cambia.org/main/r_resfoc.htm (June 1, 2004)
- Schneider K, Wells B, Dolan L, Roberts K (1997) Structural and genetic analysis of epidermal cell differentiation in Arabidopsis primary roots. Development 124: 1789–1798 [DOI] [PubMed] [Google Scholar]
- Steffens JC, Lynn DG, Kamat VS, Riopel JL (1982) Molecular specificity of haustorial induction in Agalinis purpurea (L.) Raf. (Scrophulariaceae). Ann Bot 50: 1–7 [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kawahara A, Inoue Y (2003) Ethylene promotes the induction by auxin of the cortical microtubule randomization required for low-pH-induced root hair initiation in lettuce (Lactuca sativa L.) seedlings. Plant Cell Physiol 44: 932–940 [DOI] [PubMed] [Google Scholar]
- Tomilov A, Tomilova N, Yoder JI (2004) In vitro haustorium development in roots and root cultures on the hemiparasitic plant Triphysaria versicolor. Plant Cell Tissue Organ Cult 77: 257–265 [Google Scholar]
- Wolf SJ, Timko MP (1991) In vitro root culture: a novel approach to study the obligate parasite Striga asiatica (L.) Kuntze. Plant Sci 73: 233–242 [Google Scholar]