Abstract
Nonphotochemical quenching (NPQ) of excitation energy is a well-established phenomenon in green plants, where it serves to protect the photosynthetic apparatus from photodamage under excess illumination. The induction of NPQ involves a change in the function of the light-harvesting apparatus, with the formation of quenching centers that convert excitation energy into heat. Recently, a comparable phenomenon was demonstrated in cyanobacteria grown under iron-starvation. Under these conditions, an additional integral membrane chlorophyll-protein, IsiA, is synthesized, and it is therefore likely that IsiA is required for NPQ in cyanobacteria. We have previously used fluorescence recovery after photobleaching to show that phycobilisomes diffuse rapidly on the membrane surface, but are immobilized when cells are immersed in high-osmotic strength buffers, apparently because the interaction between phycobilisomes and reaction centers is stabilized. Here, we show that when cells of the cyanobacterium Synechocystis sp. PCC 6803 subjected to prolonged iron-deprivation are immersed in 1 m phosphate buffer, NPQ can still be induced as normal by high light. However, the formation of the quenched state is irreversible under these conditions, suggesting that it involves the coupling of free phycobilisomes to an integral-membrane complex, an interaction that is stabilized by 1 m phosphate. Fluorescence spectra are consistent with this idea. Fluorescence recovery after photobleaching measurements confirm that the induction of NPQ in the presence of 1 m phosphate is accompanied by immobilization of the phycobilisomes. We propose as a working hypothesis that a major component of the fluorescence quenching observed in iron-starved cyanobacteria arises from the coupling of free phycobilisomes to IsiA.
Photosynthetic organisms have various ways of adjusting the function of their photosynthetic apparatus in response to changing light conditions. One such response has been termed nonphotochemical quenching (NPQ). NPQ is normally induced under bright illumination, and involves the dissipation of excess excitation energy as heat (for review, see Horton et al., 1996; Holt et al., 2004). It can most easily be observed as a decrease in fluorescence from chlorophyll a in response to excess illumination. NPQ has been intensively studied in green plants, where the current consensus is that it is triggered by excess acidification of the thylakoid lumen and involves the formation of quenching centers in the light-harvesting antenna. NPQ is promoted by the deepoxidation of violaxanthin to zeaxanthin in the light-harvesting antenna, which increases the rate of thermal dissipation of excitation energy. The PsbS protein of PSII is specifically involved in NPQ (Li et al., 2000).
The photosynthetic apparatus of cyanobacteria differs significantly from that of green plants. In cyanobacteria grown under standard conditions, there is no accessory chlorophyll-protein antenna. The main accessory light-harvesting complexes of cyanobacteria are the phycobilisomes, large, highly structured assemblies of phycobiliproteins associated with the cytoplasmic surface of the thylakoid membrane (Grossman et al., 1993; MacColl, 1998). However, growth of cyanobacteria under conditions of iron-starvation or oxidative stress induces the synthesis of IsiA, an integral-membrane chlorophyll-protein (Burnap et al., 1993; Li et al., 2004). IsiA forms complexes with PSI, where it acts as an additional light-harvesting antenna (Bibby et al., 2001; Boekema et al., 2001). There are also indications that IsiA may interact with PSII (Sandström et al., 2001). There is a significant additional pool of IsiA that appears to be free in the membrane, where it is able to diffuse (Yeremenko et al., 2004; Sarcina and Mullineaux, 2004).
When IsiA is present, fluorescence measurements show a phenomenon that at least superficially resembles NPQ in green plants. Exposure of cells to bright light leads to strong quenching of fluorescence. The quenching occurs on a timescale of a few minutes and is reversible (Cadoret et al., 2004; Bailey et al., 2005). The quenching can involve both the variable fluorescence (Fv) and the minimal fluorescence (F0), but F0 quenching only becomes prominent after prolonged iron-starvation (Cadoret et al., 2004; Bailey et al., 2005). Unlike NPQ in green plants, the cyanobacterial NPQ appears to be induced specifically by blue light (Cadoret et al., 2004) and appears not to be regulated by lumenal pH, since it is not inhibited by uncouplers (Cadoret et al., 2004; Bailey et al., 2005). Fluorescence quenching of phycobilisomes induced by blue light was recently reported in a PSII-deficient mutant (Rakhimberdieva et al., 2004) and this may be a related phenomenon. A very limited blue-light-induced quenching can be observed in iron-replete cells (El Bissati et al., 2000), but NPQ becomes much more significant in iron-starved cells, suggesting the involvement of IsiA (Cadoret et al., 2004).
We have previously used fluorescence recovery after photobleaching (FRAP) in the cyanobacterium Synechococcus sp. PCC7942 to investigate a connection between phycobilisome diffusion and state transitions in cyanobacteria (Joshua and Mullineaux, 2004). State transitions are a physiological adaptation mechanism that changes the distribution of energy absorbed by the phycobilisomes between PSII and PSI (van Thor et al., 1998). When cells are immersed in high-osmotic strength buffers (e.g. 0.5 m phosphate or 1 m Suc), state transitions are inhibited, and cells are locked into whichever state they were adapted prior to addition of the buffer (Mullineaux, 1993; Joshua and Mullineaux, 2004). FRAP measurements show that the phycobilisomes normally diffuse rapidly on the surface of the thylakoid membrane (Mullineaux et al., 1997; Sarcina et al., 2001), but in high-osmotic strength buffers they are immobilized (Joshua and Mullineaux, 2004). This suggests that under these conditions the interaction between phycobilisomes and reaction centers is stabilized. Phycobilisomes become very stably coupled to reaction centers, and this prevents phycobilisome diffusion and also prevents the redistribution of phycobilisomes between PSII and PSI (Joshua and Mullineaux, 2004).
Here, we show that the NPQ seen in cells of the cyanobacterium Synechocystis sp. PCC6803 after prolonged iron-deprivation is also strongly affected by a high-osmotic strength buffer, suggesting a role for phycobilisome diffusion in the induction of NPQ. As a working hypothesis, we propose that the major part of the F0 fluorescence quenching results from the coupling of free phycobilisomes to IsiA, an interpretation that is consistent both with fluorescence spectra and FRAP measurements of phycobilisome diffusion.
RESULTS
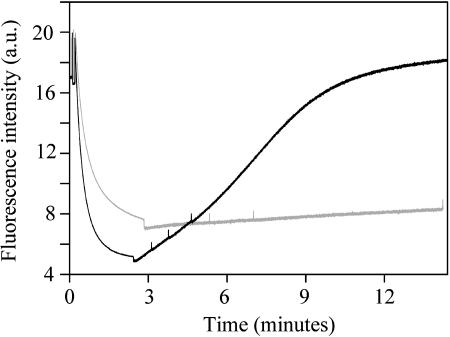
It has previously been shown that cells of Synechocystis 6803 grown under conditions of iron-starvation exhibit fluorescence quenching induced by high light (Cadoret et al., 2004; Bailey et al., 2005). The effect somewhat resembles NPQ in green plants. In the early stages of iron deprivation, the quenching is mainly of Fv (Cadoret et al., 2004; Bailey et al., 2005). After prolonged iron-starvation, there is strong quenching of F0 (Bailey et al., 2005). This effect is shown in a modulated fluorescence trace (Fig. 1). When cells are in growth medium, the fluorescence quenching is fully reversible in dim light, on a timescale of 10 to 15 min (Fig. 1).
Figure 1.
Fluorescence quenching induced by high light in iron-starved cells of Synechocystis 6803. Black line, cells in growth medium. Gray line, cells in 1 m phosphate buffer. Saturating white light was applied at the start of the measurement and switched off after 2.5 to 3 min, indicated by a dip in the trace. Spikes in the trace result from brief saturating light pulses used to monitor Fv.
Because high-osmotic strength buffers have previously been shown to inhibit state transitions (Joshua and Mullineaux, 2004), we tested the effect of immersing cells in 1 m KH2PO4/K2HPO4, pH 6.8. Phosphate buffer at this concentration would be expected to strongly stabilize phycobilisome-reaction center interaction and inhibit any adaptation processes involving redistribution of phycobilisomes (Joshua and Mullineaux, 2004). We found that the Fv quenching seen in the initial stages of iron deprivation was unaffected by 1 m phosphate buffer (data not shown). However, in cells subject to prolonged iron deprivation and exhibiting strong quenching of F0, there was a striking effect of 1 m phosphate buffer. We found that under these conditions, the quenching could be induced in essentially the same way in 1 m phosphate as in growth medium. However, in 1 m phosphate, the fluorescence quenching was almost irreversible, with little recovery of fluorescence in dim light (Fig. 1).
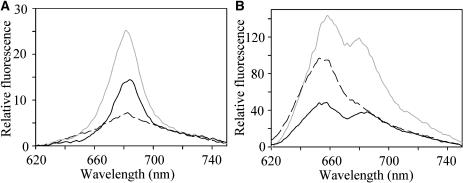
The iron-starved cells showed no loss of phycobilisomes as compared to iron-replete cells. Based on absorption spectra (not shown) deconvoluted according to the formulae of Myers et al. (1980), the ratio of phycocyanin to chlorophyll was 0.38 in iron-replete cells and 0.45 in iron-starved cells, and amounts of phycocyanin per cell were similar. We have used room temperature and 77 K fluorescence spectra to investigate the light-harvesting properties of iron-starved cells of Synechocystis 6803. Figure 2 shows room temperature fluorescence spectra for Synechocystis 6803 cells, obtained with chlorophyll excitation at 435 nm (Fig. 2A) or phycocyanin excitation at 600 nm (Fig. 2B). With chlorophyll excitation, there is a single chlorophyll α fluorescence peak at 685 nm (Fig. 2A). Fluorescence from iron-stressed cells is much higher than from iron-replete cells at the same chlorophyll concentration (Fig. 2A). With phycocyanin excitation, there is a peak at about 650 nm from phycocyanin, and a second peak at about 685 nm that may come from the chlorophyll α of PSII or IsiA (Bibby et al., 2001) or from the long-wavelength phycobilins in the phycobilisome core (Glazer, 1984). The 685-nm peak is considerably more prominent in iron-stressed cells than in iron-replete cells (Fig. 2B). Exposure to bright light leads to fluorescence quenching in iron-stressed cells, both with chlorophyll excitation (Fig. 2A) and with phycocyanin excitation (Fig. 2B), although the effect with phycocyanin excitation is larger (about 63% quenching as compared to 39% quenching with chlorophyll excitation). The larger effect with phycobilisome excitation suggests significant involvement of the phycobilisomes in the formation of the quenching state. Exposure of iron-replete cells to the same bright light led to only minor quenching of fluorescence (not shown), confirming previous reports that NPQ in cyanobacteria is significant only after iron-deprivation (Cadoret et al., 2004; Bailey et al., 2005). In iron-deprived cells, exposure to bright white light in the presence of 1 m phosphate led to changes in the fluorescence spectra (not shown) similar to those seen for iron-deprived cells in growth medium (Fig. 2).
Figure 2.
Room temperature fluorescence emission spectra for cells of Synechocystis 6803. Gray line, iron-starved cells after dark-adaptation. Black line, iron starved cells after induction of NPQ. Broken line, iron-replete cells, dark adapted. A, Excitation of chlorophyll a at 435 nm. B, Excitation of phycocyanin at 600 nm.
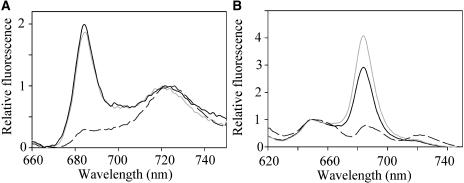
More detailed information on light-harvesting processes can be obtained from fluorescence spectra on frozen samples at 77 K. With chlorophyll excitation (Fig. 3A), there is a very sharp peak at about 685 nm that is characteristic of IsiA (Bibby et al., 2001). This peak is not seen in iron-replete cells, which show only peaks at 725 nm from PSI and much lower fluorescence at 685 to 695 nm from PSII (Fig. 3A; Mullineaux, 1994). With phycocyanin excitation, there is also very high fluorescence at about 685 nm, which is not seen in iron-replete cells (Fig. 3B). Exposure of cells to high light prior to freezing does not lead to a significant change in the shape of the fluorescence spectrum with chlorophyll excitation (Fig. 3A), but with phycocyanin excitation there is specific quenching of the peak at 685 nm (Fig. 3B). Fluorescence spectra recorded for cells in 1 m phosphate are similar to those for cells in growth medium (not shown). At 77 K, both the chlorophyll a of IsiA and the long-wavelength phycobilins of the phycobilisome core fluoresce at about 685 nm (Glazer, 1984; Bibby et al., 2001). Cells of Synechocystis 6803 lacking all reaction centers show high phycobilin fluorescence at 685 nm (Yu et al., 1999). Thus, the high 685-nm fluorescence in iron-starved cells (Fig. 3B) could indicate either a population of free, energetically uncoupled phycobilisomes, or energy transfer from phycobilisomes to IsiA, or both. The NPQ-type fluorescence quenching seen with chlorophyll excitation could result from structural changes within IsiA, as previously suggested (Cadoret et al., 2004). However, the larger quenching seen when the phycobilisomes are excited (Fig. 2) suggests that at least part of the NPQ involves the phycobilisomes. One possibility is that the fluorescence quenching results from the coupling of free phycobilisomes to IsiA. We investigated this possibility by looking at the effects of NPQ induction on the mobility of phycobilisomes in the presence of 1 m phosphate.
Figure 3.
Fluorescence emission spectra at 77 K for cells of Synechocystis 6803. Gray line, iron-starved cells after dark-adaptation. Black line, iron starved cells after induction of NPQ. Broken line, iron-replete cells, dark adapted. A, Excitation of chlorophyll a at 435 nm. Spectra are normalized to the PSI peak at 725 nm. B, Excitation of phycocyanin at 600 nm. Spectra are normalized to the phycocyanin peak at 650 nm.
We have previously shown that immersing iron-replete cells of Synechococcus 7942 in high-osmotic strength buffers prevents state transitions and the diffusion of phycobilisomes, apparently by locking the phycobilisomes onto PSII or PSI reaction centers (Joshua and Mullineaux, 2004). Thus, the phosphate effect shown in Figure 1 suggests that there may be a role for phycobilisome movement in NPQ as well as in state transitions. We used FRAP to directly probe the mobility of phycobilisomes in cells of Synechocystis 6803 under different conditions. We used Synechocystis 6803 rather than Synechococcus 7942 for this study because the quenching state can be induced much more reliably in Synechocystis; Synechococcus tends not to survive prolonged iron-starvation.
Synechococcus 7942 is an excellent organism for FRAP because it has elongated cells and a very simple, regular thylakoid membrane structure that allows quantitative estimates of diffusion rates (Sarcina et al., 2001). By contrast, Synechocystis 6803 has spherical cells and the thylakoid membranes have an irregular, unpredictable structure, often with loops of membrane extending throughout the cytoplasm (Nilsson et al., 1992). Different laboratory strains of Synechocystis 6803 have rather different cell sizes (C.W. Mullineaux, unpublished data). We used the wild type as obtained directly from the Pasteur Culture Collection, which has relatively large cells about 3 μm in diameter. Because of the irregular thylakoid membrane structure, it is not possible to obtain quantitative estimates of diffusion coefficients from FRAP measurements on Synechocystis cells (Mullineaux and Sarcina, 2002). However, in 3-μm diameter cells, it is possible to see if a fluorescent membrane component is mobile or not and to get a rough idea of timescales.
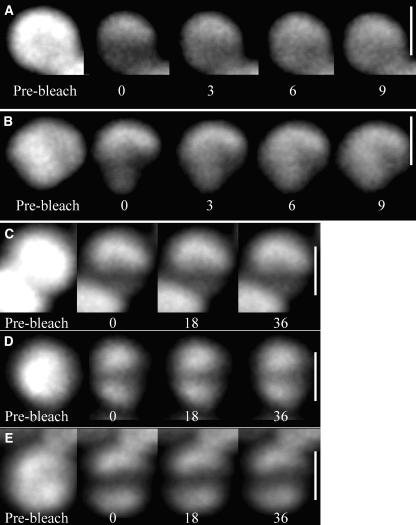
We used a laser-scanning confocal microscope with wavelength settings as previously described (Joshua and Mullineaux, 2004) to observe the diffusion of phycobilisomes in Synechocystis cells. The 633-nm laser was used to bleach phycobilisome fluorescence either in a line across the middle of the cell or at one edge of the cell. Phycobilisome fluorescence was subsequently imaged by scanning the confocal laser spot at lower power. Diffusion of phycobilisomes results in redistribution of fluorescence within the cell, with recovery of fluorescence in the bleached area. Figure 4A shows a typical FRAP image sequences for an iron-replete Synechocystis cell in growth medium. Because the cells are relatively small, the initial bleaching significantly reduces fluorescence over the entire cell, but bleaching is particularly strong in the line where the laser was scanned at high power. Phycobilisome fluorescence redistributes within a few seconds of the bleach, with essentially complete recovery of fluorescence in the strongly bleached area (Fig. 4A). This indicates that, as in other cyanobacteria (Mullineaux et al., 1997; Sarcina et al., 2001), the phycobilisomes are able to diffuse rapidly in Synechocystis cells.
Figure 4.
FRAP image sequences for cells of Synechocystis 6803. Confocal fluorescence images taken from typical FRAP sequences. Scale bar = 3 μm. Fluorescence from the phycobilisomes is imaged. After recording the prebleach image, a line was bleached across the cell by increasing the laser power and scanning the laser for about 2 s in the X-direction. The laser power was then decreased again and a series of images was recorded. For each image, the time in seconds after recording the first postbleach image is shown. A, An iron-replete cell in growth medium. B, An iron-stressed cell in growth medium. C, An iron-replete cell in 1 m phosphate. D, An iron-stressed cell in 1 m phosphate. E, An iron-stressed cell in 1 m phosphate exposed to saturating white light to induce NPQ.
The high IsiA fluorescence in iron-starved cells (Fig. 2) raises the possibility that similar FRAP measurements in iron-starved cells will monitor diffusion of IsiA as well as phycobilisomes. We therefore used data from the fluorescence spectra shown in Figure 2 to quantify the contribution made by fluorescence from IsiA directly excited by absorption from chlorophyll a. Fluorescence in the spectra shown in Figure 2 was summed for every wavelength greater than 665 nm; this approximates to the region of the spectrum that we observe in the confocal measurements. On an arbitrary scale, total fluorescence in this region from IsiA excited at 435 nm (Fig. 2A) was 1,482, whereas total fluorescence from phycobilins excited at 600 nm (Fig. 2B) was 9,847. These values were then used to calculate the fluorescence that would be observed in each case with excitation at 633 nm, using the absorption spectra of IsiA (Andrizhiyevskaya et al., 2004) and phycobilisomes of Synechocystis 6803 (C.W. Mullineaux, unpublished data). This gives calculated fluorescence values of 197 for directly excited IsiA and 1,0241 for phycobilisomes. Thus, directly excited IsiA will contribute only about 2% of the total fluorescence we observe in the confocal measurements, and we can be confident that the FRAP measurements on iron-starved cells report on phycobilisome mobility. Figure 4B shows a typical FRAP image sequence for an iron-starved Synechocystis cell. Phycobilisome diffusion occurs on similar timescales in iron-replete cells (Fig. 4A) and iron-starved cells (Fig. 4B).
We previously showed that the rate of phycobilisome diffusion in Synechococcus 7942 cells is drastically decreased when cells are immersed in high-osmotic strength buffers such as 0.5 to 1 m phosphate or 1 m Suc (Joshua and Mullineaux, 2004). We observed a similar effect in iron-replete cells of Synechocystis 6803. When cells were immersed in 1 m KH2PO4/K2HPO4, pH 6.8, there was no detectable phycobilisome diffusion on the timescale of the measurement. A typical measurement is shown in Figure 4C. Note that there is no recovery of fluorescence in the bleached zone, in contrast to the results seen when cells are in growth medium (Fig. 4A). This shows that phycobilisome diffusion can be inhibited by phosphate buffer in the same way in Synechocystis 6803 as in Synechococcus 7942. Since these buffers also prevent state transitions in Synechocystis 6803 (Joshua and Mullineaux, 2004), it is likely that phycobilisome-reaction center interactions are stabilized by high-osmotic strength buffers in the same way in Synechocystis 6803 as in Synechococcus 7942.
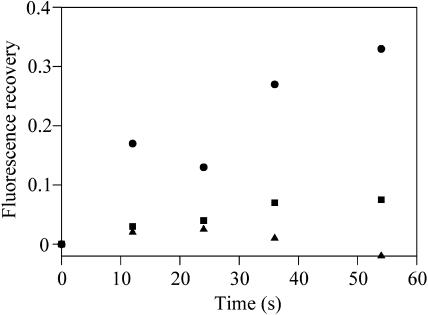
The fluorescence emission spectra for iron-starved Synechocystis 6803 cells suggest that these cells may have a population of phycobilisomes that are energetically decoupled from reaction centers (Figs. 2 and 3). If these phycobilisomes are also physically decoupled from reaction centers, we might expect that they remain mobile in phosphate buffer, since there is no phycobilisome-reaction center interaction to stabilize. Figure 4D shows a typical FRAP result for a cell from an iron-starved culture. In this case, cells were dark-adapted before mixing with phosphate buffer to keep them in the unquenched state. Exposure of the cells to the laser light prior to measurement was minimized. Since imaging and FRAP were carried out with red light at 633 nm, it is possible to visualize the cells in the unquenched state, since the cyanobacterial NPQ is induced specifically by blue light (Cadoret et al., 2004). In these cells, we observed partial recovery of the FRAP bleach over a timescale of about 60 s (Fig. 4D). In contrast to the results for cells in growth medium (Fig. 4B), recovery was clearly incomplete. Figure 5 shows quantitative data for fluorescence recovery, extracted from typical image sequences like those shown in Figure 4. Fluorescence recovery at the center of the bleach is shown. Because the initial bleach significantly decreases the total fluorescence in the cell, fluorescence at the center of the bleach will not recover to the prebleach level even if all the phycobilisomes are mobile. We therefore measured the total cell fluorescence before and after the bleach and scaled the postbleach fluorescence values accordingly. One hundred percent recovery then indicates that 100% of phycobilisome fluorescence is mobile. In iron-starved cells in phosphate buffer, the mean final fluorescence recovery in the center of the bleach was 23% ± 11%. This indicates that a fraction of the phycobilisomes, responsible on average for about 20% to 25% of the phycobilisome fluorescence from the cell, remain mobile in phosphate buffer.
Figure 5.
FRAP fluorescence recovery timecourses for phycobilisome fluorescence in cells of Synechocystis 6803 in 1 m phosphate buffer. Data is extracted from image sequences for typical cells as shown in Fig. 5. “Fluorescence recovery” is the extent to which fluorescence at the center of the bleach recovers toward the prebleach value, scaled to allow for the fact that the initial bleach significantly decreases the total fluorescence in the cell. Triangles, iron-replete cell in phosphate. Circles, iron-starved cell in phosphate. Squares, iron-starved cell in phosphate following induction of NPQ.
In phosphate buffer, the formation of the quenched state is irreversible (Fig. 1). If phycobilisome movement is important for the induction and relaxation of NPQ, we might expect that the phycobilisomes remain mobile in phosphate buffer in dark-adapted cells but are immobilized once quenching has been induced. We found this to be the case. Iron-stressed cells were mixed with 1 m phosphate buffer and adsorbed onto agar as before. The cells were then exposed to a similar treatment with bright white light as in Figure 1, before carrying out a FRAP measurement as before. Figure 4E shows a typical image sequence, with quantitative data for fluorescence recovery in Figure 5. In contrast to cells in which NPQ had not been induced (Fig. 4D), there was little recovery of the bleach over the timescale of the measurement. Similar results were obtained for cells in which NPQ was induced in liquid suspension in the presence of 1 m phosphate, before laying the cells down on agar (not shown).
DISCUSSION
The phycobilisomes of cyanobacteria are highly mobile complexes that diffuse rapidly on the surface of the thylakoid membrane (Mullineaux et al., 1997; Sarcina et al., 2001). It appears that the interaction between phycobilisomes and reaction centers is normally transient and unstable, so that a phycobilisome will frequently detach from a reaction centers, diffuse on the surface of the membrane, and reassociate with another reaction center. This allows long-range diffusion of the phycobilisomes, which can be detected by FRAP (Mullineaux et al., 1997; Sarcina et al. 2001). In contrast to the phycobilisomes, PSII reaction centers are normally immobile in the membrane (Mullineaux et al., 1997; Sarcina and Mullineaux, 2004).
State transitions in cyanobacteria are a physiological adaptation process that changes the distribution of phycobilisomes between PSII and PSI (van Thor et al., 1998). We have previously shown that cyanobacterial state transitions are inhibited by immersing cells in high-osmotic strength buffers, and that this inhibition correlates with immobilization of the phycobilisomes (Joshua and Mullineaux, 2004). These results indicate that phycobilisome diffusion is required for state transitions. It appears that high-osmotic strength buffers inhibit both state transitions and long-range phycobilisome diffusion by stabilizing the coupling of phycobilisomes to reaction centers (Joshua and Mullineaux, 2004). Based on these results, we might expect any adaptation process that depends on the redistribution of phycobilisomes to be sensitive to phosphate buffer.
Here, we have investigated the effect of phosphate buffers on NPQ, a phenomenon that in cyanobacteria becomes significant only after induction of the synthesis of the IsiA by iron-starvation (Cadoret et al., 2004; Bailey et al., 2005). The first form of NPQ to become apparent during iron deprivation affects Fv rather than F0 (Cadoret et al., 2004; Bailey et al., 2005). This form of NPQ was unaffected by 1 m phosphate, suggesting that it does not involve phycobilisome mobility. After more prolonged iron deprivation, a deep NPQ that strongly affects F0 as well as Fv becomes apparent. This form of NPQ is perturbed by 1 m phosphate buffer. Strikingly, we find that the induction of NPQ is not affected by 1 m phosphate buffer, but recovery from NPQ is completely inhibited (Fig. 1). This contrasts with the effect of phosphate buffers on state transitions, where both the state 1 transition and the state 2 transition are inhibited (Joshua and Mullineaux, 2004). However, at critical osmotic strengths (e.g. in 0.5 m Suc or 0.2–0.3 m phosphate), the transition to State 2 is inhibited much more than the transition to State 1, probably because the interaction of phycobilisomes with PSII is more easily stabilized than the interaction with PSI (Joshua and Mullineaux, 2004).
The fact that 1 m phosphate locks iron-starved cells into the quenched state suggests that in this state the phycobilisomes may be interacting with a membrane complex, an interaction that is stabilized by high-osmotic strength buffers. Fluorescence spectra with phycocyanin excitation at room temperature (Fig. 2) and at 77 K (Fig. 3) show very high fluorescence at 680 to 685 nm in dark-adapted cells. Upon induction of NPQ, the 680 to 685 nm fluorescence becomes lower, but remains high compared to iron-replete cells. This indicates that, in iron-starved cells, a proportion of the phycobilisomes are either energetically decoupled or transfer energy to IsiA. In both cases, this would result in fluorescence at 680 to 685 nm, either from the phycobilisome terminal emitters (Glazer, 1984) or from the chlorophyll a of IsiA (Bibby et al., 2001). Therefore, the two possibilities cannot be distinguished from fluorescence spectra.
If iron-starved cells contain a population of phycobilisomes that are not coupled to any membrane-integral protein complexes, we might expect that these phycobilisomes remain mobile in high-osmotic strength buffer. FRAP measurements show that this is the case. For cells in growth medium, the phycobilisomes diffuse rapidly both in iron-starved cells and in iron-replete cells (Fig. 4, A and B). However, in 1 m phosphate, the phycobilisomes are completely immobilized in iron-replete cells (Figs. 4C and 5), but in iron-starved cells a proportion of the phycobilisomes remain mobile (Figs. 4D and 5). The mobile phycobilisomes are responsible on average for about 20% to 25% of the phycobilisome fluorescence from the cell (Fig. 5). Following the induction of NPQ, phycobilisome mobility in the presence of 1 m phosphate is greatly decreased (Figs. 4E and 5).
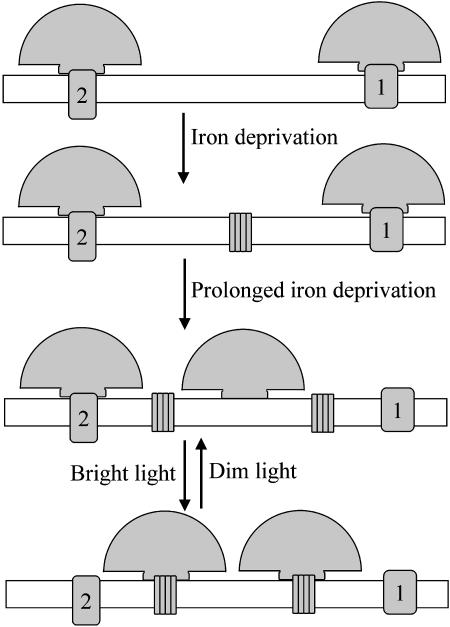
Figure 6 is a schematic illustration of the model that we propose to account for these results. Iron deprivation leads to the synthesis of IsiA (Fig. 6), which is responsible for the high, 680- to 685-nm fluorescence seen with chlorophyll excitation (Figs. 2A and 3A). After prolonged iron-deprivation, cells also contain a population of decoupled phycobilisomes (Fig. 6), responsible for the high, 680- to 685-nm fluorescence seen with phycocyanin excitation (Figs. 2B and 3B). These phycobilisomes remain mobile in phosphate buffer (Figs. 4D and 5), something that is not seen in iron-replete cells (Figs. 4C and 5). When NPQ is induced, we suggest that a signal transduction pathway triggered by bright blue light (Cadoret et al., 2004) increases the affinity of phycobilisomes for IsiA. This leads to the association of free phycobilisomes with IsiA complexes in the membrane (Fig. 6). We have no direct evidence for the association of phycobilisomes with IsiA. Because both phycobilisomes and IsiA fluoresce at 680 to 685 nm, coupling of phycobilisomes to IsiA will not lead to a shift in the fluorescence emission spectra (Figs. 2 and 3). However, our model (Fig. 6) provides a simple explanation for the observed effects of phosphate on NPQ, and the apparent requirement of IsiA for significant NPQ in cyanobacteria (Cadoret et al., 2004; Bailey et al., 2005). The induction of NPQ is not inhibited by 1 m phosphate (Fig. 1) because the decoupled phycobilisomes remain mobile under these conditions (Figs. 4D and 5). However, 1 m phosphate prevents recovery from the quenched state (Fig. 1) by effectively locking the phycobilisomes onto IsiA. The association of phycobilisomes with IsiA results in the immobilization of phycobilisomes that we observe in FRAP measurements following the induction of NPQ in the presence of phosphate (Figs. 4E and 5). Although IsiA is mobile in the membrane (Sarcina and Mullineaux, 2004) its diffusion is slow, and it is therefore likely that a stable phycobilisome-IsiA complex would be virtually immobile.
Figure 6.
Working hypothesis for the mechanism of nonphotochemical quenching of F0 in cyanobacteria. Phycobilisomes are shown as fan-shaped complexes on the thylakoid membrane surface. In iron-replete cells (top), the phycobilisomes interact with the reaction centers (1 and 2) in the membrane. Iron deprivation leads to the synthesis of IsiA, an additional chlorophyll-protein complex shown as a cluster of subunits in the membrane. After prolonged iron-deprivation, there is also a population of free phycobilisomes, neither structurally nor functionally coupled to reaction centers. Bright light induces the formation of a quenching complex between phycobilisomes and IsiA. This serves to reduce the antenna size of the reaction centers and to reduce photodamage caused by the free phycobilisomes.
The quenching of F0 induced by high light in iron-starved cells (Cadoret et al., 2004; Bailey et al., 2005) could be explained simply by the coupling of free phycobilisomes to IsiA. The intrinsic fluorescence yield of chlorophyll a is considerably lower than that of phycobilins, since chlorophyll a has a radiative lifetime of about 13 ns (Borisov and Il'ina, 1971), whereas phycobilins have radiative lifetimes of about 4 ns (Grabowski and Gantt, 1978). Therefore, a phycobilisome-IsiA complex would be expected to have a lower fluorescence yield than a free phycobilisome. However, it is also possible that IsiA forms a specifically quenching state under these conditions (Cadoret et al., 2004). The quenching of fluorescence with chlorophyll excitation (Fig. 2A) suggests that there is a quenching effect within the chlorophyll a antenna as well as an effect on the coupling of phycobilisomes. Conceivably, both effects could result from covalent modification of IsiA.
Our results suggest that NPQ in cyanobacteria and plants are rather different phenomena. In plants, the formation of quenching centers in the light-harvesting antenna of PSII has a clear role in protecting PSII from excess excitation (Horton et al., 1996; Holt et al., 2004). In cyanobacteria, the strong F0 quenching seen in deeply iron-starved cells (Fig. 1) may not involve the PSII antenna at all. The observed effects on fluorescence probably result mainly from an effect on phycobilisomes that were already decoupled from reaction centers. However, given the instability of phycobilisome-reaction center association (Fig. 4, A and B), it is likely that an increase in the affinity of IsiA for phycobilisomes would also serve to reduce the population of phycobilisomes coupled to reaction centers. This idea is illustrated in Figure 6.
It should be noted that the model illustrated in Figure 6 does not provide a complete explanation of cyanobacterial NPQ. After shorter periods of iron deprivation, cyanobacterial NPQ mainly affects Fv rather than F0 (Bailey et al., 2005), and this phenomenon is unaffected by phosphate buffers. Therefore, this form of NPQ must occur by a different mechanism that does not involve phycobilisome mobility. Furthermore, the fluorescence quenching observed with chlorophyll excitation (Fig. 2A) cannot be explained simply by phycobilisome redistribution.
We previously showed that phycobilisome mobility is essential for state transitions in cyanobacteria (Joshua and Mullineaux, 2004). Our current results demonstrate a second physiological role for phycobilisome mobility, in a form of NPQ induced in iron-deprived cyanobacteria under high light. The results emphasize that the light-harvesting function of phycobilisomes is very flexible, and the ability of phycobilisomes to associate with several different chlorophyll-protein complexes is crucial to this flexibility.
CONCLUSIONS
The induction of nonphotochemical quenching of excitation energy in iron-starved cyanobacteria requires the diffusion of phycobilisomes. We suggest that the process involves the coupling of phycobilisomes to IsiA and is triggered by a change in the affinity of IsiA for phycobilisomes. After prolonged iron-starvation, cyanobacteria contain a pool of free phycobilisomes that are neither functionally nor structurally coupled to reaction centers.
MATERIALS AND METHODS
Strains and Culture Conditions
Synechocystis sp. PCC 6803 was obtained from the Pasteur Culture Collection and grown in batch culture on BG11 medium (Castenholz, 1988) in an illuminated orbital incubator at 30°C, with white light at 30 μE m−2 s−1. For the purposes of iron-starvation, Synechocystis 6803 was grown in iron-free BG11 medium for a minimum of 20 d. Cells were transferred into fresh iron-free BG11 every 4 d.
Fluorescence Quenching Measurements
Cells of Synechocystis 6803 were resuspended in growth medium to a chlorophyll concentration of 2.5 μm, as determined from the absorption of methanol extracts at 665 nm (Porra et al., 1989). Measurements were carried out in a temperature-controlled sample holder at 30°C. Fluorescence was monitored with a Hansatech FMS-1 fluorometer (Hansatech Instruments, Norfolk, UK). All samples were dark adapted for 10 min prior to measurement. Saturating pulses were provided with white light at 3,000 μE m−2 s−1 for 1-s durations. Actinic irradiance was provided with white light at 1,500 μE m−2 s−1 irradiance. The modulated measuring light was at 594 nm. For measurements in the presence of phosphate, cells were first resuspended to 25 μm chlorophyll in growth medium and then dark-adapted before adding 9 volumes of phosphate buffer (1 m KH2PO4/K2HPO4, pH 6.8).
Fluorescence Emission Spectra
Fluorescence emission spectra were obtained in a Perkin-Elmer LS50 Luminescence Spectrometer (Foster City, CA). For 77 K spectra, samples were suspended to a total chlorophyll concentration of 5 μm. The samples were injected into silica capillary tubes of 2.5-mm internal diameter and frozen in liquid nitrogen following a 5-min incubation in the dark or in saturating white light. Room temperature spectra were recorded for cells at a chlorophyll concentration of 5 μm, in 3-mL cuvettes. Spectra were recorded either for cells adapted to the weak excitation light or exposed for 5 min to saturating white light. This was at an intensity of 1,500 μE m−2 s−1 and was provided by the same source used for the fluorescence quenching measurements. Excitation wavelengths were 435 nm (chlorophyll excitation) or 600 nm (phycocyanin excitation). Excitation and emission slit-widths were 3 nm and 5 nm, respectively, at room temperature. For 77 K spectra, both slit-widths were 5 nm.
Preparation of Samples for FRAP
Liquid cell cultures were adsorbed onto 1.5% agar containing either BG11 medium or 1 m phosphate. A small block of the agar was excised from the plate and placed in a custom-built sample holder (S. Garcia, University College London). A 0.2-mm thick coverslip was placed on top of the section of agar. The sample was maintained at 30°C using a circulating water-bath attached to the sample holder. To enable observation of cells at high magnification, a drop of immersion oil was placed between the coverslip and the objective lens.
FRAP Measurements
A laser-scanning confocal microscope (Nikon PCM2000) equipped with a red He-Ne laser (633 nm, 20 mW) was used, with a 50-μm pinhole and a 60× objective lens. Fluorescence was selected with a Schott RG665 filter, transmitting red light at wavelengths longer than about 665 nm. Cells were imaged by scanning a region of 13.8×13.8 μm with the laser power reduced to 12.5% with a neutral density filter. Images were 512×512 pixels. After recording a prebleach image, a line was bleached across the cell by scanning the confocal spot for 2 s in the X-direction with the laser at full power. The laser power was then reduced again to 12.5% and postbleach images were recorded at intervals of 3 or 6 s for up to 1 min. White light illumination to induce NPQ was with the same source used for the fluorescence quenching measurements and was at an intensity of 1,500 μE m−2 s−1.
FRAP Data Analysis
To assess the extent of fluorescence recovery after bleach, fluorescence profiles were extracted from images using Optimas 5.2 image analysis software as previously described (Mullineaux et al., 1997). Fluorescence profiles were extracted by summing pixel values across the cell in the X-direction, producing a one-dimensional fluorescence profile in the Y-direction at 90° to the bleached line across the cell. Fluorescence recovery was quantified by measuring the increase of fluorescence at the center of the bleach, relative to the prebleach fluorescence. The postbleach fluorescence values were corrected to allow for the fact that the bleach significantly reduced the total fluorescence from the cell (typically by about 30%).
Acknowledgments
We thank Dr. Anne-Lise Etienne for communicating results prior to publication.
This work was supported by the Biotechnology and Biological Science Research Council (grant to C.W.M., grant to N.H.M., and research studentship to S.J.), and by The Wellcome Trust (grant to C.W.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.061168.
References
- Andrizhiyevskaya EG, Frolov D, van Grondelle R, Dekker JP (2004) Energy transfer and trapping in the Photosystem I complex of Synechococcus PCC7942 and in its supercomplex with IsiA. Biochim Biophys Acta 1656: 104–113 [DOI] [PubMed] [Google Scholar]
- Bailey S, Mann NH, Robinson C, Scanlan DJ (2005) The occurrence of rapidly reversible non-photochemical quenching of chlorophyll α fluorescence in cyanobacteria. FEBS Lett 579: 275–280 [DOI] [PubMed] [Google Scholar]
- Bibby TS, Nield J, Barber J (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412: 743–745 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Hiffney A, Yakushevska AE, Piotrowski M, Keegstra W, Berry S, Michel K-P, Pistorius EK, Kruip J (2001) A giant chlorophyll-protein complex induced by iron-deficiency in cyanobacteria. Nature 412: 745–748 [DOI] [PubMed] [Google Scholar]
- Borisov AY, Il'ina MD (1971) The lifetime and quantum yield of fluorescence in two photochemical systems of higher plants. Biochem USSR 36: 693–695 [PubMed] [Google Scholar]
- Burnap RL, Troyan T, Sherman LA (1993) The highly abundant chlorophyll-protein complex of iron-deficient Synechococcus sp. PCC7942 (CP43') is encoded by the isiA gene. Plant Physiol 103: 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret J-C, Demoulière R, Lavaud J, van Gorkom HJ, Houmard J, Etienne A-L (2004) Dissipation of excess energy triggered by blue light in cyanobacteria with CP43' (isiA). Biochim Biophys Acta 1659: 100–104 [DOI] [PubMed] [Google Scholar]
- Castenholz RW (1988) Culturing methods for cyanobacteria. In L Packer, AN Glazer, eds, Methods in Enzymology, Vol 167. Academic Press, San Diego, pp 68–93
- El Bissati K, Delphin E, Murata N, Etienne A-L, Kirilovsky D (2000) Photosystem II fluorescence quenching in the cyanobacterium Synechocystis sp. PCC6803: involvement of two different mechanisms. Biochim Biophys Acta 1457: 229–242 [DOI] [PubMed] [Google Scholar]
- Glazer AN (1984) Phycobilisome: a macromolecular complex optimised for light energy transfer. Biochim Biophys Acta 768: 29–51 [Google Scholar]
- Grabowski J, Gantt E (1978) Excitation energy migration in phycobilisomes: comparison of experimental results and theoretical predictions. Photochem Photobiol 28: 47–54 [Google Scholar]
- Grossman AR, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev 57: 725–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt NE, Fleming GR, Niyogi KK (2004) Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry 43: 8281–8289 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Joshua S, Mullineaux CW (2004) Phycobilisome diffusion is required for light-state transitions in cyanobacteria. Plant Physiol 135: 2112–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Singh AK, McIntyre LM, Sherman LA (2004) Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp strain PCC 6803. J Bacteriol 186: 3331–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi K (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- MacColl R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124: 311–334 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW (1993) Inhibition by phosphate of light-state transitions in cyanobacterial cells. Photosynth Res 38: 135–140 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW (1994) Excitation energy transfer from phycobilisomes to Photosystem I in a cyanobacterial mutant lacking Photosystem II. Biochim Biophys Acta 1184: 71–77 [Google Scholar]
- Mullineaux CW, Sarcina M (2002) Probing the dynamics of photosynthetic membranes with fluorescence recovery after photobleaching. Trends Plant Sci 7: 237–240 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Tobin MJ, Jones GR (1997) Mobility of photosynthetic complexes in thylakoid membranes. Nature 390: 421–424 [Google Scholar]
- Myers J, Graham J-R, Wang RT (1980) Light-harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol 66: 1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson F, Simpson DJ, Jansson C, Andersson B (1992) Ultrastructural and biochemical characterisation of a Synechocystis 6803 mutant with inactivated psbA genes. Arch Biochem Biophys 295: 340–347 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedeman PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rakhimberdieva MG, Stadnichuk IN, Elanskaya IV, Karapetyan NV (2004) Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant of Synechocystis sp. FEBS Lett 574: 85–88 [DOI] [PubMed] [Google Scholar]
- Sandström S, Park YI, Öquist G, Gustafsson P (2001) CP43', the isiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus sp. PCC7942. Photochem Photobiol 74: 431–437 [DOI] [PubMed] [Google Scholar]
- Sarcina M, Mullineaux CW (2004) Mobility of the IsiA chlorophyll-binding protein in cyanobacterial thylakoid membranes. J Biol Chem 279: 36514–36518 [DOI] [PubMed] [Google Scholar]
- Sarcina M, Tobin MJ, Mullineaux CW (2001) Diffusion of phycobilisomes on the thylakoid membranes of the cyanobacterium Synechococcus 7942. Effects of phycobilisome size, temperature and membrane lipid composition. J Biol Chem 276: 46830–46834 [DOI] [PubMed] [Google Scholar]
- van Thor JJ, Mullineaux CW, Matthijs HCP, Hellingwerf KJ (1998) Light-harvesting and state transitions in cyanobacteria. Bot Acta 111: 430–443 [Google Scholar]
- Yeremenko N, Kouril R, Ihalainen JA, D'Haene S, van Oosterwijk N, Andrizhiyevskaya EG, Keegstra W, Dekker HL, Hagemann M, Boekema EJ, et al (2004) Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 43: 10308–10313 [DOI] [PubMed] [Google Scholar]
- Yu J, Wu Q, Mao H, Zhao N, Vermaas WFJ (1999) Effects of chlorophyll availability on phycobilisomes in Synechocystis sp. PCC6803. IUBMB Life 48: 625–630 [DOI] [PubMed] [Google Scholar]