Abstract
Day respiration of illuminated C3 leaves is not well understood and particularly, the metabolic origin of the day respiratory CO2 production is poorly known. This issue was addressed in leaves of French bean (Phaseolus vulgaris) using 12C/13C stable isotope techniques on illuminated leaves fed with 13C-enriched glucose or pyruvate. The 13CO2 production in light was measured using the deviation of the photosynthetic carbon isotope discrimination induced by the decarboxylation of the 13C-enriched compounds. Using different positional 13C-enrichments, it is shown that the Krebs cycle is reduced by 95% in the light and that the pyruvate dehydrogenase reaction is much less reduced, by 27% or less. Glucose molecules are scarcely metabolized to liberate CO2 in the light, simply suggesting that they can rarely enter glycolysis. Nuclear magnetic resonance analysis confirmed this view; when leaves are fed with 13C-glucose, leaf sucrose and glucose represent nearly 90% of the leaf 13C content, demonstrating that glucose is mainly directed to sucrose synthesis. Taken together, these data indicate that several metabolic down-regulations (glycolysis, Krebs cycle) accompany the light/dark transition and emphasize the decrease of the Krebs cycle decarboxylations as a metabolic basis of the light-dependent inhibition of mitochondrial respiration.
Illuminated leaves simultaneously assimilate CO2 through the photosynthetic carbon reduction cycle and lose CO2 through photorespiration and day respiration. In darkness, leaves no longer assimilate CO2 via the photosynthetic carbon reduction cycle but produce CO2 through dark respiration. Although dark respiration is known to involve glycolysis and CO2 production through pyruvate dehydrogenation and the degradative Krebs cycle (Trethewey and ap Rees, 1994; Plaxton, 1996), the carbon metabolism that is responsible for the CO2 respiratory release in the light is almost unknown. This is so because the day respiratory CO2 flux is very low and masked by the photosynthetic carbon fixation and the photorespiratory CO2 production in the light, and is thus difficult to study.
Nevertheless, it has been repeatedly shown, using either the Laisk's (Laisk, 1977) or Kok's method (Kok, 1948), that the rate of day respiration (Rd) is less than that of dark respiration (Rn; for review, see Atkin et al., 2000) so that light is known to inhibit respiration, with a Rd/Rn value (usually denoted as μ) ranging from 30% to 100% (for a recent study, see Peisker and Apel, 2001). Pioneering gas exchange measurements on mustard suggested that some enzymatic activities are inhibited in the light so that substrates accumulate (Cornic, 1973), explaining the respiratory burst when leaves are darkened: the light enhanced dark respiration. More recently, it has been shown in the unicellular alga Selenastrum minutum that pyruvate kinase (Lin et al., 1989) is inhibited by light. It is also the case of the pyruvate dehydrogenase complex that is partly inactivated by (reversible) phosphorylation in extracts from illuminated leaves (Budde and Randall, 1990; Tovar-Mendez et al., 2003). Photorespiration is also probably involved in the inhibition of pyruvate dehydrogenase as it has been shown that this enzyme is down-regulated by NH3, which is a byproduct of the photorespiratory Gly decarboxylation (Krömer, 1995). Enzymes of the Krebs cycle are also assumed to be inhibited in the light because of a high mitochondrial NADH level due to photorespiratory Gly decarboxylation (Atkin et al., 2000). Additionally, it has been shown that the mitochondrial isocitrate dehydrogenase is inhibited by the high NADPH/NADP ratios that occur in the light (Igamberdiev and Gardeström, 2003).
Although all these enzymatic data suggest that the respiratory pathway is down-regulated in the light regarding both glycolysis and the Krebs cycle, respiratory metabolic fluxes in vivo in leaves are not well known. Some labeling experiments with carbon isotopes (13C or 14C) have already been done to disentangle respiratory metabolic fluxes in vivo in the light and in the dark, but surprisingly, studies that have focused on labeling of the resulting respired CO2 are scarce. Using 14CO2 labeling techniques, Pärnik et al. (2002) suggested that CO2 production in the light is composed of (1) decarboxylation of primary products like triose phosphates and malate (between 10% and 50% from one species to another), and (2) decarboxylation of end-products like Suc and starch to a greater extent (50%–90%). 12CO2 (respiratory) production in a 13C atmosphere has been used to show that day respiration is less than night respiration, and its rate as well as the ratio μ decreased at high CO2 concentration (Pinelli and Loreto, 2003). It has thus been proposed that inhibition of CO2 production is determined by the CO2 fixation flux (Atkin et al., 1998; Pinelli and Loreto, 2003). The ability of leaves to oxidize some metabolites through respiration has also been investigated with feeding experiments using labeled organic compounds. Supplying 13C-enriched Glc to myrtle leaves in the light with CO2-free air did enrich the CO2 subsequently respired in the dark, although the CO2 was not completely labeled (Affek and Yakir, 2003). Nevertheless, the 13C amount in the light-respired CO2 was not measured in that study.
So already published data do not show what the respiratory metabolic pathway is actually in illuminated leaves; that is, what the metabolic fluxes associated with the day respiratory CO2 production are. We address this question here by feeding illuminated French bean (Phaseolus vulgaris) leaves with positional 13C-enriched Glc and pyruvate and measuring the resulting 13C-enrichment in both day and dark-respired CO2 and in intermediary respiratory compounds, using isotope ratio mass spectrometry and nuclear magnetic resonance (NMR), respectively. The isotopic analysis of CO2 respired in the dark and the measurement of the carbon isotope discrimination during photosynthesis allows us to calculate the 13C-content in dark- and light-respired CO2, respectively. Respiration of supplied 13C1- and 13C3-enriched Glc is found to be almost completely inhibited by light. By contrast, decarboxylation of 13C1-enriched pyruvate through the pyruvate decarboxylation is much less inhibited by light, unlike 13C-2-pyruvate. These data are supported by NMR spectra obtained from illuminated leaves and taken as a whole, they suggest that (1) the Krebs cycle and glycolysis are strongly inhibited by light, with little interconversion between triose phosphates and hexose phosphates through the triose phosphate isomerase and aldolase reactions, and (2) the pyruvate dehydrogenation is only partly inhibited by light, with the acetyl-CoA molecules being directed toward purposes other than respiration.
THEORY
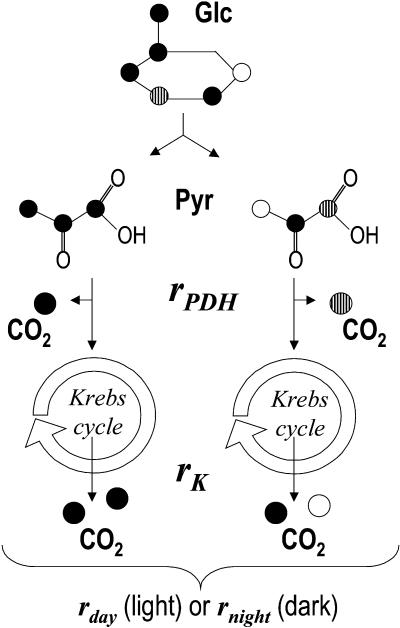
This “Theory” section describes the mathematical background used to calculate the Rd decarboxylations when detached leaves were supplied with 13C-enriched molecules (Fig. 1).
Figure 1.
Metabolic model used in this paper for decarboxylations of 13C-enriched substrates and respiratory variables taken into account (rPDH and rK and the sum, rday or rnight). rPDH and rK are the rates of decarboxylation of 13C-enriched substrates through the PDH reaction and the Krebs cycle, respectively. rday and rnight are the sum of rPDH and rK in the light and in the dark, respectively. Different symbols are used for different carbon atom positions in order to see the pathways to CO2 production.
In the following, the isotope composition (δ13C) and the isotope ratio (13C/12C) are denoted as δ and R, respectively, and the percentage of 13C is denoted as λ. λ is simply deduced from δ through the following relationship:
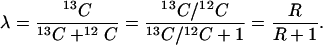 |
As 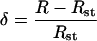 where Rst is the 13C/12C ratio in the Pee Dee reference material (Rst = 0.0112372), we have:
where Rst is the 13C/12C ratio in the Pee Dee reference material (Rst = 0.0112372), we have:
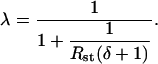 |
(1) |
Rate of 13C-Enriched Substrate Decarboxylation in the Light (rday)
The on-line discrimination value was obtained using the method of Evans et al. (1986):
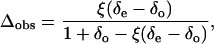 |
where the isotope compositions of air entering and leaving the cuvette are δe and δo, respectively. ξ is equal to ce/(ce − co) where ce and co are the CO2 molar fractions in the entering and leaving air, respectively.
The Δobs value obtained before the substrate addition is denoted as Δ. It is assumed that after substrate addition, the air leaving the leaf cuvette is the sum of the CO2 left by photosynthetic discrimination and additional CO2 released from substrate respiration. The latter flux is denoted as rday. If the leaf area is denoted as S and cfixed is the CO2 amount (in μL/L) fixed by photosynthesis, the mass balance equation for CO2 is as follows:
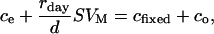 |
where d (L/s) is the air flow through the leaf cuvette and VM the molar volume. Thus, we have:
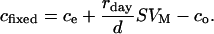 |
(2) |
Case of Feeding with a Compound That Has a Homogeneous Isotope Composition
Homogeneous isotopic compounds are not involved in this study, but the calculation is explained here as it gives a basis to understand the next step, that is, the use of compounds with a heterogeneous isotope composition.
The isotope composition of the substrate fed to the leaf is denoted as δs. Isotopic mass balance is so that the 13C amount entering the cuvette (ceλe) plus that from the additional CO2 release (rdaySVMλs/d) is equal to the sum of the 13C amount fixed by the leaf (cfixed λfixed) and the 13C amount leaving the cuvette (coλo). That is,
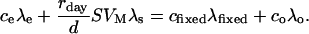 |
(3) |
Note that the similar relationship with deltas is not correct as the strong 13C-enrichment does not allow one to neglect R compared to 1 so that λ ≠ R. λfixed is obtained with the usual relationship 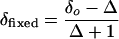 and Equation 1, where Δ is obtained before feeding by on-line isotopic measurements (Evans et al., 1986). Substituting Equation 2 in Equation 3 gives:
and Equation 1, where Δ is obtained before feeding by on-line isotopic measurements (Evans et al., 1986). Substituting Equation 2 in Equation 3 gives:
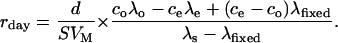 |
(4) |
It should be noted that possible 12C/13C fractionations that can occur during the absorption of labeled compounds are neglected here. The effect of such a fractionation is clearly negligible, that is, in the per mil order of magnitude, compared to the labeling level, which is in the percent order of magnitude or more.
Case of Feeding with a Compound That Has a Nonhomogeneous Positional Isotope Composition
The underlying assumption of the previous paragraph is that the substrate is isotopically homogeneous. However, the isotope composition of the feeding substrates used in these experiments is nonhomogeneous so that its different carbon atom positions do not have the same Δ values. In such a case, the metabolic reactions that are responsible for the decarboxylation of the carbon atoms and their rates should be taken into account. For example, this occurs when Glc or pyruvate is added: the C-1 atom of pyruvate is decarboxylated by pyruvate dehydrogenase, while the C-2 and C-3 positions are decarboxylated by the Krebs cycle (Fig. 1). Similarly, the C-3 and C-4 atom positions of Glc are decarboxylated by the pyruvate dehydrogenase reaction, the other being decarboxylated by the Krebs cycle. Advantage can then be taken from this with positionally 13C-enriched substrates; 13C-1-pyruvate would specifically enrich the CO2 produced by pyruvate dehydrogenase, while 13C-2-pyruvate would specifically enrich the CO2 that comes from the Krebs cycle. The same applies to positional 13C-enrichment in Glc.
The additional decarboxylations through the pyruvate dehydrogenase (PDH) reaction and the Krebs cycle are denoted as rPDH and rK, respectively (Fig. 1). With the relationship rday = rPDH + rK, Equation 2 still works. For 13C-1-pyruvate, Equation 3 becomes:
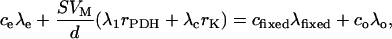 |
(5) |
where λ1 is the 13C percentage in the C-1 position of the labeled pyruvate. λc is the 13C percentage of the other (unlabeled) positions. For 13C-2-pyruvate, we have:
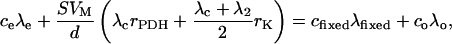 |
(6) |
where λ2 is the 13C percentage in the C-2 (labeled) position of pyruvate. λc (unlabeled positions) is the same as λc of Equation 5 (13C-1 enrichment). It can be seen that the two conditions of 13C-enrichment give two Equations (5 and 6) so that rPDH and rK can be deduced by a substitution procedure. A similar procedure is used for the positional 13C-enrichment of Glc.
Rate of 13C-Enriched Substrate Decarboxylation in Darkness
The CO2 that is produced in darkness after a light period with 13C-enriched substrate feeding comes from respiratory oxidation of new photosynthates (13C percentage in the fixed carbon λfixed), photosynthates from the previous light period in the greenhouse (13C percentage λprevious), and additional C coming from the 13C-enriched substrate fed to the leaf (13C percentage λs). It has been previously shown that the contribution of new photosynthates to dark respiration after 3-h light in French bean is 40% (Nogués et al., 2004). So the 13C percentage in photosynthates feeding respiration is given by λp = 0.4 λfixed + 0.6 λprevious. It should be noted that possible variations in the coefficients due to some physiological reasons do only introduce a minor error in the estimate of the 13C-enriched substrate decarboxylation rnight because of the strong 13C-enrichment in the substrate.
The total CO2 production in the dark is denoted as Rn. The 13C percentage in dark respired CO2 (denoted as λglobal) is calculated with the δ13C value and Equation 1. The 13C mass balance gives the following relationship:
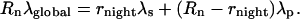 |
(7) |
Rearranging, it gives:
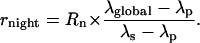 |
(8) |
When substrates do not have a homogeneous isotopic distribution (positional enrichment), Equation 7 is completed to:
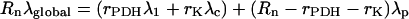 |
(9) |
for the 13C-1-pyruvate feeding. And similarly, for the 13C-2-enrichment, it gives:
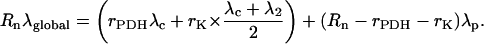 |
(10) |
Equations 9 and 10 allow one to extract rPDH and rK with a substitution procedure. The method is similar for 13C-Glc.
RESULTS
On-Line Carbon Isotope Discrimination of Leaves Fed with 13C-Enriched Substrates
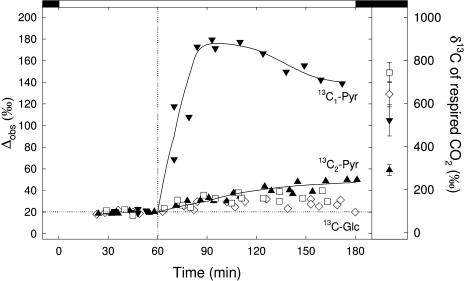
The carbon isotope discrimination during photosynthesis of detached French bean leaves before and after addition of 13C-enriched carbohydrates is shown in Figure 2. Before adding substrates, the carbon isotope discrimination was around 20‰ in all cases. This is in accordance with the pi/pa value around 0.7 (data not shown). At t = 60 min, 13C-enriched substrates were added. The overall signature of Glc was 5,500‰ and that of pyruvate was 2,500‰. When 13C1-pyruvate was supplied, the carbon isotope discrimination then increased to Δobs = 180‰ as a consequence of 13C1-enriched pyruvate decarboxylation. The carbon isotope discrimination then slightly decreased and reached approximately 140‰. The on-line carbon isotope discrimination value increased much less with 13C2-enriched pyruvate, with a maximum value of Δobs around 50‰, indicating that decarboxylations following Pyr dehydrogenation (i.e. Krebs cycle) had very small rates. Glc was hardly decarboxylated in the light, thus with a very small increase in Δobs (up to 35‰–40‰ only).
Figure 2.
Left, Development of the on-line carbon isotope discrimination (Δobs) of detached bean leaves before and after feeding with Glc that is 13C-enriched in C-1 (⋄) or C-3 (□), or pyruvate that is 13C-enriched in C-1 (▾) or C-2 (▴). In both positional enrichment cases, the overall δ13C values of Glc and pyruvate are 5,500‰ and 2,500‰, respectively. The detached leaves are first put in distilled water and the light is turned on. The vertical dotted line indicates the moment at which the substrate (Glc or pyruvate) is supplied (t = 60 min). The horizontal dotted line represents the mean photosynthetic fractionation before substrate feeding (Δobs approximately equal to 20‰). The gas exchange conditions were 350 μL L−1 CO2 in 21% O2, 22°C, and 450 μmol m−2 s−1 light. Note that the Δobs measurements only begin after photosynthesis stabilizes that is, after approximately 30 min in the light. At t = 180 min, the light is switched off for dark-respired CO2 measurements. The trends of the on-line isotope discrimination with Pyr feeding are indicated with a solid line. Right, δ13C value (in per mil) of the CO2 respired in the dark just after having switched off the light. Same symbols as for the left section.
Rates of Decarboxylation in the Light
The rates of decarboxylation of the 13C-enriched substrates (see Fig. 1 that summarizes the parameters considered) in the light were calculated using the carbon isotope discrimination Δobs and are given in Table I. These calculations used a two variable model (see “Theory” section) that is, rPDH, the rate of decarboxylation through the PDH, and rK, the decarboxylation through the Krebs cycle. These rates may be compared to the overall Rd. As expected from conclusions of Figure 2, the decarboxylation rate of pyruvate through the PDH reaction was around 0.05 to 0.06 μmol m−2 s−1, the other decarboxylation rates being very low, under 0.01 μmol m−2 s−1. Although the effect of decarboxylation of 13C1-enriched Pyr was striking in Figure 2, the decarboxylation rate was low compared to day respiration (Rd, around 0.6 μmol m−2 s−1) simply because the labeling was strong in Pyr. It is noteworthy that the decarboxylation rates were negligible compared to the overall Rd, so that we argue that the respiratory pathway was not artefactually enhanced in our feeding experiment. Accordingly, the overall Rd of fed leaves was similar to that of the control. Nevertheless, the decarboxylation CO2 flux could have been somewhat underestimated because of refixation (see below).
Table I.
Respiration rate and calculated decarboxylations (in μmol m−2 s−1) of Glc and pyruvate supplied to detached French bean leaves through the PDH and the Krebs cycle in the light and in the dark (see “Theory” section for calculation details)
Day respiration rates were measured as the slope of Γ/riRn relationships (see “Gas Exchange Measurements” section) and are thus total day respiratory fluxes in the light. Decarboxylation data are mean and se of three independent measurements. The mean inhibition of decarboxylation by light was calculated as the ratio mean light decarboxylation/mean dark decarboxylation.
| Control | Pyruvate | Glc | ||
|---|---|---|---|---|
| Light | ||||
| Rd | Day respiration rate | 0.632 ± 0.125 | 0.625 ± 0.150 | 0.539 ± 0.143 |
| rPDH | Decarboxylation from the PDH reaction | 0.058 ± 0.009 | 0.004 ± 0.001 | |
| rK | Decarboxylation from the Krebs cycle | 0.005 ± 0.004 | 0.009 ± 0.004 | |
| Darkness | ||||
| Rn | Dark respiration rate | 1.178 ± 0.039 | 1.266 ± 0.071 | 1.331 ± 0.115 |
| rPDH | Decarboxylation from the PDH reaction | 0.079 ± 0.023 | 0.070 ± 0.015 | |
| rK | Decarboxylation from the Krebs cycle | 0.116 ± 0.011 | 0.236 ± 0.050 | |
| iPDH | Mean inhibition of the PDH reaction by light | 27% | 94% | |
| iK | Mean inhibition of the Krebs cycle by light | 95% | 96% |
Rates of Decarboxylation in Darkness
After 180 min (see Fig. 2), light was switched off and the carbon isotope composition of the CO2 evolved in the first 30 min of darkness was measured. The δ13C value of respired CO2 with 13C1- and 13C3-Glc feeding was approximately 740‰ and 640‰, respectively, and the δ13C value of respired CO2 with 13C1- and 13C2-pyruvate feeding was approximately 520‰ and 290‰, respectively (Fig. 2, right). The decarboxylation rates calculated using these values are shown in Table I. rPDH was around 0.070 μmol m−2 s−1 with either Glc or pyruvate and rK with Glc was around the double (0.2 μmol m−2 s−1) of that with pyruvate. Clearly, these decarboxylations witness that leaves oxidized molecules via glycolysis in the dark. A similar result was already obtained by Stitt and ap Rees (1978) with 14CO2. When compared to the day decarboxylation values, the inhibition by light was around 95% for both rPDH and rK with Glc and around 30% and 95% for rPDH and rK with pyruvate, respectively. In other words, light inhibited only partially the pyruvate dehydrogenase reaction and almost stopped the Krebs cycle. In the light, the respiratory breakdown of Glc into CO2 did not occur through the Krebs cycle or the pyruvate dehydrogenase reaction, simply demonstrating that the Glc molecules could not reach these metabolic steps in illuminated leaves.
Metabolic Pathways That Consumed 13C-Enriched Glc
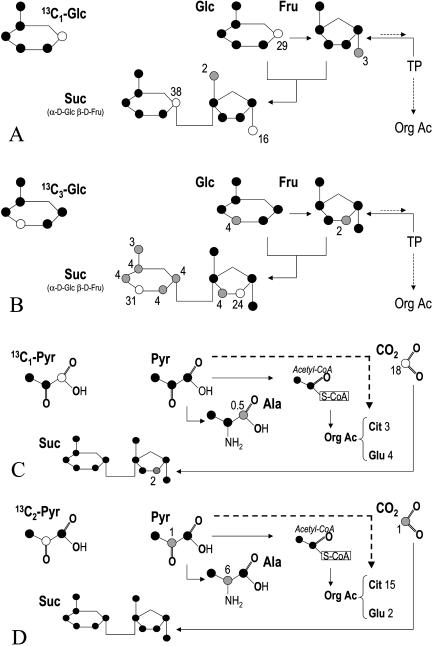
The weakness of Glc or pyruvate decarboxylation in the light raises the question of the fate of these molecules in the leaf. That is why starch purification on sample of the experiment of Figure 2 was made and NMR analysis of illuminated leaves fed with positional, fully 13C-labeled substrates (99% 13C in a given position) was done. The results are shown in Table II and Figure 3, respectively.
Table II.
Amount and carbon isotope composition (δ13C) of starch from detached leaves supplied with 13C-enriched Glc (δ13C 5,500‰) or 13C-enriched Pyr (δ13C 2,500‰), 15 mmol L−1 for 2 h in the light with 350 μL L−1 CO2 at −51‰ (see Fig. 1) after 1 h in the light without feeding
Data are mean and sd of three independent measurements.
| Conditions | Amount | δ13C |
|---|---|---|
| μg mg−1 fresh weight | ‰ | |
| Control (greenhouse) | 13.1 ± 5.3 | −28.5 ± 0.5 |
| No feeding | 15.1 ± 5.0 | −31.4 ± 1.3 |
| 13C1-Glc | 15.0 ± 3.3 | −1.9 ± 9.0 |
| 13C3-Glc | 13.6 ± 2.9 | −9.0 ± 9.5 |
| 13C1-Pyr | 11.7 ± 5.4 | −16.5 ± 3.4 |
| 13C2-Pyr | 12.2 ± 3.2 | −29.9 ± 1.6 |
Figure 3.
Distribution of 13C from NMR analysis of leaves fed with 13C1- or 13C3-Glc (A and B), 13C1- or 13C2-pyruvate (C and D) for 2 h in the light at ambient CO2. In each case, the compound supplied to leaves is indicated in the top left of each section. Simplified metabolic pathways are represented in order to emphasize the relationships between metabolites. Cit, Citrate; TP, triose phosphates; Org Ac, organic acids. The dashed line stands for the production of organic acids from pyruvate via the phosphoenolpyruvate carboxylation. The bottom continuous line in C and D stands for the refixation of decarboxylated CO2. Carbon atoms are colored in accordance with their labeling level: black, no labeling, gray, low labeling, white, strong labeling. For each labeled carbon, numbers are in percent of 13C that is in the sample. Carbon atoms that are considered to be labeled are those which proportion of 13C (13C/12C + 13C) is equal or more than 2% (the natural abundance of 13C is approximately 1.1%).
13C-enriched Glc supplied to leaves was directed to Suc synthesis; the 13C content of Suc as a whole approached 60% in both (C-1 and C-3) enrichment conditions (Fig. 3, A and B). When 13C3-Glc was used, the scrambling of carbon atoms appeared, with a small labeling of all the carbon atom positions in the Glc moiety of Suc (13C content around 4%). This presumably came from the pentose phosphates cycle. Accordingly, this effect was not seen with 13C1-Glc because the first steps of the cycle are the dehydrogenation and decarboxylation of the C-1 carbon atom of Glc.
Surprisingly, starch was also labeled by Glc (Table II); with 13C1-Glc and 13C3-Glc, the δ13C value of starch was strongly higher (−1.9‰ and −9‰, respectively) than in unfed leaves (−31.4‰). In other words, Glc could feed starch synthesis. Its contribution should have nevertheless been low as the starch amount was not significantly different between fed and unfed leaves (Table II). The contribution of feeding Glc (in percent of total starch amount), denoted here as σ, can be calculated with the following mass-balance relationship:
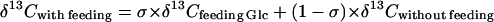 |
that gives σ = 0.5% ± 0.2% and 0.4% ± 0.2% for 13C1-Glc and 13C3-Glc, respectively. This contribution was very low, but was clearly seen in starch (Table II) simply because the δ13C value of the labeling Glc was very high. This result indicates that Glc molecules from the cytoplasm (or, more generally, C from the fed Glc) could reach the chloroplastic compartment and enter the starch synthetic pathway.
The enriched carbon atoms of Glc were almost not redistributed to other positions by metabolic pathways; when supplied with 13C1-Glc, the C-6 carbon atoms in leaf Glc, Fru, or Suc were not labeled (Fig. 3A). Similarly, when supplied with 13C3-Glc, the C-4 carbon atoms in leaf Glc and Fru were not labeled and the corresponding positions in Suc were only weakly labeled (4% of the 13C content; Fig. 3B). In other words, the scrambling of carbon atoms through the triose phosphates and hexose phosphates interconversion (with triose phosphate isomerase and aldolase) was very low. This result is consistent with the gas exchange measurements of Figure 2 in which glycolysis appeared to be stopped so that Glc was not (or very weakly) decarboxylated.
Metabolic Pathways That Consumed 13C-Enriched Pyruvate
When leaves were fed with 13C2-Pyr (99% of 13C in C-2), Ala was (weakly) labeled in C-2, indicating that Pyr had been aminated and citrate was labeled, strongly suggesting that some of the Pyr molecules entered the Krebs cycle (Fig. 3D). Surprisingly, when fed with 13C1-Pyr, leaves had only a few labeled carbon atoms (Fig. 3C). Ala appeared as only weakly labeled in C-1, but this originated mainly from the low detectability of carboxyl (−COOH) carbon atoms with NMR. Moreover, the CO2 produced in the light accounted for an important 13C loss; using a decarboxylation rate of 0.05 μmol m−2 s−1 (Table I), there was a 13C loss of 350 μmol 13C m−2 during the light treatment (2 h),while approximately 1,950 μmol Pyr m−2 entered the leaf (=Pyr concentration × transpiration rate = 15 mol L−1 × 18 μL m−2 s−1 × 2 h light), that is, approximately 18% of the 13C supplied in C-1. A similar calculation gives, with 13C2-Pyr, a 13C loss of only approximately 1%.
Refixation of Decarboxylated CO2
The rate of the decarboxylation of 13C-enriched substrates in the light may have been underestimated by possible refixation of CO2. If so, the absolute rate of refixation is expected to be important when 13C1-Pyr was supplied to leaves as decarboxylation was high in that case (Table I). Nevertheless, Glc and Fru were not labeled and Suc was only weakly labeled in the C-3 position of the Fru moiety (Fig. 3C). Although it is difficult to quantify such small quantities, this labeling in Suc accounted, as a maximum, for 0.5 μmol g−1 of 13C, that is, a 13C (re)fixation rate of 0.009 μmol m−2 s−1 during 2 h of the treatment in the light. When 13C1-Pyr was supplied to leaves, starch was also labeled (the isotope signature is around −20‰, compared to −31‰ expected; Table II) and this was also a consequence of refixation. The amount of 13C-enriched carbon in starch was calculated to be 0.2% (similar calculation as for section “Metabolic Pathways That Consumed 13C-Enriched Glc”). The starch amount and the percentage of carbon in starch were 0.015 mg mg−1 fresh weight and approximately 40%, respectively. This gives a refixation rate (of labeled carbon) of 0.008 μmol m−2 s−1. The overall refixation rate would thus have been, as a maximum, 0.017 μmol m−2 s−1. This means that the total decarboxylation rate of 13C1-Pyr would have been 0.058 (see Table I) + 0.017 = 0.075 μmol m−2 s−1 and so, the inhibition of the PDH reaction by light would have been approximately 5% only. The effect of refixation in the other case (13C2-Pyr) was clearly negligible: starch was not labeled (Table II) and no labeling could be seen in Suc, Glc, or Fru (Fig. 3D). As the rates of decarboxylation were similar with C-1 and C-3 13C-enriched Glc, refixation was likely to be negligible in both cases.
DISCUSSION
The respiratory metabolism in illuminated leaves is inhibited compared to darkness, as repeatedly shown by gas-exchange measurements (for review, see Atkin et al., 2000). However, the metabolic basis of such an inhibition is not well known. We addressed this question by feeding experiments using 13C-enriched substrates and followed the 13C atoms with isotope ratio mass spectrometry and NMR to determine which metabolic pathways are inhibited in the light.
The Pyruvate Dehydrogenase Activity in the Light
The two main steps responsible for the respiratory CO2 production are the dehydrogenation of Pyr (PDH reaction) and the Krebs cycle (Fig. 1). The carbon atoms that are decarboxylated are not the same in both: the PDH reaction decarboxylates the C-1 of Pyr, while the Krebs cycle decarboxylates the two others. Feeding illuminated leaves with 13C-enriched Pyr in C-1 significantly enriched the respiratory CO2 produced in the light, as revealed by the strong modification of the photosynthetic carbon isotope discrimination measured on-line, from the steady value of 20‰ to approximately 160‰ (Fig. 2). Clearly, the PDH reaction consumes the Pyr molecules, and so is not totally inhibited in the light. When compared to the dark decarboxylation rate, the calculated inhibition is 27% (Table I). Refixation of decarboxylated CO2 can nevertheless occur and lead to an overestimation of the inhibition level. Indeed, there was a small 13C enrichment in Suc (Fig. 3) as well as in starch (Table II) and a calculation (see “Results”) gives a refixation rate of 0.017 μmol m−2 s−1; that is, a total decarboxylation rate of Pyr of 0.075 μmol m−2 s−1. The refixation rate obtained here stands for 22% of the (total) decarboxylation of 13C-enriched Pyr, a value that is in accordance with calculations that use 14C data by Gerbaud and André (1987) on sunflower leaves (between 15% and 22%), but a little low compared to Pinelli and Loreto (2003) who found a refixation rate of 40% in mint leaves maintained at 350 μL L−1. In this study, we looked at only 13C enrichment in the two main compounds of CO2 fixation; that is, Suc and starch. Nevertheless, we recognize that some 13C atoms in other compounds were missed, leading to a small underestimation of the refixation rate. Taking our value as correct, this would give a light inhibition value of the PDH reaction of only 5%.
However, it is not possible in our experiment to distinguish the chloroplastic PDH activity from the mitochondrial one so that our values do represent the total cellular PDH activity. It has been shown that the mitochondrial PDH is partly inhibited in illuminated leaves by phosphorylation (Budde and Randall, 1990). In addition, photorespiratory produced NH3 is also assumed to inhibit this enzyme (Krömer, 1995). By contrast, the chloroplastic PDH is not regulated by phosphorylation and is assumed to be active in the light (Plaxton, 1996). The decarboxylation rate measured in this study (Table I) is likely to be the sum of both PDH activity in which the chloroplastic enzyme plays the major role.
Furthermore, the PDH reaction may be artefactually activated by the amount of Pyr (thermodynamic mass action law) introduced in the leaf. We nevertheless think that this effect is minor; the pyruvate concentration in the feeding solution is only 15 mmol L−1 and although it is difficult to detect carboxyl carbon atoms by NMR, it was not possible to see the corresponding C-1 atom of Pyr on the NMR spectrum, indicating that the Pyr concentration in the leaf is certainly less than 2 mmol L−1 in the cell.
Glycolysis and the Krebs Cycle Are Inhibited in the Light
In contrast with 13C1-Pyr, when leaves were fed with 13C2-Pyr, the photosynthetic carbon isotope discrimination was hardly modified (Fig. 2), indicating that there was nearly no decarboxylation of the C-2 carbon atom of Pyr. In other words, the Krebs cycle activity was very low and so there is a very small 13CO2 production. The calculated decarboxylation rate is 0.005 μmol m−2 s−1 only in the light; that is, the Krebs cycle is inhibited by 95% compared to the dark decarboxylation rate (Table I). This is consistent with the NMR results: (1) the intermediary products of the Krebs cycle such as succinate and fumarate are not detected, Glu (that is the amino acid that corresponds to α-ketoglutarate) was hardly labeled, (2) the two carboxyl atoms of citrate were 13C-labeled so that citrate stood for 15% of the sample 13C content, i.e. each carboxyl carbon atom represented 7.5% only of the 13C content. These data are in accordance with previous observations of Hanning and Heldt (1993) that mitochondria extracted from illuminated leaves had a low metabolic flux throughout the Krebs cycle. In addition, the mitochondrial matrix in the light is supposed to be reduced because of the photorespiratory Gly decarboxylation that lead to a high NAD(P)H/NAD(P)+ ratio. Some enzymes of the Krebs cycle are inhibited by the high NADH/NAD+ ratios (for review, see Siedow and Day, 2000), and it has been recently found that isocitrate dehydrogenase from pea is inhibited by high NADPH/NADP+ ratios, a feature that occurs in illuminated mitochondria (Igamberdiev and Gardeström, 2003).
The fact that the Krebs cycle is slowed down in the light raises the question of the fate of acetyl-CoA molecules produced by the PDH reaction. In our study, acetyl-CoA probably accumulated a little: the decrease in the photosynthetic on-line discrimination after 115 min (Fig. 2) suggests that the PDH reaction was less active. This was likely because of the retroinhibition of Pyr dehydrogenase by its product acetyl-CoA (Harding et al., 1970; Rapp et al., 1987), as the Krebs cycle activity that consumes acetyl-CoA was severely diminished in the light. A significant part of the acetyl-CoA molecules was directed to fatty acid production in the chloroplast (Ohlrogge and Jaworski, 1997). Accordingly, the mutant line of Arabidopsis (Arabidopsis thaliana) that produces the antisense RNA of the PDH kinase (thus enhancing the mitochondrial PDH reaction), accumulated 14C-labeled fatty acids when 14C-Pyr was fed to (photosynthetic) stems (Marillia et al., 2003), strongly suggesting that fatty acid synthesis can act as outfall for acetyl-CoA molecules.
13C-enriched Glc was only weakly decarboxylated in the light whatever the position of the 13C-enrichment was (Fig. 2). Thus, Glc molecules could hardly reach the PDH step, very likely because they could not enter glycolysis. Instead, the Glc molecules were directed to Suc synthesis (Fig. 3). Further, when fed with 13C1-Glc, the Fru moiety in Suc was only very weakly labeled in C-6, and similarly, when fed with 13C3-Glc, the Fru moiety in Suc was hardly labeled in C-4 (Fig. 3). Clearly, this shows that the C-1/C-6 and C-3/C-4 interconversion through the triose phosphates isomerase reaction was minor. In other words, only a small fraction (less than approximately 5% of the 13C-Glc fed to the leaf) of the Glc molecules reached this step, strongly suggesting a high metabolic resistance to the glycolytic breakdown of hexoses. Noteworthy, these results are in accordance with the regulation of the enzymes responsible for the phosphorylation/dephosphorylation of Fru-6-P to Fru-2,6-bisphosphate: (1) in the light, the high triose phosphates/inorganic phosphate decreases the Fru-2,6-bisphosphate concentration, promoting the dephosphorylation of Fru-1,6-bisphosphate in the cytosol (Stitt, 1990), and (2) in the chloroplast, phosphofructokinase is thought to be inhibited in the light (Plaxton, 1996). The rationale of such enzymatic regulations is that hexose molecules are prevented from entering the glycolytic breakdown, and so respiration does not consume Suc as soon as it is synthesized in the cytoplasm.
Cytoplasmic Glc Contributes to Starch Synthesis in the Light
When fed with 13C-enriched Glc (at 5,500 per mil), leaves produced 13C-labeled starch (Table II), with a δ13C value around 30‰ higher than without Glc feeding, indicating that Glc contributed to starch synthesis. The contribution was nevertheless low, around only 0.5% of the starch amount (that is, approximately 0.075 μg mg−1 fresh weight) was labeled (see the “Result” section for calculations). The flux of starch synthesis from 13C-enriched Glc during the 2 h of feeding treatment in the light was then 0.075 μg mg−1/2 h ∼ 0.038 μg mg−1 h−1; that is, 0.045 μmol C m−2 s−1, while the total starch synthetic flux was around 2 μg mg−1/3 h ∼ 0.7 μg mg−1 h−1 (Table II); that is, 0.84 μmol C m−2 s−1. So, Glc fed 0.045/0.84 ∼ 5% of starch synthesis in the leaf during the feeding experiment. The rate of starch synthesis from 13C-enriched Glc is consistent with that found in potato by Quick et al. (1995): chloroplasts extracted from intact leaves and illuminated with 14C-Glc (in a medium containing HCO3−) synthesized starch from 14C-Glc with a rate of approximately 0.04 μmol C m−2 s−1 (recalculated assuming a realistic chlorophyll amount of 0.4 g Chl m−2).
It has also been found that other carbon sources than photosynthetic CO2 can feed starch synthesis in intact illuminated leaves: Nogués et al. (2004) showed that it was not possible to completely label starch with CO2 and while the starch amount increased, the δ13C value of starch reached a plateau.
These observations might be paralleled with the presence of a diffusion-driven Glc phosphate translocator on the inner chloroplast membrane (Schäfer et al., 1977; Quick et al., 1995), which can feed starch synthesis with cytoplasmic hexose molecules, although the associated flux appears to be quantitatively minor in this study.
Concluding Remarks
To our knowledge, this paper describes the first in vivo study on the metabolic basis of inhibition by light of leaf respiration. Clearly, the main inhibited steps are the entrance of hexose molecules into the glycolytic pathway and the Krebs cycle. We nevertheless recognize that our experiments were made in typical conditions (21% O2, 350 μL L−1 CO2, 22°C) and the results may be influenced by environmental parameters. Indeed, it has been shown that there lies no inhibition of respiration by light in some physiological conditions (Sharp et al., 1984, and refs. therein). Two parameters are of particular interest: temperature, which is known to enhance respiratory enzymatic activities, and the photorespiratory rate (oxygen partial pressure), which has an effect on the redox status of the mitochondria. Further experimental data are now needed to investigate the effect of such environmental conditions on day respiratory metabolism. Moreover, the fact that the Krebs cycle appears to be slowed down in the light raises the question of energy in leaf cells and more precisely, of NADH feeding of the respiratory chain. Although one may suggest that photorespiration or the cytoplasmic-mitochondrial malate shuttle have such a role, further metabolic studies are needed to determine their respective contribution in vivo.
MATERIALS AND METHODS
Plant Material
French bean (Phaseolus vulgaris) L. cv Contender plants were grown from seed in 1-L pots of potting mix in a greenhouse, as described by Tcherkez et al. (2003). Minimum photosynthetic photon flux density during a 16-h photoperiod was maintained at approximately 500 μmol m−2 s−1 by supplemental lighting. Temperature and vapor pressure deficit were maintained at approximately 25.5°C/18.5°C and 1.4/1.2 kPa day/night, respectively. Carbon isotope composition (δ13C) of CO2 in the greenhouse air was −9.5‰ ± 0.3‰. The first trifoliar fully expanded leaves were used for all measurements.
Gas Exchange Measurements
Closed System (Dark Respiration)
The respiration chamber was placed in a closed system, which was directly coupled to an elemental analyzer (EA) NA-1500 (Carlo-Erba, Milan) through a 15-mL loop, as described by Tcherkez et al. (2003). Briefly, molar fractions of respiratory CO2 were measured with an infrared gas analyzer (IRGA; Finor, Maihak, Germany) placed in the closed system that was first flushed with CO2-free air. The loop was shunted when CO2 reached around 300 μL L−1 and the gas inside the loop was introduced into the EA with helium for gas chromatography. The connection valve between the elemental analyzer and the isotope ratio mass spectrometer (VG Optima, Micromass, Villeurbanne, France) was opened when the CO2 peak emerged from the EA.
Open System (Photosynthesis and On-Line Carbon Isotope Discrimination)
The assimilation chamber was connected in parallel to the sample air hose of the LI-6400 (LI-COR, Lincoln, NE). This aluminum chamber ([20 × 12 × 6] 10−6 m3) had a clear Plexiglas lid that allowed us to accommodate the middle leaflet (typical leaf surface approximately 0.01 m2). Two fans placed in the chamber gave a boundary layer conductance to water of approximately 6.7 mol m−2 s−1. Leaf temperature was controlled at 20°C with circulating water from a cooling water bath to the jacket of the leaf chamber and was measured with a copper-constantan thermocouple plugged to the thermocouple sensor connector of the LI-6400 chamber/IRGA. Ingoing air was dried (at approximately 1 mmol H2O mol−1) and passed through the chamber at a rate of 1 L min−1, monitored by the LI-6400. Molar fractions of CO2 were measured with the IRGA of the LI-6400. Light was supplied by a 500-W halogen lamp (Massive N. V., Kontich, Belgium). The lamp was placed about 30 cm above the chamber and 5 cm of deionized water and 1 cm of glass in the container filtered the radiation. The photosynthetic photon flux density at leaf level inside the chamber was maintained at 450 μmol m−2 s−1 during the labeling period. Inlet CO2 was obtained from a gas cylinder (Air Liquide, Grigny, France) with a δ13C of −51.2‰ ± 0.2‰. The outlet air of the chamber was regularly shunted and was sent to the loop to measure its isotope composition and thus the on-line carbon isotopic discrimination (Δobs). The gas inside the loop was introduced into the EA for gas chromatography as described above. Δobs during photosynthesis was measured following the method described by Evans et al. (1986; see “Theory”).
Day Respiration Measurements
Day respiration was measured with a LICOR-6400 open system, according to the method described in Peisker and Apel (2001). Briefly, A/ci curves are done at different light levels and the linear fit gives the CO2 compensation point Γ and the internal leaf resistance ri. Γ is plotted as a function of the product riRn and the slope of the linear regression is μ = Rd/Rn and so gives Rd. In bean, the effect of light on the Γ/ri relationship was slight and did not modify significantly the estimate of μ.
Starch Extraction Procedure
The extraction procedures for starch were similar to that described by Tcherkez et al. (2003). Leaf powder was suspended with 1 mL of distilled water in an Eppendorf tube (Eppendorf Scientific, Hamburg, Germany). After centrifugation, the pellet was washed four times with 95% ethanol at room temperature and starch was extracted with HCl solubilization and precipitated with cold methanol. After lyophilization, starch is transferred to tin capsules (Courtage Analyze Service, Mont Saint-Aignan, France) for isotope analysis.
NMR
Perchloric acid (PCA) extracts were prepared from 5 g of frozen leaf material as described by Aubert et al. (1996) for phloem cells. Spectra were obtained on a spectrometer (AMX 400) equipped with a 10-mm multinuclear probe tuned at 161.9 and 100.6 MHz for 31P- and 13C-NMR, respectively. The deuterium resonance of 2H2O (100 μL added per mL of extract) was used as a lock signal.
Conditions for 13C-NMR acquisition utilized 19-μs pulses (90°) at 6-s intervals and a sweep width of 20 kHz. Broad-band decoupling at 2.5 W during acquisition and 0.5 W during the delay was applied using the Waltz sequence; the signal was digitized using 32,000 data points zero-filled to 64,000 and processed with a 0.2-Hz line broadening. 13C-NMR spectra are referenced to hexamethyldisiloxane at 2.7 ppm. Mn2+ ions were chelated by the addition of 1 mmol L−1 1,2-cyclohexylenedinitrilotetraacetic acid. The assignments of resonance of 13C peaks were carried out according to Gout et al. (1993). Identified compounds were quantified from the height of their resonance peaks using fully relaxed conditions for spectra acquisition (pulses at 20-s intervals). Peak intensities were normalized to a known amount of the reference compound (maleate) that is added to the sample (internal standard). A carbon atom is here considered to be labeled when its estimated positional 13C proportion 13C/(13C + 12C) is more than 2% (the natural abundance is nearly 1.1%).
13C-Enriched Molecules
The positional 13C-labeled molecules (99% 13C in the considered position) were purchased to Eurisotop (Saclay, France). Pyruvate was dissolved in distilled water and pH was corrected to 6.8 with NaOH. To obtain non-fully labeled solutions, the labeled compounds were mixed with industrial Glc (δ13C = −9‰) or pyruvate (δ13C = −21‰) from Sigma. The resulting overall composition of the Glc and pyruvate solutions was checked to be 5,500‰ and 2,500‰, respectively. In other words, the 13C-enriched carbon atom position had a composition of 47,750‰ and 8,000‰, respectively (the other positions being at −9‰ and −21‰ for Glc and Pyr, respectively). The final concentration was 0.015 mol L−1 in all cases. The solutions were poured in an Eppendorf tube and fed to the leaves through the transpiration stream.
Acknowledgments
Guillaume Tcherkez acknowledges Salvador Nogués for his help for preliminary works on dark respiration.
This work was supported by the European Community's Human Potential Programme (grant no. HPRN–CT–1999–00059, NEtwork for Terrestrial ecosystems CARbon Budget, to J.G.) and by the Centre National de la Recherche Scientifique (to G.C. and R.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062141.
References
- Affek HP, Yakir D (2003) Natural abundance carbon isotope composition of isoprene reflects incomplete coupling between isoprene synthesis and photosynthetic carbon flow. Plant Physiol 131: 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Evans JR, Siebke K (1998) Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust J Plant Physiol 25: 437–443 [Google Scholar]
- Atkin OK, Millar AH, Gärdestrom P, Day DA (2000) Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis, Physiology and Metabolism. Kluwer Academic Publishers, London
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrate. J Cell Biol 133: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde RJA, Randall DD (1990) Pea leaf mitochondrial PDH complex is inactivated in vivo in a light-dependent manner. Proc Natl Acad Sci USA 87: 673–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G (1973) Etude de l'inhibition de la respiration par la lumière chez la moutarde blanche Sinapis alba L. Physiol Veg 11: 663–679 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Gerbaud A, André M (1987) An evaluation of the recycling in measurements of photorespiration. Plant Physiol 83: 933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R (1993) The C-13 nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem 286: 3986–3992 [PubMed] [Google Scholar]
- Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol 103: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding RW, Caroline DF, Wagner RP (1970) The pyruvate dehydrogenase complex from the mitochondrial fraction of neurospora crassa. Arch Biochem Biophys 138: 653–661 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Gardeström P (2003) Regulation of NAD and NADP dependent isocitrate dehydrogenases by reduction levels of pyridine nucleosides in mitochondria and cytosol of Pea leaves. Biochim Biophys Acta 1606: 117–125 [DOI] [PubMed] [Google Scholar]
- Kok B (1948) A critical consideration of the quantum yield of Chlorella photosynthesis. Enzymologia 13: 1–56 [Google Scholar]
- Krömer S (1995) Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 46: 45–70 [Google Scholar]
- Laisk AK (1977) Kinetics of Photosynthesis and Photorespiration in C3 Plants. Nauka, Moscow
- Lin M, Turpin DH, Plaxton WC (1989) Pyruvate kinase isozymes from the green alga Selenastrum minutum. Kinetic and regulatory properties. Arch Biochem Biophys 269: 228–238 [DOI] [PubMed] [Google Scholar]
- Marillia EF, Micallef BJ, Micallef M, Weninger A, Pedersen KK, Zou J, Taylor DC (2003) Biochemical and physiological studies of Arabidopsis thaliana lines with repressed expression of the mitochondrial pyruvate dehydrogenase kinase. J Exp Bot 54: 259–270 [DOI] [PubMed] [Google Scholar]
- Nogués S, Tcherkez G, Cornic G, Ghashghaie J (2004) Respiratory carbon metabolism following illumination in intact French bean leaves using 13C/12C isotope labeling. Plant Physiol 136: 3245–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 109–136 [DOI] [PubMed] [Google Scholar]
- Pärnik TR, Voronin PY, Ivanova HN, Keerberg OF (2002) Respiratory CO2 fluxes in photosynthesising leaves of C3 species varying in rates of starch synthesis. Russ J Plant Physiol 49: 729–735 [Google Scholar]
- Peisker M, Apel H (2001) Inhibition by light of CO2 evolution from dark evolution: comparison of two gas exchange methods. Photosynth Res 70: 291–298 [DOI] [PubMed] [Google Scholar]
- Pinelli P, Loreto F (2003) 12CO2 emission from different metabolic pathways measured in illuminated and darkened C3 and C4 leaves at low, atmospheric and elevated CO2 concentration. J Exp Bot 54: 1761–1769 [DOI] [PubMed] [Google Scholar]
- Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47: 185–214 [DOI] [PubMed] [Google Scholar]
- Quick WP, Scheibe R, Neuhaus HE (1995) Induction of hexose-phosphate translocator activity in spinach chloroplasts. Plant Physiol 109: 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp BJ, Miernyk JA, Randall DD (1987) Pyruvate dehydrogenase complexes from Ricinus communis endosperm. J Plant Physiol 127: 293–306 [Google Scholar]
- Schäfer G, Heber U, Heldt HW (1977) Glucose transport into spinach chloroplasts. Plant Physiol 60: 286–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Matthews MA, Boyer JS (1984) Kok effect and the quantum yield of photosynthesis: light partially inhibits dark respiration. Plant Physiol 75: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN, Day DA (2000) Respiration and photorespiration. In B Buchanan, W Gruissem, R Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- Stitt M (1990) Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol 41: 153–185 [Google Scholar]
- Stitt M, ap Rees T (1978) Pathways of carbohydrate oxidation in leaves of Pisum sativum and Triticum aestivum. Phytochemistry 17: 1251–1256 [Google Scholar]
- Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark respired CO2 in French bean. Plant Physiol 131: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Mendez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270: 1043–1049 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, ap Rees T (1994) The role of the hexose transporter in the chloroplasts of Arabidopsis thaliana L. Planta 195: 168–174 [Google Scholar]