Abstract
A large class of proteins with cytosolic functional domains is anchored to selected intracellular membranes by a single hydrophobic segment close to the C-terminus. Although such tail-anchored (TA) proteins are numerous, diverse, and functionally important, the mechanism of their transmembrane insertion and the basis of their membrane selectivity remain unclear. To address this problem, we have developed a highly specific, sensitive, and quantitative in vitro assay for the proper membrane-spanning topology of a model TA protein, cytochrome b5 (b5). Selective depletion from membranes of components involved in cotranslational protein translocation had no effect on either the efficiency or topology of b5 insertion. Indeed, the kinetics of transmembrane insertion into protein-free phospholipid vesicles was the same as for native ER microsomes. Remarkably, loading of either liposomes or microsomes with cholesterol to levels found in other membranes of the secretory pathway sharply and reversibly inhibited b5 transmembrane insertion. These results identify the minimal requirements for transmembrane topogenesis of a TA protein and suggest that selectivity among various intracellular compartments can be imparted by differences in their lipid composition.
Keywords: endoplasmic reticulum, membrane proteins, protein targeting, protein translocation, Sec61 translocon
Introduction
In eukaryotic cells, the membranes that delimit organelles provide not only a compartment within which to sequester components, but also a membrane surface on which proteins with cytosolic activities can be spatially localized. These cytosolic activities are often carried by membrane proteins endowed with a single C-terminal hydrophobic domain capable of insertion into the lipid bilayer. Such tail-anchored (TA) proteins are found on all intracellular membranes exposed to the cytosol, and are involved in a remarkably diverse range of physiologic processes ranging from intracellular trafficking to protein degradation and programmed cell death (reviewed in Borgese et al, 2003). Thus, deciphering the molecular details underlying the selective membrane insertion and trafficking of TA proteins is critical to understanding a wide range of cell biological and physiological processes. Despite this importance, the mechanisms utilized by TA proteins to arrive at and insert into their target membrane are largely unresolved.
The ER is the target of most newly synthesized TA proteins, since all those destined to compartments of the secretory pathway first insert into this membrane and then travel to their final residence by vesicular trafficking (Jäntti et al, 1994; Kutay et al, 1995; Linstedt et al, 1995; Pedrazzini et al, 1996). Insertion of membrane proteins into the ER has been studied most extensively for non-TA proteins, whose translocation and integration occurs by a ribosome-dependent cotranslational mechanism. A hydrophobic domain, either a signal sequence or a transmembrane segment, is recognized upon its emergence from the ribosome by the signal recognition particle (SRP). After targeting of this ribosome–nascent chain–SRP complex to the ER membrane, interactions between the Sec61 translocation channel and transmembrane segments mediate their subsequent cotranslational integration into the lipid bilayer (Rapoport et al, 1996). TA proteins stand apart from other membrane proteins, because their membrane anchor is still buried inside the ribosome when the stop codon is reached. Thus, it is clear that TA protein targeting and insertion into the lipid bilayer must occur by a ribosome-independent pathway after synthesis of the entire protein has been completed. What remains unknown is whether qualitatively distinct components and mechanisms are involved in the insertion of TA and non-TA membrane proteins into the lipid bilayer.
Studies attempting to address this issue have yielded differing results. For one commonly used model TA protein, cytochrome b5 (b5), functional analyses using yeast mutants defective in the Sec61 system showed no impairment of b5 insertion (Yabal et al, 2003), arguing that the phenomenon occurs independently of the known Sec61 functions. However, these results did not exclude the possibility that the Sec61 translocon does function in b5 insertion, but by a mechanism not affected by the analysed mutations. For another widely studied TA protein, synaptobrevin, some investigators found that its insertion in vitro can proceed independently of either the Sec61 complex or SRP receptor (Kutay et al, 1995), although at least one ER membrane protein has been implicated (Kutay et al, 1995; Kim et al, 1997). On the other hand, cross-linking experiments revealed a transient association of the in vitro synthesized protein with Sec61 (Abell et al, 2003), as well as an unexpected post-translational interaction with SRP54 (Abell et al, 2004). While a functional role for the Sec61 complex remains to be established, synaptobrevin binding to membranes in vitro was decreased in the absence of either SRP or its receptor (Abell et al, 2004).
One problem in interpreting in vitro studies on TA protein insertion is that most of the reported results are based on membrane-binding assays that may not necessarily measure correct transmembrane integration. This problem is well exemplified by the studies with b5. There has been general agreement that both the purified protein isolated from tissues and the in vitro synthesized polypeptide are capable of binding to a wide range of natural and artificial membranes, including protease-treated microsomes and protein-free liposomes (reviewed in Borgese et al, 2003). However, whereas the native protein in vivo is inserted in the ER with transmembrane topology (Kuroda et al, 1996; Pedrazzini et al, 2000), the purified protein can associate tightly with lipid bilayers in a hairpin conformation, that is, with the N and C termini both exposed to the outside of the vesicles (Dailey and Strittmatter, 1981; Arinc et al, 1987). The capability of b5 to bind to lipid bilayers with an unphysiological topology may explain the discrepancy between its specific ER localization in vivo (D'Arrigo et al, 1993) and its promiscuity in in vitro assays (Remacle, 1978; Kim et al, 1997).
To overcome the difficulties inherent in binding assays, we previously used an epitope-tagged b5 containing an N-glycosylation site close to the C-terminus. By adopting glycosylation as a stringent criterion for ER translocation, a transmembrane topology for b5 could be demonstrated in vivo and in vitro (Pedrazzini et al, 2000; Borgese et al, 2001; Yabal et al, 2003). One drawback of this assay is that it requires all of the components for the N-glycosylation reaction to be present and active in the membrane. For this reason, the glycosylation assay is not useful for in vitro work with proteoliposomes reconstituted from fractionated microsomal extracts, an approach that has been of fundamental importance for the identification of membrane components involved in cotranslational translocation (Nicchitta et al, 1991; Görlich and Rapoport, 1993). The application of this same approach to the problem of TA protein insertion therefore necessitates an appropriately specific and quantitative translocation assay.
Here, we have developed a rigorous assay for transmembrane integration of a TA protein based on protection from proteolysis of the translocated C-terminal tail. This assay allowed us to quantitatively assess both the efficiency and kinetics of topologically correct b5 insertion into proteoliposomes reconstituted from systematically fractionated ER microsomal components. The results from this analysis, together with the demonstration of efficient transmembrane insertion into protein-free liposomes, argue strongly against either essential or even stimulatory membrane protein requirements. Instead, insertion efficiency is strikingly modulated by alterations in lipid composition of the vesicles, a finding that may explain the long-observed in vivo targeting specificity of b5, as well as of other ER-directed TA proteins.
Results
Assay design and characterization
We devised and tested a protease protection approach to selectively and directly detect the properly inserted C-terminal tail of a model TA protein (Figure 1A). The substrate we chose is a b5 variant, called b5-Nglyc, that has been well characterized in vitro and in vivo (Pedrazzini et al, 2000; Yabal et al, 2003). The C-terminus of this substrate contains not only a glycosylation site that facilitates optimization and validation of the protease assay, but also a 19-residue sequence (from the N-terminus of bovine opsin) that is recognized by a monoclonal antibody (Adamus et al, 1991) (see Figure 1A). In this assay, post-translational translocation of the C-terminus of b5-Nglyc across the membrane of a closed vesicle should result in protease protection of a ∼5 kDa peptide that contains three of five 35S-methionine residues, an intact opsin epitope, and, depending on the membranes used, a single high-mannose carbohydrate tree (which would increase the apparent size by ∼4 kDa). While glycosylation serves as an independent marker of translocation, it is important that it not be required if the assay is to be useful in fractionated proteoliposomes. Hence, immunological detection of the protected C-terminal peptide should serve as a specific and quantitative marker of proper b5-Nglyc topogenesis.
Figure 1.
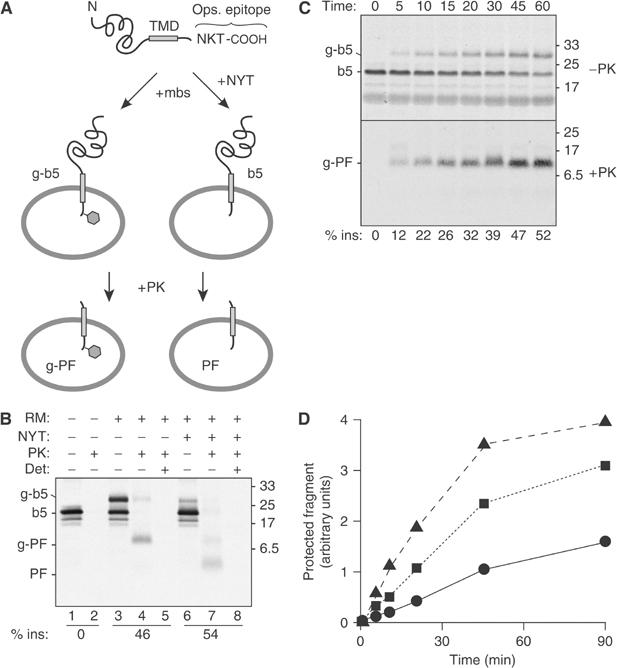
Protease protection assay for transmembrane insertion of a TA protein. (A) Illustration of the model TA protein construct (b5-Nglyc) and assay design. (B) In vitro translated b5-Nglyc was post-translationally incubated with ER-derived RMs in the absence or presence of a tripeptide inhibitor of glycosylation (NYT). The samples were then divided for digestion with PK in the presence or absence of the detergent TX100. Equal amounts of each sample were subsequently immunoprecipitated with antibodies against the C-terminal opsin tag prior to analysis by SDS–PAGE and autoradiography. The positions of the primary translation product (b5), glycosylated b5 (g-b5), and their respective protease-protected fragments (PF and g-PF) are indicated to the left. The efficiency of insertion (% ins) is indicated below the respective lanes. (C) Time course of glycosylation and translocation of b5-Nglyc. Translated b5-Nglyc was incubated with RMs for the indicated times. For each time point, total nondigested and PK-digested immunoprecipitated samples are shown in the upper and lower panel, respectively. A nonglycosylated PF band could not be detected even on longer exposures of the autoradiograph. (D) Time course of generation of b5-Nglyc PF with different loads of pig RM: 0.1 eq/μl (•); 0.35 eq/μl (▪); 0.7 eq/μl (▴). In all figures, numbers on the side of the panels indicate the position and size (in kDa) of Mr markers.
These expectations were borne out by the experiment illustrated in Figure 1B. Approximately 50% of the ∼20 kDa b5-Nglyc polypeptide (indicated as b5 in lane 1) generated by in vitro translation in reticulocyte lysate (RL) was converted by post-translational incubation with ER-derived rough microsomes (RMs) into a glycosylated form (g-b5, lane 3). When the translation mix without added RM was exposed to proteinase K (PK), no immunoprecipitable b5-Nglyc fragments were detected (lane 2), even after gross overexposure of the autoradiograph (not shown). By contrast, post-translational incubation with RM prior to PK digestion generated an ∼9 kDa protected fragment (g-PF, lane 4) that was immunoprecipitated by the opsin antibody. The g-PF band was not seen if detergent was included during the PK digestion, demonstrating that its generation is dependent on an intact membrane (lane 5). The identification of g-b5 and g-PF as glycosylated products was verified by their markedly reduced generation when parallel reactions were performed in the presence of a tripeptide (NYT) that competitively inhibits glycosylation (lanes 6–8). In this case, PK treatment generated a ∼5.5 kDa PF (lane 7), which, like g-PF, was digested in the presence of detergent (lane 8). These results not only demonstrated the high sensitivity and specificity of the assay, but also indicated that glycosylation itself is not required to stabilize the transmembrane topology of b5-Nglyc (lane 7 versus 4). In addition, analysis of the nonimmunoprecipitated products (Supplementary Figure S1) demonstrated the absence of topologically incorrect b5-Nglyc transmembrane integration.
Since PK (at the 0.25 mg/ml concentration we routinely used) can substantially digest substrates within seconds after addition (data not shown), we reasoned that the protection assay could also be used to monitor the kinetics of b5-Nglyc insertion. A comparison of the time course of glycosylation and of PF generation (Figure 1C) showed that the two phenomena proceeded in parallel, since no unglycosylated peptide was detected even at early time points. These results demonstrate that the rate-limiting step for glycosylation in RM is translocation across the microsomal membrane. Furthermore, insertion under these conditions progresses steadily for over 30 min, achieving the maximal translocation of ∼50% only after ∼45 min. This moderate efficiency and kinetics allows both easily detectable translocation and any potential changes that may be caused by manipulations to membrane composition. Indeed, changing the amount of RM in the reaction results in readily detectable changes in transmembrane insertion of b5-Nglyc, with increasing rate and efficiency at higher concentrations of vesicles (Figure 1D).
Since glycosylation in RM proceeds essentially simultaneously with insertion, glycosylation efficiency (which is readily evaluated in the undigested sample) provides a direct measure of insertion efficiency. Accordingly, the immunoprecipitated g-PF band for the RM sample represents a quantitative standard for this level of insertion. This means that the g-PF band can be compared to the PF and/or g-PF bands of parallel samples to judge their insertion efficiencies relative to this standard. Thus, as long as an insertion reaction with RM is analysed in parallel, b5-Nglyc insertion can be readily quantified even in the absence of glycosylation (e.g., in reconstituted proteoliposomes, as shown in Figures 2 and 3, and Supplementary Figure S5).
Figure 2.
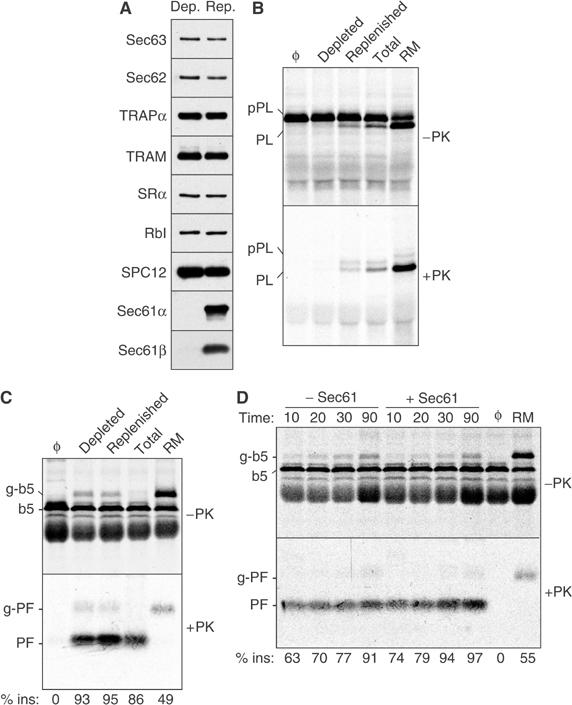
Transmembrane integration of b5-Nglyc is unaffected by Sec61 depletion. (A) Proteoliposomes reconstituted from a Sec61-depleted extract or a depleted extract replenished with purified Sec61 complex were analysed by immunoblotting against various translocation-related ER proteins (SRα, α subunit of the SRP receptor; RbI, ribophorin I; TRAM, translocating chain-association membrane protein; TRAPα, α subunit of the translocon-associated protein; SPC, signal peptidase complex). (B) The Sec61-depleted and replenished proteoliposomes from panel A were assayed for cotranslational translocation of pPL. Equal aliquots of the translocation reaction were either left untreated (top panel) or digested with PK (bottom panel) prior to analysis by SDS–PAGE. Translocation reactions containing native RM, proteoliposomes made with a total RM extract (Total), or without added vesicles (Φ) are also included for comparison. The positions of precursor (pPL) and signal-cleaved PL are indicated. (C) The various membranes analysed in panel B were assayed for b5-Nglyc translocation using the protease protection assay. (D) The time course of b5-Nglyc translocation into Sec61-depleted and replenished proteoliposomes was assayed by protease protection. Reactions lacking vesicles (Φ) or containing RM are also shown.
Figure 3.
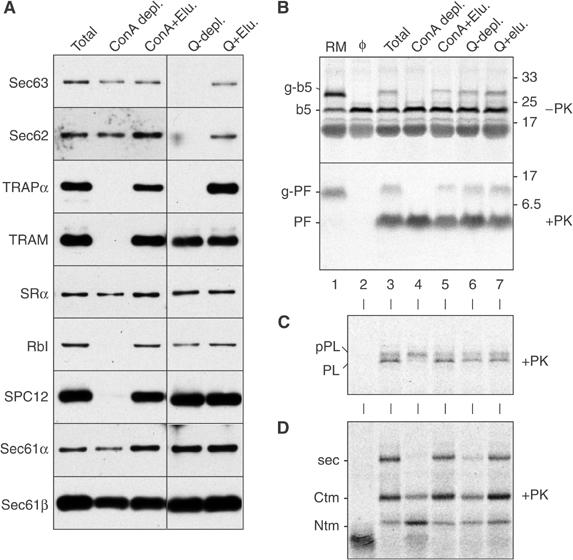
Proteoliposomes made with differently depleted extracts support b5-Nglyc transmembrane insertion with equal efficiency. (A) Immunoblot analysis of proteoliposomes reconstituted from either a total RM extract, ConA-depleted extract, Q-Sepharose-depleted extract, or the depleted extracts replenished with the material eluted from the corresponding resins (+Elu.). Abbreviations are as in the legend to Figure 2. The various proteoliposomes from panel A were analysed for post-translational translocation of b5-Nglyc (B) or cotranslational translocation of pPL (C) and PrP (D). For pPL and PrP, only the PK digested samples are shown. The PF band indicative of b5-Nglyc translocation into proteoliposomes was not observed if detergent was included during the protease digestion reaction (Supplementary Figure S4). The positions of the fully translocated form of PrP (termed secPrP) and the protected fragments generated from two transmembrane forms (termed CtmPrP and NtmPrP) are indicated by ‘sec', ‘Ctm', and ‘Ntm', respectively (Fons et al, 2003).
Analysis of b5 translocation upon Sec61 depletion
To examine the membrane requirements for b5 translocation, it is necessary to reassemble translocation-competent proteoliposomes using components solubilized from RM. Using conditions that had previously been used to dissect the requirements for cotranslational translocation, we first examined the role of the Sec61 complex, the only known protein-conducting channel of the mammalian ER (Rapoport et al, 1996). A microsomal detergent extract was immunodepleted of the Sec61 complex and then reconstituted into proteoliposomes either before or after replenishment with purified Sec61 complex to the original levels. By immunoblot analysis, both Sec61α and Sec61β were undetectable in the immunodepleted proteoliposomes, while other RM components were unaffected (Figure 2A and Supplementary Figure S2).
As shown in Figure 2B, translocation of the cotranslational signal peptide-containing substrate preprolactin (pPL) into the Sec61-depleted proteoliposomes was sharply impaired, as judged by a lack of both signal peptide cleavage and protease protection. Replenishment with Sec61 allowed pPL translocation to levels comparable to unfractionated proteoliposomes (which are still much less efficient than native RMs). In sharp contrast to pPL, translocation of the C-terminus of b5-Nglyc proceeded equally well across Sec61-depleted, replenished, or unfractionated proteoliposomes (Figure 2C), as revealed by generation of the ∼5.5 kDa PF. Although reconstituted proteoliposomes are rather inefficient in N-glycosylation (Görlich et al, 1992), longer exposures revealed a small but equal degree of glycosylation of b5-Nglyc in the depleted or replenished proteoliposomes (and corresponding g-PF after PK digestion). This independently confirmed a lack of effect of Sec61 depletion on b5-Nglyc transmembrane integration.
We also evaluated the possibility that slow but eventual complete insertion of b5-Nglyc was responsible for the lack of effect after the 1 h translocation reaction in Figure 2C. However, a time course of b5-Nglyc translocation into the depleted and replenished proteoliposomes revealed comparable efficiencies and rates of insertion in both cases (Figure 2D). Of note, Figure 2C and D revealed that the overall efficiency of b5-Nglyc translocation into the proteoliposomes was even higher than that seen with native RM. This is markedly different from cotranslational translocation (Figure 2B), which is far less efficient in the proteoliposomes than in RM, despite the two preparations containing comparable amounts of various translocon components (Supplementary Figure S2). This suggested that features of the membrane required for TA protein insertion are functionally reconstituted far more efficiently than the components needed for cotranslational translocation. This observation is discussed further below, after presentation of the data of Figure 4.
Figure 4.
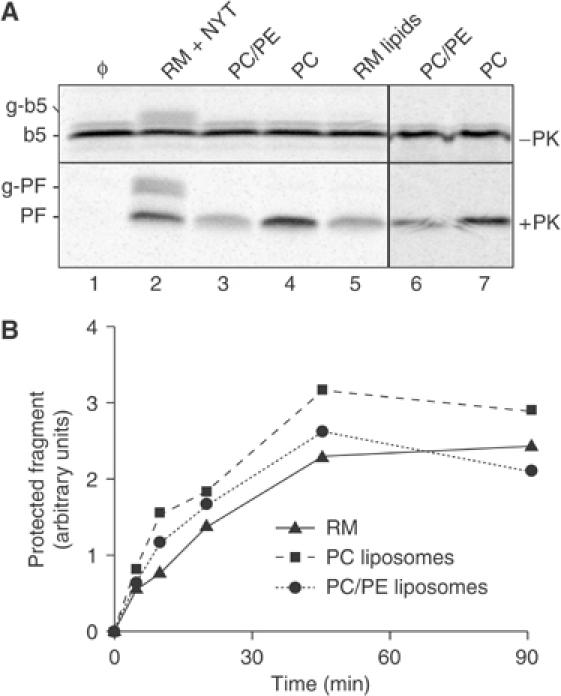
Post-translational translocation of b5-Nglyc into protein-free liposomes. (A) Standard b5-Nglyc translocation reactions were performed for 90 min with protein-free liposomes prepared from PC, a mixture of PC and PE in a 4:1 ratio, or total lipids extracted from RM (RM lipids). Liposomes were made either using extrusion of a detergent-free lipid suspension (lanes 3–5) or by removal of detergent (DBC method, lanes 6 and 7). Reactions in the absence of added vesicles (Φ) or with pig RMs in the presence of NYT were analysed in parallel. A total of 10 μg phospholipid per 10 μl reaction volume was used in lanes 2–7. The PF band indicative of b5-Nglyc translocation into liposomes was not observed if detergent was included during the protease digestion reaction (Supplementary Figure S4). (B) Time course of b5-Nglyc translocation into protein-free liposomes (prepared by extrusion) compared with pig RMs. The amount of PF generated by the standard protease protection assay was quantified by phosphorimaging and plotted on the y-axis. For the RM sample, the sum of PF and g-PF, the latter due to incomplete inhibition of glycosylation by the NYT tripeptide, is given.
Evaluation of membrane protein requirements during b5 translocation
Having excluded the involvement of the Sec61 complex, we next turned to other microsomal proteins that might facilitate b5-Nglyc insertion. We first fractionated microsomal detergent extracts by chromatographic procedures based either on anion exchange (Q-sepharose) or on affinity depletion of glycoproteins by immobilized concanavalin A (ConA), and used the depleted extracts to reconstitute proteoliposomes. Western blot analysis of the proteoliposomes demonstrated that numerous proteins known or implicated in translocation were depleted in at least one of the samples by more than 90% (Figure 3A and Supplementary Figure S3). However, we could not detect any difference in b5-Nglyc translocation into the fractionated proteoliposomes compared to the unfractionated controls (Figure 3B, lanes 4 and 6 versus lane 3), and replenishment of the depleted proteoliposomes with material eluted from the respective resins had no effect on the extent of translocation (lanes 5 and 7). Insertion efficiencies into the proteoliposomes (more than 90% in each case) were again better than into RM (∼60%). Notably, the ribophorin I depletion mediated by ConA (panel A) did impair glycosylation of b5-Nglyc (lane 4), as expected.
The functional effectiveness of the fractionation and reconstitution procedures was further verified by analyses of the classical pPL substrate and of Prion protein (PrP) translocation. The latter differs from pPL in its requirement for the TRAP complex for complete translocation into the ER lumen, in the secPrP form (Fons et al, 2003). The absence in ConA-depleted proteoliposomes of the signal peptidase complex (Figure 3A) was functionally correlated to lack of pPL signal cleavage, but not its translocation (Figure 3C, lane 4). Similarly, complete translocation of PrP across the membrane (secPrP form, indicated as sec in Figure 3D) was inhibited in both the ConA- and Q-depleted proteoliposomes (Figure 3D, lanes 4 and 6), both of which lack the TRAP complex (Figure 3A). Thus, while the fractionation procedures clearly influenced various translocon-related activities, the proper transmembrane insertion of b5-Nglyc was unchanged.
Proteoliposomes made from numerous other ion-exchange fractions or protease-digested detergent extracts also supported efficient transmembrane insertion of b5-Nglyc (Supplementary Figure S5). Taken together, these results suggested that no single microsomal protein or lipid species was specifically required for b5-Nglyc translocation. Furthermore, it appears that bulk proteins do not stimulate or inhibit b5-Nglyc insertion nonspecifically. This conclusion is based on the collective observations in Figures 2 and 3, and Supplementary Figure S5 that protein mixtures of vastly different composition and abundance have little influence on b5-Nglyc insertion when incorporated into lipid vesicles.
Analysis of translocation across protein-free liposomes
The above results together suggested that the C-terminus of in vitro synthesized b5-Nglyc can be translocated completely across the lipid bilayer without the aid of any proteins. To test this hypothesis, we analysed b5-Nglyc translocation into protein-free liposomes of differing composition. Phosphatidylcholine (PC), a mixture of PC and phosphatidylethanolamine (PE) in the ratio approximating that in ER membranes (Colbeau et al, 1971), or total lipids extracted from RM, were used to prepare the liposomes using either the detergent removal method or a detergent-free extrusion procedure (see Materials and methods). All preparations were present at the same phospholipid concentration (1 μg phospholipids/μl) so as to provide comparable membrane surface areas. As shown in Figure 4, with all liposome preparations, we obtained translocation efficiencies comparable to that obtained with native RM at the same phospholipid concentration, although the PC/PE mixture and the total RM lipids appeared somewhat less efficient than pure PC liposomes. Determination of the time course of translocation (Figure 4B) confirmed that the protein-free liposomes were as efficient as native RM in translocating the C-terminus of b5-Nglyc.
This finding offers an explanation to the finding that insertion of b5-Nglyc into proteoliposomes was actually more efficient than into the RM from which they were derived (e.g., Figures 2C, D, and 3B). Proteoliposomes are resuspended in one-fifth the volume of the starting RM to compensate for the reduced recovery of proteins during the solubilization, fractionation, and reconstitution procedures (see Materials and methods). Thus, they contain protein levels comparable to that found in the RM (see Supplementary Figures S2 and S3), although they likely contain more actual vesicles than the RM because the lipids are recovered more efficiently than the proteins. Since insertion can occur into protein-free liposomes (Figure 4), the increase in vesicle content in the proteoliposome preparations would readily explain the higher insertion efficiency relative to RM. Indeed, insertion efficiency can also be increased simply by increasing the RM concentration in the assay (Figure 1D).
The results showing equally efficient translocation across protein-free bilayers and native RM membranes appear at variance with in vivo studies showing that b5 and b5-Nglyc are selectively targeted to the ER (Borgese et al, 2003). Since the in vitro analyses argue against membrane protein(s) providing the requisite specificity for ER insertion, we considered alternative explanations. One important difference between the ER bilayer and the other membranes of the secretory pathway is in cholesterol content, which is low in the ER relative to downstream compartments of the exo- and endocytic systems (van Meer, 1989). We therefore investigated the effect of cholesterol on b5-Nglyc transmembrane integration.
First, RMs were loaded with cholesterol using cholesterol–methyl-β-cyclodextrin (MβCD) complexes and then tested for their ability to translocate b5-Nglyc. Strikingly, cholesterol loading of the RMs with an extra 0.06 molar ratio (relative to phospholipids) severely reduced their capacity to translocate b5-Nglyc relative to RMs incubated with cholesterol-free MβCD (Figure 5A, lanes 1 and 2). A higher molar ratio of cholesterol (0.09) resulted in near complete inhibition of b5-Nglyc translocation (lane 3). Importantly, the 0.06 and 0.09 molar ratios of added cholesterol only increase the estimated 0.05 molar ratio in native ER membranes (Colbeau et al, 1971) by ∼2–3-fold. Cholesterol assays on the treated RMs confirmed the expected increase in content (data not shown). The inhibitory effect of cholesterol on b5-Nglyc translocation was readily reversed by treatment of the cholesterol-loaded RM with excess MβCD to remove the added cholesterol (lanes 4 and 5).
Figure 5.
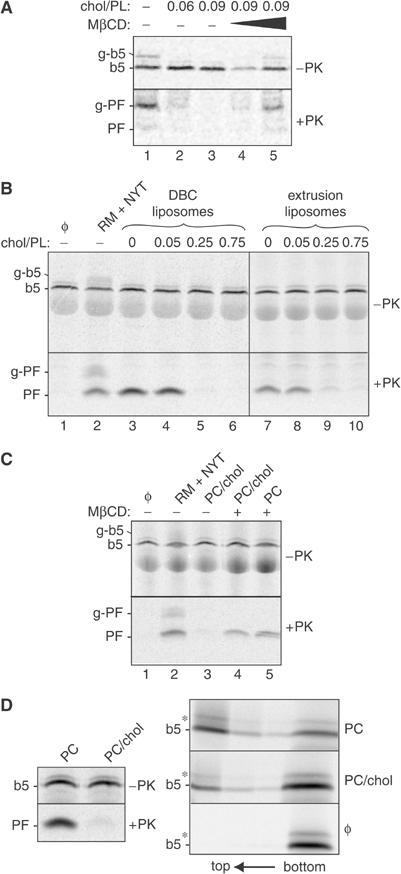
Inhibition of b5-Nglyc transmembrane integration by membrane cholesterol. (A) Pig RMs loaded with cholesterol added in a molar ratio to microsomal phospholipids as indicated were tested for translocation using the protease protection assay. An aliquot of RMs loaded with the highest amount of cholesterol (at 0.09 molar ratio) was incubated in a second step with either nine- or 17-fold excess MβCD (lanes 4 and 5, respectively) to extract the cholesterol before carrying out b5-Nglyc insertion. (B) Protein-free PC liposomes containing different molar ratios of cholesterol, as indicated, were prepared either by the DBC-Biobeads method (lanes 3–6) or by extrusion (lanes 7–10), and tested for b5-Nglyc insertion by protease protection. All 10 μl samples, including the one with native RMs (lane 2), contained 10 μg of total lipids. (C) PC/cholesterol liposomes (0.75 molar ratio) prepared by extrusion were either incubated in buffer (lane 3), or in buffer containing a 30-fold molar excess of MβCD over cholesterol (lane 4). In parallel, PC liposomes lacking cholesterol were also treated with MβCD (lane 5). Removal of cholesterol from the PC/cholesterol liposomes restores b5-Nglyc translocation to the levels seen with pure PC liposomes (lane 5) and native RMs (lane 2). (D) Alkaline sucrose gradient flotation detects tightly bound, nontranslocated b5-Nglyc. In vitro translated b5-Nglyc was post-translationally incubated with PC or PC/cholesterol liposomes (0.75 molar ratio) prepared by extrusion. Half of each sample was subjected to the protease protection assay (left panel), while the other half was analysed by flotation through alkaline discontinuous sucrose gradients (right panel). Lipid vesicles float to the top two fractions of the gradient. Note that, in the absence of added vesicles, b5-Nglyc was not detected in these fractions. The asterisk indicates a nonspecific band.
Next, we tested the effect of including cholesterol in protein-free liposomes. Cholesterol to phospholipid molar ratios were chosen to reflect those reported for rough ER (∼0.05—Colbeau et al, 1971), Golgi membranes (∼0.25—Fleischer et al, 1974; Brugger et al, 2000), or plasma membrane (∼0.75—Colbeau et al, 1971). Inclusion of cholesterol at ER amounts had no effect on the translocation reaction (Figure 5B, lanes 4 and 8), while higher levels were severely inhibitory (lanes 5, 6, 9, and 10). The effects of cholesterol were seen regardless of whether the liposomes were prepared by detergent removal (lanes 3–6) or by extrusion (lanes 7–10). If cholesterol-containing liposomes were incubated with excess MβCD, their translocation capacity was restored to the levels obtained with cholesterol-free liposomes or native RM (Figure 5C).
The striking effect of cholesterol on b5-Nglyc insertion was surprising in view of previous reports of efficient binding of b5 to cholesterol-containing membranes (Remacle, 1978; Kim et al, 1997). To investigate the basis of the discrepancy between our results and previous reports, we directly compared the protease protection assay for b5-Nglyc with parallel determinations of its alkaline-resistant binding to membranes (Figure 5D). The protease protection assay again showed that inclusion of cholesterol in the liposomes (in 0.75 molar ratio to phospholipids) nearly completely abolished b5-Nglyc transmembrane integration (top panel). By striking contrast however, in the same sample, tight, alkaline-resistant binding of b5-Nglyc to the vesicles floating to the top of a sucrose gradient was only modestly reduced (bottom panel).
Quantification of the data of Figure 5D revealed that the ratio of insertion efficiencies into liposomes lacking versus containing cholesterol was ∼24:1, as judged by the PK protection assay. Yet, the same samples showed a mere 2.7:1 ratio when assayed by alkaline-resistant binding, indicating that the vast majority of b5-Nglyc floated with the PC/cholesterol liposomes was associated in a manner that did not have the correct membrane-spanning topology. These results indicate that the previous conclusion of nonselective b5 insertion into liposomes must be tempered to distinguish between transmembrane and nontransmembrane modes of association.
Discussion
Despite the wide functional diversity and current interest in TA proteins, membrane factors that influence or regulate their post-translational insertion have been elusive. This has been in large part due to assays that have been insufficiently sensitive and specific to allow methodical, quantitative analyses. In the present study, we have developed a protease protection assay that scores only a membrane-inserted TA protein whose C-terminus has been translocated across the bilayer. Application of this assay to the systematic evaluation of the role of membrane components in b5-Nglyc transmembrane integration revealed a high efficiency of insertion of this TA protein into cholesterol-poor membranes, with a surprisingly sharp inhibition at levels of the sterol only slightly higher than those found in native ER membranes. The striking specificity for cholesterol-poor membranes may at least partially explain the restricted in vivo targeting of b5 to ER membranes, as the higher cholesterol content of downstream membranes of the exo- and endocytic systems (Colbeau et al, 1971; van Meer, 1989) could hinder TA protein insertion. Beyond this specificity factor, several independent approaches argued against the existence of any single membrane protein that is required for translocation of the C-terminus of b5-Nglyc. In particular, numerous components of the classical ER translocation machinery, including the Sec61 complex, did not functionally influence b5-Nglyc insertion. While the in vitro translocation of small polypeptide domains across pure lipid vesicles had been demonstrated for a few bacterial membrane proteins (reviewed in van Dalen and de Kruijff, 2004), to our knowledge, this is the first time that the phenomenon has been clearly documented in a eukaryotic system. Our results define the minimal requirements for translocation of a C-terminal transmembrane domain across the lipid bilayer and provide insight into one mechanism for achieving selectivity in membrane insertion.
Although it has long been known that b5 can bind to protein-free liposomes (Enoch et al, 1979; Kim et al, 1997), the requirements for physiologically relevant insertion and a molecular basis for the membrane selectivity observed in vivo have remained a matter of debate. In hindsight, the majority of previous seemingly contradictory observations can now be reconciled if two key aspects are considered. First, there are clearly multiple modes of b5 binding to membranes that range from a relatively ‘loose' binding to at least two ways of tight integration into the bilayer (Enoch et al, 1979; Dailey and Strittmatter, 1981; Pedrazzini et al, 2000). Among these, the physiologically relevant topology is a transmembrane orientation with the C-terminus translocated across the lipid bilayer (Kuroda et al, 1996; Pedrazzini et al, 2000). Since the topology of the in vitro synthesized and inserted protein was not assessed in previous work, distinctions between modes of b5 insertion that might exist among different types of membranes would have been largely obscured. As demonstrated here (Figure 5D), insertion (as measured by binding assays) can appear to be far more promiscuous than actually is the case when only the physiologically relevant transmembrane topology is considered.
The second key experimental parameter is the source of b5 that is used in the translocation assays: b5 purified from its native source (liver) versus de novo synthesized b5 in a cytosolic extract (e.g., RL). It is known that purified b5 in the absence of detergent forms protein micelles (Spatz and Strittmatter, 1971), while in vitro synthesized b5 most likely associates with chaperones that prevent its aggregation. This difference may explain why purified b5 binds to dimyristoyl PC liposomes in a ‘hairpin' conformation (Dailey and Strittmatter, 1981; Arinc et al, 1987), while the in vitro translated protein inserts into PC liposomes with transmembrane topology (Figure 4). The different results obtained with purified b5 and with the in vitro synthesized protein suggest that cytosolic factors may play a critical role in transmembrane insertion of b5 into the membrane. The identification of these cytosolic factors can now be pursued using the assay developed in this study, and represents a main goal of our future research.
An important question is whether the findings with b5-Nglyc are applicable to other TA proteins. Some TA proteins (e.g., synaptobrevin (Kutay et al, 1995; Kim et al, 1997; Abell et al, 2004) and the yeast vacuolar protein Nyv1p (Steel et al, 2002)) require a membrane-associated trypsin-sensitive component to post-translationally bind to microsomes, whereas others (e.g., Bcl2 (Kim et al, 1997)) may be similar to b5 in their lack of requirement for microsomal membrane protein(s). One way to reconcile the available observations is to postulate that the actual translocation step across the membrane occurs without any membrane factors (as demonstrated here for b5) and proceeds similarly for all TA proteins. In this view, some substrates would need factors in the cytosol and/or membrane to efficiently bring the TA protein in close proximity to the lipid bilayer. In addition to lipid composition, such factors could further refine the targeting specificity of TA proteins. Thus, TA polypeptides which do not strictly require membrane proteins for their insertion would be targeted by factors that increase the kinetics of delivery to the membrane. Rapid delivery to the target membrane may be particularly important for some, but not all TA proteins, and could hinder inappropriate insertion into otherwise permissive bilayers. This may be important for avoiding insertion of b5 into mitochondrial membranes, which, like the ER, is thought to be relatively cholesterol-poor. Alternatively, other lipid differences between the ER and mitochondrial outer membrane may provide a source of discrimination in a manner analogous to cholesterol. Future work using the methods developed in this study to examine TA protein insertion into fractionated and reconstituted mitochondrial outer membranes will be needed to address this issue.
The framework discussed in our study is entirely consistent with recent observations that SRP and its membrane receptor facilitate the insertion of various TA proteins to differing degrees (Abell et al, 2004). Similarly, the requirement for a trypsin-sensitive membrane component for insertion of some, but not other, TA proteins could also be reconciled. Thus, the argument that multiple parallel pathways operate in TA protein insertion (High and Abell, 2004) may not be necessary. Instead, a core translocation pathway may exist in which several accessory proteinaceous factors have differing levels of importance for different subclasses of TA proteins. This model is analogous to the Sec61-mediated pathway, which consists of the core protein-conducting channel and accessory factors (Rapoport et al, 1996). For cotranslational translocation, the Sec61 channel plus SRP and its receptor are necessary for all substrates (Görlich and Rapoport, 1993), but sufficient for only a very few (such as the model protein pPL). Additional components, like the TRAM protein (Görlich et al, 1992) and the TRAP complex (Hartmann et al, 1993; Fons et al, 2003), are required for most proteins. In the case of Sec61-mediated post-translational translocation in yeast, four additional membrane components plus the lumenal chaperone Kar2p play essential or stimulatory roles (Panzner et al, 1995). It therefore seems plausible that b5, like pPL for signal-sequence-driven translocation, represents a model substrate that allows the definition of the core principles of the TA protein insertion pathway. The future challenge will be to determine the ways in which such a core pathway has been embellished to accommodate the very wide range of TA proteins that must be properly compartmentalized in all eukaryotic cells.
Materials and methods
In vitro translocation assays
Constructs encoding b5-Nglyc, pPL, and PrP behind the SP6 promoter have been described (Hegde et al, 1998; Pedrazzini et al, 2000). Transcription and translation of proteins in the RL system were as described (Fons et al, 2003). For a standard b5-Nglyc translocation reaction, translation was stopped by addition of cycloheximide to 0.3 mg/ml, and the ribosomes were removed by sedimentation (55 000 r.p.m., 30 min in the Beckman TLA100.3 rotor). Independent experiments confirmed that these spin conditions quantitatively remove ribosome-associated nascent chains (data not shown). In all, 10 μl of the supernatant was incubated at 32°C for 1 h with the specified vesicles. Unless otherwise indicated, RM and proteoliposomes were used at a final concentration of 0.1 and 0.5 equivalents (eq)/μl (see Walter and Blobel (1983) for a definition). In Figures 4 and 5, samples contained equal amounts of phospholipids (1 μg/μl—assayed according to Ames and Dubin, 1960), corresponding to 0.35 eq of native RM/μl). Where indicated, the tripeptide NYT was included at 100 μM. After incubation, a small aliquot of each sample was removed and directly analysed by SDS–PAGE to confirm equal amounts of translation product in each tube and to assess the degree of glycoyslation (for b5-Nglyc) or of signal cleavage (for pPL). The remainder of each sample was digested with 0.25 mg/ml PK for 30 min at 0°C. PK was inhibited by addition of PMSF to 2 mM, and the reaction mixtures were transferred to 100 μl of boiling 1% SDS/0.1 M Tris, pH 8.8. IP buffer (50 mM Hepes-K+, pH 7.6, 100 mM NaCl, 1% Triton X-100 (TX100)) was added to 1 ml before immunoprecipitation with anti-opsin monoclonals (8 μg IgG/ml). Identical results were obtained when translation reactions were treated with puromycin before translocation (data not shown), confirming that insertion occurred post-translationally. For time-course experiments, aliquots were transferred at the indicated time points from the translocation sample to tubes containing pre-aliquotted PK and then treated as above. Translocation assays of pPL and PrP have been described (Hegde et al, 1998).
Preparation of proteoliposomes
RMs from dog or pig pancreas were prepared according to Walter and Blobel (1983). A detergent extract of RM membrane proteins using deoxy Big CHAP (DBC) was prepared as described previously (Fons et al, 2003). Fractionation of DBC extracts by immunodepletion, ConA chromatography, and ion exchange chromatography has been described (Görlich and Rapoport, 1993; Hegde et al, 1998; Fons et al, 2003). Each of the various fractionated detergent extracts above was reconstituted into proteoliposomes by detergent removal using BioBeads SM (BioRad) in the presence of lipids (a 4:1 mixture of PC and PE purchased from Avanti Polar Lipids) as described (Fons et al, 2003). The final membrane preparations obtained from the equivalent of 100 μl of starting RM were resuspended in 20 μl of storage buffer (50 mM Hepes, pH 7.5, 100 mM KAc, 250 mM sucrose, 2 mM MgCl2, 1 mM DTT), frozen in liquid N2, and stored at −80°C.
Preparation of protein-free liposomes
Total RM lipids were extracted with a 2:1 mixture of chloroform:methanol or with 3:2 hexane:isopropanol. Total phospholipid phosphorus in the extracts was determined by the Ames method (Ames and Dubin, 1960). To prepare liposomes, phospholipid mixtures (PC, PC/PE 4:1, or total RM lipids) with or without cholesterol (from Sigma) were mixed in the desired ratios with a known amount of tritiated PC (Amersham Biosciences) as a tracer. The organic solvent was evaporated under a stream of nitrogen and the samples were desiccated overnight under vacuum. The lipid films were resuspended at 10 mg/ml (4 mg/ml for RM lipids) by overnight mixing in 15% glycerol, 50 mM Hepes, pH 7.5, 10 mM DTT. For extrusion, the lipid suspension was passed 10 times through two stacked 1 μm polycarbonate Nucleopore filters (Costar) using an extruder from Lipex Biomembranes. For the detergent-removal procedure, the lipid suspensions were supplemented with 2% DBC and the samples treated with BioBeads using the same procedure as for the proteoliposomes described above. The liposomes resulting from both methods were sedimented at 70 000 r.p.m. for 30 min in the TLA 100.3 rotor and resuspended in 20 μl of storage buffer. The overall yield was assessed by measuring the recovery of tritiated PC.
Cholesterol loading and unloading
RMs were loaded with cholesterol by incubation for 20 min at 30°C with cholesterol–MβCD (from Sigma) complex (Nilsson et al, 2001). Pig RMs (26 μg phospholipids/10 μl) were incubated with the complex (at final concentrations of 8.5 mM MβCD/0.85 mM cholesterol or 12.5 mM MβCD/1.25 mM cholesterol), or with 12.5 mM MβCD without cholesterol for 20 min at 30°C. To extract cholesterol from RMs and liposomes, the vesicles were incubated for 20 min at 30°C with MβCD in upto 30-fold molar excess over the sterol. Cholesterol content was assayed with the kit from MBL International Corporation.
Alkaline sucrose gradients
In all, 25 μl of b5-Nglyc translocation reactions was treated with an equal volume of 0.2 M Na2CO3 at pH 11.5 for 30 min on ice and then brought to 1.5 M sucrose, 0.1 M Na2CO3 in a final volume of 0.5 ml. The samples were layered under a discontinuous sucrose gradient composed of 2 ml of 1.2 M and 1.5 ml of 0.25 M sucrose, both containing 0.1 M Na2CO3. After centrifugation overnight (40 000 r.p.m., 4°C, Beckman SW 55 rotor), 1 ml fractions were collected from the top, precipitated with TCA and then analysed by SDS–PAGE.
SDS–PAGE and quantification of b5-Nglyc insertion efficiency
SDS–PAGE was on 12 or 13% Tris-tricine gels. Gels were imaged with the Storm phosphorimager (Amersham) and band intensities quantified with ImageQuant software. Alternatively, gels were exposed to film, the autoradiograms were digitized using Adobe Photoshop® software and band intensities determined with NIH Image software.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Acknowledgments
We are grateful to Teresa Sprocati and Sara Colombo for assistance and helpful discussion, to Paul Hargrave (University of Florida) for his kind gift of hybridoma cells producing anti-opsin mAbs (R2–15), and to Massimo Masserini and Silvia Sesana (University of Milano Bicocca) for their help in the preparation of liposomes by extrusion. NB was supported by grants from Telethon (N. GGP04129) and Ministero Università e Ricerca (PRIN 2002 and 2003). RSH and SS are supported by the NIH intramural program. MM is a Biocentrum Helsinki Fellow and was supported by the Academy of Finland (grants 38017 and 178444). MY is a student in the Helsinki Graduate School in Biotechnology and Molecular Biology, and was supported by a FEBS summer fellowship.
References
- Abell BM, Jung M, Oliver JD, Knight BC, Tyedmers J, Zimmermann R, High S (2003) Tail-anchored and signal-anchored proteins utilise overlapping pathways during membrane insertion. J Biol Chem 278: 5669–5678 [DOI] [PubMed] [Google Scholar]
- Abell BM, Pool MR, Schlenker O, Sinning I, High S (2004) Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J 23: 2755–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus G, Arendt A, Hargrave PA (1991) Genetic control of antibody response to bovin rhodopsin in mice: epitope mapping of rhodopsin structure. J Neuroimmunol 34: 89–97 [DOI] [PubMed] [Google Scholar]
- Ames BN, Dubin DT (1960) The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem 235: 769–775 [PubMed] [Google Scholar]
- Arinc E, Rzepecki LM, Strittmatter P (1987) Topography of the C-terminus of cytochrome b5 tightly bound to dimyristoylphophatidylcholine vesicles. J Biol Chem 262: 15563–15567 [PubMed] [Google Scholar]
- Borgese N, Colombo S, Pedrazzini E (2003) The tale of tail-anchored proteins: coming from the cytosol and looking for a membrane. J Cell Biol 161: 1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Gazzoni I, Barberi M, Colombo S, Pedrazzini E (2001) Targeting of a tail-anchored protein to endoplasmic reticulum and mitochondrial outer membrane by independent but competing pathways. Mol Biol Cell 12: 2482–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger B, Sandhoff R, Wegenhingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT (2000) Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol 151: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A, Nachbaur J, Vignais PM (1971) Enzyme characterization and lipid composition of rat liver subcellular membranes. Biochem Biophys Acta 249: 462–492 [DOI] [PubMed] [Google Scholar]
- D'Arrigo A, Manera E, Longhi R, Borgese N (1993) The specific subcellular localization of two isoforms of cytochrome b5 suggests novel targeting pathways. J Biol Chem 268: 2802–2808 [PubMed] [Google Scholar]
- Dailey HA, Strittmatter P (1981) Orientation of the carboxyl and NH2 termini of the membrane-binding segment of cytochrome b5 on the same side of phopholipid bilayers. J Biol Chem 256: 3951–3955 [PubMed] [Google Scholar]
- Enoch HG, Fleming PJ, Strittmatter P (1979) The binding of cytochrome b5 to phospholipid vesicles and biological membranes. Effect of orientation on intermembrane transfer and digestion by carboxypeptidase Y. J Biol Chem 254: 6483–6488 [PubMed] [Google Scholar]
- Fleischer B, Zambrano F, Fleischer S (1974) Biochemical characterization of the golgi complex of mammalian cells. J Supramol Struct 2: 737–750 [DOI] [PubMed] [Google Scholar]
- Fons RD, Bogert BA, Hegde RS (2003) Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J Cell Biol 160: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Hartmann E, Prehn S, Rapoport TA (1992) A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature 357: 47–52 [DOI] [PubMed] [Google Scholar]
- Görlich D, Rapoport TA (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75: 615–630 [DOI] [PubMed] [Google Scholar]
- Hartmann E, Görlich D, Kostka S, Otto A, Kraft R, Knespel S, Burger E, Rapoport TA, Prehn S (1993) A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur J Biochem 214: 375–381 [DOI] [PubMed] [Google Scholar]
- Hegde RS, Voigt S, Lingappa VR (1998) Regulation of protein topology by trans-acting factors at the endoplasmic reticulum. Mol Cell 2: 85–91 [DOI] [PubMed] [Google Scholar]
- High S, Abell BM (2004) Tail-anchored protein biosynthesis at the endoplasmic reticulum: the same but different. Biochem Soc Trans 32: 659–662 [DOI] [PubMed] [Google Scholar]
- Jäntti J, Keränen S, Toikkanen J, Ehnholm C, Södderlund H, Olkkonen VM (1994) Membrane insertion and intracellular transport of yeast syntaxin Sso2p in mammalian cells. J Cell Sci 107: 3623–3633 [DOI] [PubMed] [Google Scholar]
- Kim PK, Janiak-Spens F, Trimble WS, Leber B, Andrews DW (1997) Evidence for multiple mechanisms for membrane binding and integration via carboxyl-terminal insertion sequences. Biochemistry 36: 8873–8882 [DOI] [PubMed] [Google Scholar]
- Kuroda R, Kinoshita J-y, Honsho M, Mitoma J-y, Ito A (1996) In situ topology of cytochrome b(5) in the endoplasmic reticulum membrane. J Biochem Tokyo 120: 828–833 [DOI] [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilgen G, Hartmann E, Wiedenmann B, Rapoport TA (1995) Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J 14: 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Foguet M, Renz M, Seelig HP, Glick BS, Hauri H-P (1995) A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA 92: 5102–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta CV, Migliaccio G, Blobel G (1991) Biochemical fractionation and assembly of the membrane components that mediate nascent chain targeting and translocation. Cell 65: 587–598 [DOI] [PubMed] [Google Scholar]
- Nilsson I, Ohvo-Rekilvä H, Slot JP, Johnson AE, von Heijne G (2001) Inhibition of protein translocation across the endoplasmic reticulum membrane by sterols. J Biol Chem 276: 41748–41754 [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81: 561–570 [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Villa A, Borgese N (1996) A mutant cytochrome b5 with a lengthened membrane anchor escapes from the endoplasmic reticulum and reaches the plasma membrane. Proc Natl Acad Sci USA 93: 4207–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini E, Villa A, Longhi R, Bulbarelli A, Borgese N (2000) Mechanism of residence of cytochrome b(5), a tail-anchored protein, in the endoplasmic reticulum. J Cell Biol 148: 899–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA, Jungnickel B, Kutay U (1996) Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem 65: 271–303 [DOI] [PubMed] [Google Scholar]
- Remacle J (1978) Binding of cytochrome b5 to membranes of isolated subcellular organelles from rat liver. J Cell Biol 79: 291–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L, Strittmatter P (1971) A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acids. Proc Natl Acad Sci USA 68: 1042–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel GJ, Brownsword J, Stirling CJ (2002) Tail-anchored protein insertion into yeast ER requires a novel posttranslational mechanism which is independent of the SEC machinery. Biochemistry 41: 11914–11920 [DOI] [PubMed] [Google Scholar]
- van Dalen A, de Kruijff B (2004) The role of lipids in membrane insertion and translocation of bacterial proteins. Biochim Biophys Acta 1694: 97–109 [DOI] [PubMed] [Google Scholar]
- van Meer G (1989) Lipid traffic in animal cells. Ann Rev Cell Biol 5: 247–275 [DOI] [PubMed] [Google Scholar]
- Walter P, Blobel G (1983) Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol 96: 84–93 [DOI] [PubMed] [Google Scholar]
- Yabal M, Brambillasca S, Soffientini P, Pedrazzini E, Borgese N, Makarow M (2003) Translocation of the C terminus of a tail-anchored protein across the endoplasmic reticulum membrane in yeast mutants defective in signal peptide-driven translocation. J Biol Chem 278: 3489–3496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5


