Abstract
Heat shock proteins (Hsps) have provoked interest not only because of their involvement in human diseases but also for their potential as biomarkers of environmental pollution. Whereas the former interest is covered by numerous reports, the latter is an exciting new field of research. We report the isolation of the full-length cpn60 messenger ribonucleic acid (mRNA) and partial genomic deoxyribonucleic acid from the free-living, environmental sentinel nematode Plectus acuminatus, a species used in classical ecotoxicity tests. Although the primary sequence displays high identity scores to other nematodes and human Cpn60 (75% and 70%, respectively), the intron-exon structure differs markedly. Furthermore, although mRNA levels remained constant after exposure to ZnCl2 (0–330 μM) under laboratory conditions, protein levels increased significantly in a dose-dependent manner. In conclusion, this first account of molecular genetic similarities and differences of Cpn60 in a neglected nematode taxon provides a valuable insight into its potential uses in gene-based ecotoxicological risk assessment exercises.
INTRODUCTION
Nematodes are 1 of the most dominant and abundant groups of multicellular organisms. Bacterivorous nematodes play a major role in decomposition processes of organic matter by stimulating N mineralization and nutrient cycling (Anderson et al 1981). The most commonly studied member of the phylum Nematoda, class Chromadorea is undoubtedly Caenorhabditis elegans. With a fully sequenced and exquisitely annotated genome, a vast amount of information has consequently been rapidly accumulating. Unfortunately, there remains a multitude of neglected nematode species that are increasingly becoming underrepresented (because of the lack of data), despite their biological importance and value. For example, the nonparasitic soil-inhabiting bacterivorous nematode Plectus acuminatus is a member of the order Araeolaimida. It is highly abundant in the temperate regions of the world and has a life cycle that is notably different to C elegans, namely an egg to egg period of approximately 3 weeks (opposed to 3 days) and an average life span of 3 months (opposed to 21 days) at 20°C.
Given that Plectus is a terrestrial bacterial feeder that inhabits the interstitial water of soil particles (Houx and Aben 1993), it is subjected directly to the dissolved fraction of contaminants in soils through contact or indirectly through the food source. It follows that these organisms in particular require efficient response pathways. Toxicity tests have shown that the effect levels to several contaminants was comparable with other invertebrates suggesting that P acuminatus may be an ideal indicator species for exposure assessment and biomonitoring (Kammenga et al 1996). Although no genetic data is available for this species, the effects of metal toxicosis have been monitored at the protein level, using unspecific monoclonal antibodies to human stress proteins to evaluate the responses to increasing concentrations of cadmium and copper (Kammenga et al 1998).
Heat shock proteins (Hsps) are a multigene family first discovered to be activated by thermal stress but later found to be induced by a wide variety of other factors, including physical, chemical, and biological stress (Nover 1991). The ubiquitous 55–65 kDa Hsps have been found to occur almost exclusively in organelles of endosymbiotic origin (mitochondria, chloroplasts) (Hemmingsen et al 1988; Cheng et al 1989) and are commonly referred to as Cpn60, Hsp60, stress-60, or chaperonins because of their function as molecular chaperones. Moreover, they facilitate the synthesis, folding, assembly and intracellular transport of proteins, reduce protein denaturation and aggregation, and aid in protein renaturation (Ellis and van der Vies 1991; Parsell and Lindquist 1993). They consist of large oligomeric structures composed of 2 stacked heptameric rings of identical or closely related rotationally symmetric 60-kDa subunits to form a central cavity (Hendrix 1979; Hohn et al 1979; McMullin and Hallberg 1988). The central cavity binds intermediately folded polypeptides and thereby prevents incorrect associations within and between polypeptide chains during protein folding and protects preexisting proteins under cellular stress. In most cases, adenosine triphosphate and a single heptameric ring of 10-kDa subunits, the cochaperonin Cpn10 (Hsp10, GroES), are required for folding of the polypeptide intermediates and release from the chaperonin (Martin et al 1993). Chaperonins are also involved in secretion and (membrane) translocation of a number of protein precursors and aid damaged and misfolded proteins to refold correctly to their native conformation or otherwise, making them sensitive to proteolytic digestion (Gething and Sambrook 1992; Parsell and Lindquist 1993). Its role in autoimmune diseases has been studied extensively (Wick et al 2001, 2004; Quintana et al 2002) and there is evidence for a possible involvement in prion diseases (Ranford and Henderson 2002).
This article not only aims to redress the lack of genetic information of a neglected nematode species by describing the molecular cloning of the full-length transcripts of the P acuminatus Hsp cpn60 and actin, but also highlights partial cpn60 intron-exon structures and the measurement of differential transcriptional and translational responses of Cpn60 to zinc exposure.
RESULTS AND DISCUSSION
Identification of Cpn60 in P acuminatus
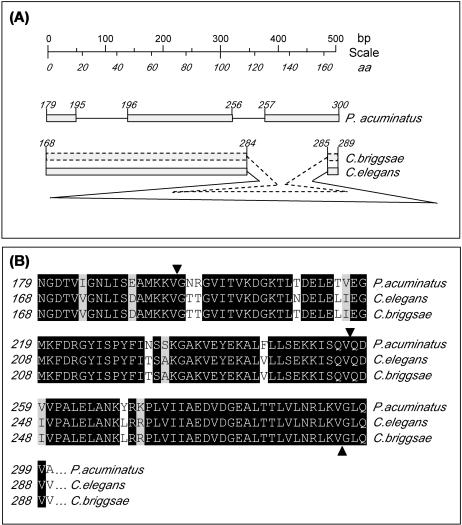
Cpn60 is a member of the large and phylogenetically conserved family of stress proteins (Lindquist and Craig 1988). Consequently it was possible to isolate the messenger ribonucleic acid (mRNA) sequence of the P acuminatus using degenerate cpn60 primers. The final contig of a subset of independent rapid amplification of complementary deoxyribonucleic acid (DNA) ends–polymerase chain reaction (PCR) yielded the full-length sequence of 2068 bp, comprising 96 bp 5′ UTR and 229 bp 3′ UTR. The sequence is accessible from the European Molecular Biology Laboratory (EMBL)/Genbank depositories under accession no. AJ130877. The 580 amino acid Cpn60 has a predicted weight of 61.8 kDa with an isoelectric point of 6.2. Using MitoProt II for Unix (Claros and Vincens 1996), it was possible to predict the mitochondrial signal sequence indicating, with a probability of P = 0.96, that Cpn60 is indeed exported to the mitochondria (see Fig 1, panel A). The presence of a putative targeting signal that is responsible for the translocation into the mitochondrial matrix has also been reported in Cpn60 of Drosophila melanogaster (Kozlova et al 1997), Trypanosoma cruzi (Giambiagi-de Marval et al 1993), Culicoides variipennis (Abdallah et al 2000) and Paracentrotus lividus (Gianguzza et al 2000) and others. The deduced amino acid translation displays a considerable sequence identity (75%) and similarity (87%) to the Cpn60s from C elegans and C briggsae (Fig 1, panel B). The phylogenetic conservation of Cpn60 is highlighted by the fact that the P acuminatus sequence is remarkably conserved to the human paralog with an identity of 70% and a similarity 83% (Fig 1, panel B) and other organisms from vertebrates to plants (Fig 1, panel C).
Fig 1. .
Identification of cpn60 from the nematode Plectus acuminatus. An adapter ligated complementary deoxyribonucleic acid (cDNA) library was constructed from 7000 P acuminatus individuals using the Marathon™ cDNA Amplification Kit (CLONTECH Laboratories, Palo Alto, CA, USA). Cpn60 was amplified, with degenerative primers, in a touchdown polymerase chain reaction and forthcoming bands purified, cloned (pGEM-T, Promega, Southampton, Hampshire, UK), and sequenced. The full-length P acuminatus cpn60 sequence was determined on both strands of the 5′ fragment, the 3′ fragment, and the 5′-3′ overlap region resulting in a final contig of 2068 bp (European Molecular Biology Laboratory/Genbank accession no. AJ130877). The translation is given in panel A with the putative N-terminal mitochondrial targeting signal boxed. Homology searches using the BlastN and BlastP algorithms (Altschul et al 1990; Gish and States 1993) identified the sequence to be closely related to 2 further nematodes (panel A). Pairwise comparisons of the deduced amino acid sequence were calculated from 3 nematode species and the human Cpn60, where the first number represents percent identity and the second, percent similarity scores (panel B). A ClustalW multiple alignment was used to construct a Cladogram of the P acuminatus Cpn60 amino acid sequence and 12 further Cpn60s from a variety of species to highlight phylogenetic relationships (panel C)
The genomic organization of cpn60
A 506-bp fragment of genomic DNA was amplified from the central region cpn60 gene, cloned, and sequenced on both strands to provide an insight into the genomic organization of P acuminatus. It should be noted that although it was never the primary aim to extract extensive sequencing data of the cpn60 genomic locus, the fragment obtained nevertheless provided valuable information. In contrast to the single intron encountered in C elegans and C briggsae, the Plectus cpn60 contains 2 introns. However, the combined intronic size of 139 bp of both Plectus introns (86 and 53 bp, respectively) is nevertheless significantly shorter than the single intron in C elegans (1365 bp) and C briggsae (717 bp). Although there is an approximate 2-fold difference in size of both Caenorhabditis intronic sequences, the splice sites perfectly match each other. In contrast, Plectus introns are spliced at different locations (Fig 2, panel A). Although this only provides a diminutive view of the general genomic organization, the lack of “splice site conservation” highlights the evolutionary distance of the 2 classes, both belonging to the phylum Nematoda.
Fig 2. .
Identification of nonconserved splicing in Plectus acuminatus Cpn60. Amplification of cpn60 from P acuminatus genomic deoxyribonucleic acid identified intron-exon structures that markedly differed to the boundaries encountered in Rhabditidae nematodes (panel A). The cartoon is drawn to scale and numbers in italics represent amino acid identifiers. Note that the amplified region of P acuminatus cpn60 contains 2 short introns, compared with 1 in the Caenorhabditis species, with the C elegans intron being significantly larger. Correct splicing was confirmed in silico (panel B), where splice sites are annotated by filled triangles
cpn60/Cpn60 expression in P acuminatus
Because Cpn60 operates in a multifactorial manner, its transcriptional activation is equally complex and is activated by numerous triggers, ranging from heat shock to exposure to heavy metals. Direct evidence of this statement is provided by a report that demonstrated an induction of HSP60 and HSP70 protein levels after exposure to cadmium and copper in P acuminatus (Kammenga et al 1998). Although, at the time, limited by the lack of genetic information available, the study not only highlighted the conserved nature of the protein family but also the potential uses of cross-reactive mammalian antibodies in environmental and toxicological testing regimens.
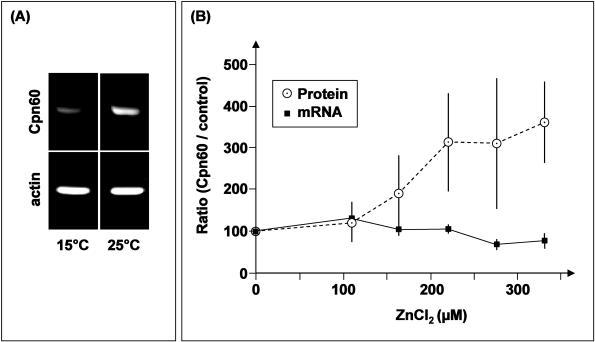
With the molecular genetic composition of P acuminatus cpn60 elucidated (see above), it was feasible to design specific primers and measure transcriptional responses by reverse transcriptase (RT)–PCR. In a semiquantitative approach, it was possible to verify that the transcription of Plectus cpn60 is indeed responsive to temperature stress, in a marginal but clearly detectable manner (Fig 3, panel A), an observation that could be confirmed by fully quantitative PCR (data not shown). This, in itself, is of course not a significant, nor novel, finding, but merely a confirmatory statement that supports the many publications that cpn60 expression is temperature dependent (Yoshida et al 1995; Martin et al 2002; Kingsley et al 2003).
Fig 3. .
Quantification of Plectus acuminatus Cpn60 transcript and protein levels. The cpn60 response at the messenger ribonucleic acid (mRNA) level was determined by quantitative polymerase chain reaction (PCR) (LightCycler, Idaho Technology, Idaho Falls, ID, USA). Conditions were set and optimized as described by Stürzenbaum et al (1998) and Stürzenbaum (1999), and every PCR product was assessed for its amplification specificity by real-time melting curve analysis (Ririe et al 1997), ensuring that only true amplification products were included in the quantification. All amplifications were normalized to actin, which was isolated as described in Figure 1 (accession no. AJ012665). Cpn60 protein levels were determined using the mouse monoclonals anti-human Cpn60 and anti–Globodera rostochiensis tropomyosin as an internal control on Western blots as described in De Boer et al (1996). Gray values of the blot bands were measured by densitometric image analysis (Applied Imaging, Tyne & Wear, UK) and processed using the MIDAS software (Applied Imaging). All measurements (at the mRNA and protein level) were taken after a 24-hour exposure period, mean values were calculated from 4 independent replicate analyses and assessed for statistical significance by one-way analysis of variance. Note that at the transcriptional level, a distinct response was obtained to heat shock (panel A) but not to zinc exposure (panel B). In contrast, a zinc exposure dependent translational response was clearly visible (panel B)
Because protein levels have been shown previously to be sensitive to exposure of cadmium and copper (Kammenga et al 1998), a zinc exposure regimen (ranging from 0 to 330 μM Zn) was devised, and nematodes subsequently split to allow the measurement of mRNA and protein levels on the same pool of animals. Consequently, it was surprising to find that mRNA levels remained remarkably constant (after signal normalization to actin) upon exposure to the zinc concentration gradient, particularly because protein levels were seen to rise significantly (P = 0.001) with increasing supplementation of zinc (Fig 3, panel B).
However, it should be noted that each family of Hsps comprises multiple closely related isoforms (Sanders 1993), and several Cpn60 isoforms have been detected in P acuminatus (Kammenga et al 1998). Given that we know very little about the interspecific cross-reactivity of the commercial antibody used in this study, it is important to state that the cpn60 mRNA measured by quantitative RT-PCR may indeed not represent the identical pool of Cpn60 measured at the protein level. Nevertheless, it does highlight the notion that mRNA levels–responses may not always equal protein levels–responses. Particularly with the family of Hsps, this may be an ill-defined assumption. Differential degradation, stability or posttranslational modifications may well influence the abundance or ratio of mRNA and protein levels. For example, Ornatsky et al (1995) report that whereas cpn60 mRNA levels did not alter in chronically stimulated rats skeletal muscles, Cpn60 protein level increased 3.2-fold. In contrast, T cruzi cpn60 mRNA levels were responsive to temperature exposure, whereas the protein level remained essentially constant (Sullivan et al 1994).
It is doubtful that the documented differential responses of mRNA and protein expression are entirely independent from each other, but rather a transiently elevated response on a different time scale, where the protein response may lag behind the mRNA response but ultimately sustain for considerably longer periods. This is exemplified in human proximal tubule cells, where cadmium-induced elevation of cpn60 mRNA returned to the control levels within 8 hours but proteins levels required 24 hours after removal of cadmium before returning to basal levels (Somji et al 2000). Similarly, changes in hsp70 transcription was more sensitive than stress protein accumulation in the gray garden slug Deroceras reticulatum exposed to cadmium- or zinc-enriched food (Köhler et al 1998). Given that the measurements were taken after a 24-hour exposure period, it is conceivable that the mRNA levels may have surpassed the transcriptional upregulation and returned to basal level expression, resulting in an apparent lack of response. Alternatively, a hypersensitive response to laboratory setup or handling may have resulted in a continuous elevation of cpn60 transcripts— even in control exposures. In any case, either of the above possibilities warrants further investigations.
Overall this first account of the molecular genetic makeup of a neglected nematode phylum of direct environmental relevance highlights clear similarities and differences of the Cpn60 family. The primary sequence may be highly conserved, but the intron-exon structure proves to be more complex compared with Caenorhabditis. The marked difference between Cpn60 mRNA and protein levels in response to zinc exposure provides a valuable insight into the potential exploitation of cpn60 in gene-based ecotoxicological risk assessment exercises, namely the results shown here beg for caution when using cpn60 mRNA responses as a stress marker.
The sequence data reported in this article have been submitted to the EMBL/Genbank libraries under the accession numbers AJ012665, AJ130877, and AJ130947.
Acknowledgments
We thank Dr G. Smant, Laboratory of Monoclonal Antibodies, Wageningen, The Netherlands, for his expertise. This work was supported by the Royal Society and the Natural Environmental Research Council, NERC (both to S.R.S.), and the EU Environment and Climate Research Programme, contract ENV4-CT96-0222 (to J.E.K.).
REFERENCES
- Abdallah MA, Pollenz RS, Nunamaker RA, Murphy KE. Identification and characterization of a cDNA clone encoding the heat shock protein (Hsp60) from the biting midge, Culicoides variipennis sonorensis Wirth and Jones. Biochem Genet. 2000;38:157–165.0006-2928(2000)038[0157:IACOAC]2.0.CO;2 [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2.0022-2836(1990)215[0403:BLAST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Anderson RV, Coleman DC, Cole CV, Elliott ET. Effect of the nematodes Acrobeloides spp and Mesodiplogaster lheritieri on substrate utilization and nitrogen and phosphorus mineralization in soil. Ecology. 1981;62:549–555.0012-9658(1981)062[0549:EOTNAS]2.0.CO;2 [Google Scholar]
- Cheng MY, Hartl FU, and Martin J. et al. 1989 Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 337:620–625. [DOI] [PubMed] [Google Scholar]
- Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x.0014-2956(1996)241[0779:CMTPMI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- De Boer JM, Overmars HA, and Pomp HR. et al. 1996 Production and characterization of monoclonal antibodies to antigens from second-stage juveniles of the potato cyst-nematode, Globodera rostochiensis. Fundam Appl Nematol. 19:545–554. [Google Scholar]
- Ellis RJ, van der Vies SM. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541.0066-4154(1991)060[0321:MC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gething M-J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0.0028-0836(1992)355[0033:PFITC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giambiagi-de Marval M, Lees RA, Monteiro BAG, Carvalho JFO, Gottesdiener K, De Castro FT, Rondinelli E. The heat-shock response in Trypanosoma cruzi and Crithidia fasciculata. Cienc Cult (Sao Paulo) 1993;45:216–222.0009-6725(1993)045[0216:THRITC]2.0.CO;2 [Google Scholar]
- Gianguzza F, Ragusa MA, Roccheri MC, Di Liegro I, Rinaldi AM. Isolation and characterization of a Paracentrotus lividus cDNA encoding a stress-inducible chaperonin. Cell Stress Chaperones. 2000;5:87–89. doi: 10.1379/1466-1268(2000)005<0087:iacoap>2.0.co;2.1466-1268(2000)005[0087:IACOAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish W, States DJ. Identification of protein coding regions by database similarity search. Nature Genet. 1993;3:266–272. doi: 10.1038/ng0393-266.1061-4036(1993)003[0266:IOPCRB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, Hendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0.0028-0836(1988)333[0330:HPABPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hendrix RW. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979;129:375–392. doi: 10.1016/0022-2836(79)90502-3.0022-2836(1979)129[0375:PAPOGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hohn T, Hohn B, Engel A, Wurtz M. Isolation and characterization of the host protein groE involved in bacteriophage assembly. J Mol Biol. 1979;129:359–373. doi: 10.1016/0022-2836(79)90501-1.0022-2836(1979)129[0359:IACOTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Houx NWH, Aben WJM 1993 Bioavailability of pollutants to soil organisms via the soil solution. Sci Total Environ. 1(Suppl). 387–395. [Google Scholar]
- Kammenga JE, Arts MSJ, Oude-Breuil WJM. HSP60 as a potential biomarker of toxic stress in the nematode Plectus acuminatus. Arch Environ Contam Toxicol. 1998;34:253–258. doi: 10.1007/s002449900314.0090-4341(1998)034[0253:HAAPBO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kammenga JE, Van Koert PHG, Riksen JAG, Korthals GW, Bakker J. A toxicity test in artificial soil based on the life-history strategy of the nematode Plectus acuminatus. Environ Toxicol Chem. 1996;15:722–727.1552-8618(1996)015[0722:ATTIAS]2.0.CO;2 [Google Scholar]
- Kingsley RJ, Afif E, and Cox BC. et al. 2003 Expression of heat shock and cold shock proteins in the gorgonian Leptogorgia virgulata. J Exp Zool Part A. 296:98–107. [DOI] [PubMed] [Google Scholar]
- Köhler H-R, Belitz B, Eckwert H, Adam R, Rahman B, Trontelj P. Validation of hsp70 stress gene expression as a marker of metal effects in Deroceras reticulatum (Pulmonata): correlation with demographic parameters. Environ Toxicol Chem. 1998;17:2246–2253.1552-8618(1998)017[2246:VOHSGE]2.0.CO;2 [Google Scholar]
- Kozlova T, Perezgasga L, Reynaud E, Zurita M. The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locusl(1)10Ac and is differentially expressed during fly development. Dev Genes Evol. 1997;207:253–263. doi: 10.1007/s004270050113.0949-944X(1997)207[0253:TDMHOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Martin CC, Tsang CH, Beiko RG, Krone PH. Expression and genomic organization of the zebrafish chaperonin gene complex. Genome. 2002;45:804–811. doi: 10.1139/g02-044.0831-2796(2002)045[0804:EAGOOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Martin J, Geromanos S, Tempst P, Hartl FU. Identification of nucleotide-binding regions in the chaperonin proteins GroEL and GroES. Nature. 1993;366:279–282. doi: 10.1038/366279a0.0028-0836(1993)366[0279:IONRIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McMullin TW, Hallberg RL. A highly evolutionary conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988;8:371–380. doi: 10.1128/mcb.8.1.371.0270-7306(1988)008[0371:AHECMP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L 1991 The Heat Shock Response. CRC Press, Boca Raton, FL. [Google Scholar]
- Ornatsky OI, Connor MK, Hood DA. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem J. 1995;311:119–123. doi: 10.1042/bj3110119.0264-6021(1995)311[0119:EOSPAM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253.0066-4197(1993)027[0437:TFOHPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J Immunol. 2002;151:3422–3428. doi: 10.4049/jimmunol.169.6.3422.0022-1767(2002)151[3422:IOAABA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ranford JC, Henderson B. Chaperonins in disease: mechanisms, models, and treatments. Mol Pathol. 2002;55:209–213. doi: 10.1136/mp.55.4.209.1366-8714(2002)055[0209:CIDMMA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154–160. doi: 10.1006/abio.1996.9916.0003-2697(1997)245[0154:PDBAOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: An environmental perspective. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074.1040-8444(1993)023[0049:SPIAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Somji S, Todd JH, Sens MA, Garrett SH, Sens DA. Expression of heat shock protein 60 in human proximal tubule cells exposed to heat, sodium arsenite and CdCl2. Toxicol Lett. 2000;115:127–136. doi: 10.1016/s0378-4274(00)00183-1.0378-4274(2000)115[0127:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stürzenbaum SR. Benchmarks: transfer RNA reduces the formation of primer artifacts during quantitative PCR. BioTechniques. 1999;27:50–52. doi: 10.2144/99271bm08.0736-6205(1999)027[0050:BTRRTF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stürzenbaum SR, Kille P, Morgan AJ. Identification of heavy metal induced changes in the expression patterns of the translationally controlled tumour protein (TCTP) in the earthworm Lumbricus rubellus. Biochim Biophys Acta. 1998;1398:294–304. doi: 10.1016/s0167-4781(98)00077-3.0006-3002(1998)1398[0294:IOHMIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Olson CL, Winquist AG, Engman DM. Expression and localization of Trypanosoma cruzi hsp60. Mol Biochem Parasitol. 1994;68:197–208. doi: 10.1016/0166-6851(94)90165-1.0166-6851(1994)068[0197:EALOTC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644.0732-0582(2004)022[0361:AAIMIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22:665–669. doi: 10.1016/s1471-4906(01)02089-0.1471-4906(2001)022[0665:AAAADA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Yanagi H, Yura T. Cloning and characterization of the mitochondrial HSP60-encoding gene of Schizosaccharomyces pombe. Gene. 1995;167:163–166. doi: 10.1016/0378-1119(96)82966-0.0378-1119(1995)167[0163:CACOTM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]