Abstract
Heat shock proteins (Hsps) are overexpressed in a wide range of human cancers and are implicated in tumor cell proliferation, differentiation, invasion, metastasis, death, and recognition by the immune system. We review the current status of the role of Hsp expression in cancer with special emphasis on the clinical setting. Although Hsp levels are not informative at the diagnostic level, they are useful biomarkers for carcinogenesis in some tissues and signal the degree of differentiation and the aggressiveness of some cancers. In addition, the circulating levels of Hsp and anti-Hsp antibodies in cancer patients may be useful in tumor diagnosis. Furthermore, several Hsp are implicated with the prognosis of specific cancers, most notably Hsp27, whose expression is associated with poor prognosis in gastric, liver, and prostate carcinoma, and osteosarcomas, and Hsp70, which is correlated with poor prognosis in breast, endometrial, uterine cervical, and bladder carcinomas. Increased Hsp expression may also predict the response to some anticancer treatments. For example, Hsp27 and Hsp70 are implicated in resistance to chemotherapy in breast cancer, Hsp27 predicts a poor response to chemotherapy in leukemia patients, whereas Hsp70 expression predicts a better response to chemotherapy in osteosarcomas. Implication of Hsp in tumor progression and response to therapy has led to its successful targeting in therapy by 2 main strategies, including: (1) pharmacological modification of Hsp expression or molecular chaperone activity and (2) use of Hsps in anticancer vaccines, exploiting their ability to act as immunological adjuvants. In conclusion, the present times are of importance for the field of Hsps in cancer, with great contributions to both basic and clinical cancer research.
INTRODUCTION
Levels of the heat Hsp molecular chaperones are elevated in many cancers, and Hsp overexpression signals a poor prognosis in terms of survival and response to therapy in specific cancer types (Ciocca et al 1993; Cornford et al 2000; Blagosklonny 2001; van de Vijver et al 2002; van 't Veer et al 2002). Elevated Hsp expression in malignant cells plays a key role in protection from spontaneous apoptosis associated with malignancy as well as the apoptosis generated by therapy, mechanisms which may underlie the role of Hsp in tumor progression and resistance to treatment (Volloch and Sherman 1999; Nylandsted et al 2000a, 2000b; Ciocca et al 2003; Gyrd-Hansen et al 2004). Hsp transcription requires activated heat shock transcription factor 1 (hsf1), which is itself overexpressed in cancer and plays a role in invasion and metastasis (Wu 1995; McMillan et al 1998; Hoang et al 2000; Wang et al 2004). However, the molecular mechanisms linking increased Hsp and HSF1 expression to tumor progression are not currently understood. Because Hsps are induced only under stress conditions in normal cells, some aspects of the malignant phenotype apparently cause Hsp dysregulation (Lindquist and Craig 1988).
Heat shock proteins
Hsps were first discovered as a cohort of proteins that are powerfully induced by heat shock and other chemical and physical stresses in a wide range of species (Lindquist and Craig 1988; Georgopolis and Welch 1993). The Hsps have been subsequently characterized as molecular chaperones, proteins, which have in common the property of modifying the structures and interactions of other proteins (Beckmann et al 1990; Gething and Sambrook 1992; Netzer and Hartl 1998; Freeman and Yamamoto 2002). Molecular chaperone function dictates that the Hsps often interact in a stoichiometric manner with their substrates, necessitating high intracellular concentrations of the proteins (Lindquist and Craig 1988; Georgopolis and Welch 1993). As proteins that shift the balance from denatured, aggregated protein conformation toward ordered, functional conformation, Hsps are particularly in demand when proteins are disordered by heat shock, oxidative stress, or other protein-damaging events (Lindquist and Craig 1988; Hightower 1991; Gething and Sambrook 1992; Georgopolis and Welch 1993). The Hsp28, 40, 70, and 110 genes have therefore evolved a highly efficient mechanism for mass synthesis during stress, with powerful transcriptional activation, efficient messenger RNA (mRNA) stabilization, and selective mRNA translation (Voellmy 1994). Hsp27, 70, 90, and 110 increase to become the dominantly expressed proteins after stress (Hickey and Weber 1982; Landry et al 1982; Li and Werb 1982; Subjeck et al 1982; Henics et al 1999; Zhao et al 2002). Hsp gene transcription is regulated by transcription factors belonging to the heat shock factor (HSF) family that ensure prompt transcriptional activation in stress and equally precipitous switch-off after recovery (Sorger and Pelham 1988; Wu 1995). The hsf gene family includes heat shock transcription factor 1 (hsf1), the molecular coordinator of the heat shock response, as well as 2 less well-characterized genes, heat shock transcription factor 2 (hsf2) and heat shock transcription factor 4 (hsf4) (Rabindran et al 1991; Schuetz et al 1991; Nakai et al 1997). In addition to the Hsps induced by heat, cells also contain a large number of constitutively expressed Hsps (Tang et al 2005). Recent studies have shown that the constitutive Hsps are found in a variety of multiprotein complexes containing both Hsps and cofactors (Buchner 1999). These include Hsp10 and Hsp60 complexes that mediate protein folding and Hsp70- and Hsp90-containing complexes that are involved in both generic protein-folding pathways and in specific association with key regulatory proteins within the cell (Netzer and Hartl 1998; Pratt and Toft 2003). Hsp90 plays a particularly versatile role in cell regulation, forming complexes with a large number of cellular kinases, transcription factors, and other molecules.
Mechanisms for Hsp elevation
Hsp expression is tailored for induction by the stress response, and the proximal signal for Hsp induction is apparently the accumulation of denatured proteins (Voellmy 2004). It is thus rather a mystery as to how the Hsps become overexpressed in cancer. One hypothesis is that the physiopathological features of the tumor microenvironment (low glucose, pH, and oxygen) tend toward Hsp induction. Whether this is true or not, we do not know. However, one recent study indicates that when cells are transferred from tissue culture to growth as xenografts in vivo, Hsp expression declines markedly (Tang et al 2005). Because the elevated Hsp levels associated with malignancy tend to persist when cells are grown in tissue culture, they may well be related to the genetic changes associated with tumor progression (Tang et al 2005). Oncoproteins may appear during carcinogenesis (eg, mutated p53), and these mutated and conformationally altered proteins may elicit an Hsp response. However, the exact mechanisms are yet to be determined although they likely involve molecular changes common to a wide range of cancer cells with the potential to feed into the signaling mechanisms that lead to HSF1 activation.
In this short review, we will provide an overview of the current status of the Hsps in cancer with special emphasis on the clinical setting. This is not an easy task because there are more than 200 types of cancers and there are several Hsp families, each with multiple members (Tang et al 2005). Finally, the process of carcinogenesis involves a complex array of genetic and epigenetic alterations, which contribute to cancer pathogenesis (Hahn and Weinberg 2002), leading ultimately to a unique cancer tissue (often with mixed cancer clones) within a unique molecular milieu and in this may change dramatically the Hsp context and behavior. Therefore, we need to define whether altered expression of the Hsps at genomic or proteomic level is of importance to cancer prevention, diagnosis, prognosis, prediction, and treatment.
IMPLICATIONS IN DIAGNOSIS
In these cases, Hsp expression has been analyzed in relation to the histopathological characteristics of the tumor tissues (eg, tumor type, grade of differentiation), with the expression of other molecules (eg, estrogen receptors, c-myc, mutated p53), and with patient parameters like sex and age. In addition, levels of circulating Hsps and anti-HSP antibodies have been correlated with patient and tumor characteristics. Table 1 summarizes the publications regarding the diagnostic implications of the Hsps in cancer.
Table 1.
Heat shock proteins in cancer: diagnostic implications
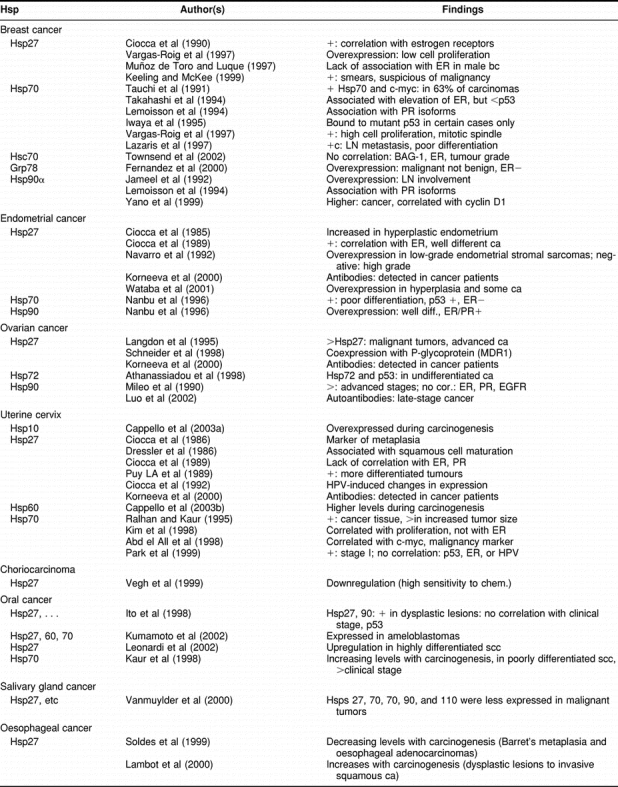
Because of space limitations, we cannot perform a detailed analysis of each study (eg, adequacy of the number and homogeneity of samples incorporated). However, the following main conclusions can be made. (a) Diagnostic implications—Hsps are overexpressed in a wide range of malignant cells and tissues. Therefore, Hsp detection is not useful in diagnostic immunopathology (there are other more restricted molecular markers to identify the lineage of origin of cancer tissues: carcinoma, sarcoma, lymphoma, etc). However, it might be useful to apply in a panel of immunopathology antibodies, the detection of αBcrystallin for identification of renal cell carcinomas (Pinder et al 1994), and the detection of Hsp27 or Hsp90 for identification of Reed-Sternberg cells (Hsu and Hsu 1998). The presence of Hsps and antibodies to the Hsps in the serum of cancer patients is still a new research area. Although it seems that autoantibodies to certain Hsps are of significance as tumor markers in osteosarcomas, ovarian cancer and others, at present, we need more studies to draw a clear conclusion on this important subject. The same applies to the study of the polymorphism of the Hsp70-2 gene. (b) Carcinogenesis—Hsp expression levels can help indicate the presence of abnormal changes during the process of carcinogenesis (in certain tissues). For example, Hsp27 is overexpressed in hyperplastic endometrium, and this protein appears as a marker of squamous metaplasia in the uterine cervix; Hsp10 and Hsp60 are related with the process of carcinogenesis of the uterine cervix and colon; and Hsp70 is associated with carcinogenesis of the oral epithelium and as a marker of early hepatocellular carcinoma. In oesophageal carcinomas, Hsp27 decreases during the carcinogenesis that ends in adenocarcinomas but increases during the carcinogenesis that ends in squamous carcinomas. Then, Hsps can be used as subrogate biomarkers of certain cancers. (c) Differentiation—Hsp expression correlates with the degree of differentiation in certain tissues. Hsps associated with higher differentiation are: Hsp27 and Hsp90 in endometrial carcinomas, Hsp27 in squamous carcinomas (uterine cervix, oral epithelium), and Hsp27 as a marker of keratinocyte differentiation in the skin. In contrast, Hsps associated with poor differentiation are Hsp70 in cancers of the breast, ovary, and oral epithelium, Grp78 in lung carcinomas, and Hsp27 in astrocytomas. At present, we do not have a clear explanation for these disparities and associations. Hsp70 has been involved not only with poor tumor differentiation but also with increased cell proliferation (breast, uterine cervix, lung), lymph node metastasis (breast, colon), increased tumor size (uterine cervix), presence of mutated p53 (breast, endometrium), and higher clinical stage (oral, colon, melanoma). (d) Associations with other molecules—In general, several Hsps are coexpressed in cancer tissues; in addition, certain Hsps can be significantly associated with other molecules. For example, Hsp27 has been associated with ERα in female breast carcinomas and endometrial carcinomas, but this protein did not appear associated with ERα in male breast carcinomas, cervical uterine carcinomas, hepatocellular carcinomas, and meningiomas (tissues that may express ERα). It is interesting that Hsp27, which was first described as an estrogen-regulated protein, is significantly associated with ERα in the female breast and endometrium (Table 1). These 2 organs are under strong estrogen and progesterone regulation. On the other hand, Hsp70 has been described as an important molecule in the assembly and trafficking of steroid receptors, and in breast cancer, Hsp70 has been found associated with ERα (Takahashi et al 1994). It is of interest to mention that Hsp70 can increase ERα transcriptional activity and growth in MCF-7 breast cancer cells (Spears and Barnes 2003), which in turn may explain the increased cell proliferation found in breast tumor biopsy samples that express Hsp70 (Vargas-Roig et al 1997). In addition, Hsp70 has been associated and complexed with mutant p53 in cancer cell lines (Lehmann et al 1991). This association has been studied in several cancer tissues, and the results have shown this association in certain cases only.
IMPLICATIONS IN THE PROGNOSIS
We have seen that expression of certain Hsps can be correlated with the carcinogenic process as well as with the degree of differentiation and cell proliferation, and moreover, they have been implicated in the regulation of apoptosis. Therefore, it was reasonable to study the prognostic implications of Hsps, and they emerged as useful in certain cancer types. The prognosis of a particular cancer patient is very important in the clinic to individualize cancer treatments, to plan the patient's follow-up, and to answer questions from the patient or relatives. Overtherapy with cytotoxic drugs can be avoided in cancer patients if they are correctly identified as having good prognosis and vice versa (Table 2). Again, because of space limitations, we cannot perform a detailed analysis of each study; however, the following conclusions can be made about the Hsps that have been studied most. (a) Hsp27— breast cancer is one of the sites where numerous studies on prognostic factors have been reported. To date, at the proteomic level, we can establish a categorization of several prognostic factors that, integrated with the traditional clinicopathological factors, provide a very good idea of the disease outcome (Gago et al 1998). Hsp27 is not among the list of useful prognostic factors in breast cancer (Oesterreich et al 1996). Hsp27 expression has been associated with poor prognosis in ovarian, gastric, liver and prostate cancer, and osteosarcomas. In contrast, Hsp27 expression has been associated with good prognosis in endometrial adenocarcinomas, oesophageal cancer, and in malignant fibrous histiocytomas. Although there are fewer studies in other cancers, the data suggest that Hsp27 has no prognostic value in head and neck squamous cancer, bladder and renal cancer, and leukemia (except when associated with other markers). There are contradictory data in oral cancer and ovarian cancer. (b) Hsp70—Hsp70 expression is correlated with poor prognosis in breast cancer, endometrial cancer, uterine cervical cancer, and transitional cell carcinoma of the bladder. This is consistent with the Hsp70 associations with poor differentiation, lymph node metastasis, increased cell proliferation, block of apoptosis, and higher clinical stage, which are markers of poor clinical outcome. In contrast, high Hsp70 expression was correlated with good prognosis in oesophageal cancer, pancreatic cancer, renal cancer, and melanoma. Hsp70 expression showed no correlation with prognosis in ovarian cancer, oral cancer, head and neck squamous cancer, gastric and prostate cancer, and leukemia. (c) Hsp90—Hsp90 expression in cancer tissues and presence of autoantibodies to Hsp90 have been correlated with poor prognosis in breast cancer. In contrast, Hsp90 expression is associated with good prognosis in endometrial cancer. Loss of Hsp90 (and Hsp60) expression has been associated in bladder carcinoma with invasive recurrence risk. Hsp90 expression was of no prognostic value in ovarian and oral cancer.
Table 2.
Heat shock proteins in cancer: prognostic implications

It is evident that we need more studies on Hsps to confirm whether they have prognostic value in specific cancers. At this point, it is tempting to speculate that the unique molecular context or milieu present in each cancer type drives the correlations of Hsps with the disease prognosis. For example, in breast cancers, Hsp90 can be chaperoning the oncoprotein HER-2/neu and the mutated p53 protecting these molecules from degradation by the proteasome, which is good for the tumor but bad for the patient. In contrast, in endometrial cancers Hsp90 may be chaperoning progesterone receptors contributing to its maturation, maintaining a more differentiated and less aggressive tumor phenotype with a better response to synthetic progestational agents. Therefore, depending of the cancer type, each Hsp has unique associations with the prognosis of the disease.
PREDICTIVE IMPLICATIONS
There are studies exploring the use of the Hsps to predict the response (or lack of response) of a set of cancer patients to a specific treatment(s) (Table 3). These studies are very important because they may tailor the treatment strategy to individual cancer patients. These studies may be aided by a clearer understanding of the molecular link between the malignant phenotype and Hsp expression although such studies are currently at a very early stage. The following conclusions can be deduced from the published data (considering only those papers that presented a relatively large number of patients, with an homogeneous treatment, and with a clinical follow-up). (a) Hsp27—Although the expression of Hsp27 correlated with that of ERα in breast cancer, detection of Hsp27 does not predict the response to tamoxifen. Hsp27 expression was found in 31% of prostate cancer patients refractory to hormone therapy (Bubendorf et al 1999), although we need more studies on this important subject. Regarding chemotherapy, Hsp27 overexpression has been correlated with a shorter disease-free survival in advanced breast cancer patients who received neoadjuvant chemotherapy (Vargas-Roig et al 1998). This clinical implication of Hsp27 expression with resistance to chemotherapy is in agreement with studies performed in ovarian cancer, in head and neck cancer, esophageal squamous cell carcinoma, and leukemia (in association with other molecular markers). Hsp27 has shown no predictive value to chemotherapy in rectal cancer, malignant fibrous histiocytoma, and central nervous system tumors (induction radiochemotherapy). Regarding the brain tumors, we should point out that glioblastoma multiforme is rather resistant to radiochemotherapy and that these tumors already show a high expression of Hsp27 (as well as other Hsps) and that a further elevation in Hsp content may not correlate with a more resistant phenotype. We need more studies to know whether Hsp27 is related to radioresistance or radiosensitivity in cancer. The molecular mechanisms involving Hsp27 and other Hsps in resistance to cancer therapies can be explained in several ways: (1) as molecular chaperones they can confer cytoprotection by repairing more efficiently the damaged proteins resulting from cytotoxic drug administration, (2) protecting cancer cells from apoptosis (Arrigo et al 2002), (3) protecting the microvasculature inside tumors, because Hsp27 is found in endothelial cells (Ciocca et al 2003), and (4) enhancing DNA repair (Mendez et al 2003; Nadin et al 2003). (b) Hsp70—Although Hsp70 expression is associated with ERα expression in breast cancer, Hsp70 (like Hsp27) did not show predictive value for tamoxifen administration. In contrast, Hsp70 is emerging as a predictor of resistance to chemotherapy in breast cancer. Moreover, high Hsp70 levels predicted lower response of breast cancers to radiation and hyperthermia. In a recent study in multiple myeloma cells using oligonucleotide arrays, Chauhan et al (2003) identified several members of the Hsp family (including Hsp70) among the molecules conferring resistance to the conventional treatment with dexamethasone. Interestingly, they reported a new compound that overcomes dexamethasone resistance, which decreased the levels of Hsp27, Hsp70, and Hsp90 in the myeloma cells. Cancer cells have several defense mechanisms against cytotoxic drugs, which may be redundant, and in order to predict more accurately the resistance of cancer cells to certain therapies, it will be necessary to examine alternative pathways. Moreover, at the proteomic level, we will need to examine not only the expression but also the localization of the Hsps because this seems to be an important factor in their predictive value (Vargas-Roig et al 1998). Depending on the type of cancer (and their molecular profile and interactions), the Hsps can have a more marginal predictive role; for instance, in lung cancer, Hsp70 showed a weak predictive value compared with other molecules (Volm and Rittgen 2000). On the other hand, in osteosarcomas, Hsp70 predicted a better response to neoadjuvant chemotherapy (Trieb et al 1998), which may be explained by the different molecular context of these tumors. However, we need more studies regarding the predictive value of Hsps in cancer to deduce the precise significance of HSP expression.
Table 3.
Heat shock proteins in cancer: predictive implications
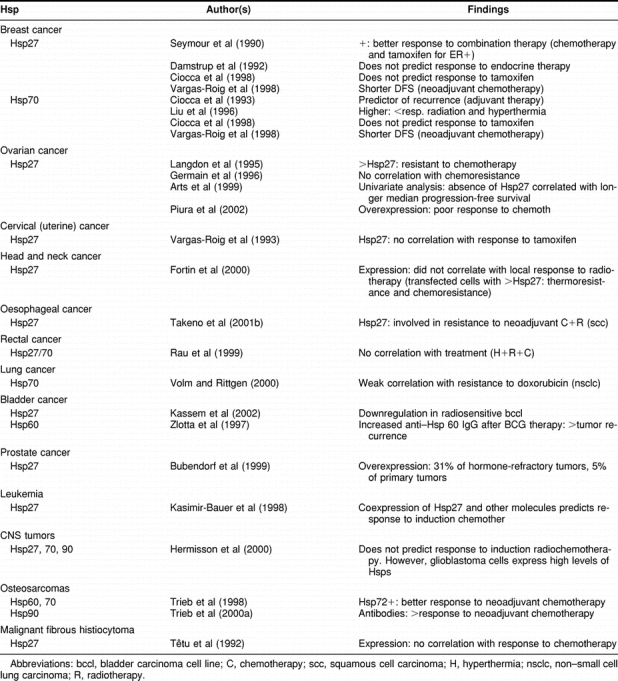
IMPLICATIONS IN THE TREATMENT
This is an exciting new door for the field of Hsps in cancer. Hsp and the HSF family could provide a true Achilles heel for cancer therapy because they seem to be required for cell survival during tumor progression and metastasis (Volloch and Sherman 1999; Hoang et al 2000; Nylandsted et al 2000a, 2000b; Jones et al 2004). Hsps or HSFs may be targeted by drugs and new classes of drugs targeting Hsps are beginning to be deployed, most notably at this time targeting Hsp90 (Neckers and Ivy 2003). The elevated Hsps may also provide a tempting target for immunotherapy protocols because they are able to chaperone tumor antigens and act as biological adjuvants to break tolerance to tumor antigens and cause immune killing by cytotoxic CTL and tumor regression (Arnold-Schild et al 1999; Belli et al 2002; Manjili et al 2002; Noessner et al 2002; Srivastava 2002; Todryk et al 2003; Castelli et al 2004; Daniels et al 2004). Dependence on the selective advantages for growth offered by the protective effects of Hsps may thus render tumor cells vulnerable to detection through immunosurveillance and killing by chaperone-based immunotherapy. Table 4 shows the preclinical and the few clinical studies implicating Hsps in cancer treatment.
Table 4.
Heat shock proteins in cancer: treatment implications
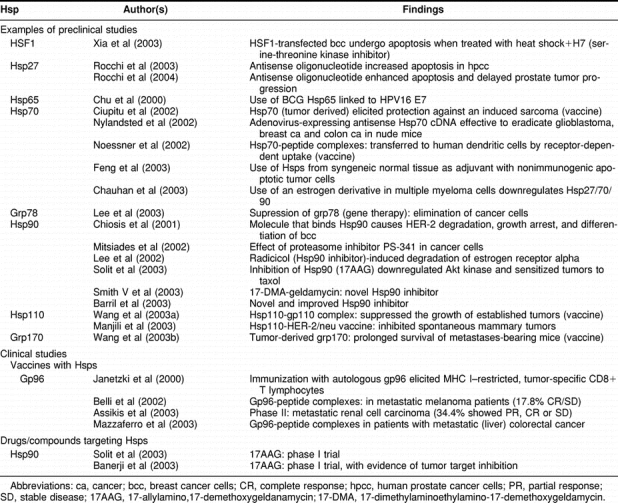
Agents that modify the molecular levels or molecular capabilities of the Hsps—this is achieved by the inhibition of Hsp90 by the natural product geldanamycin or the geldanamycin analog 17AAG. These drugs target the nucleotide-binding site in the N-terminal domain of Hsp90, the same as the adenosine triphosphate–binding site (inhibition of the adenosine triphosphatase activity) causing inhibition of the binding of Hsp90 to the client proteins (Workman 2002). These proteins are stress response, or survival-related, or mutated proteins that without the binding to Hsp90 are not properly folded (less stable) and destroyed by the proteasome. For instance, Hsp90 has been shown to bind mutant p53 more avidly than wild-type p53 (Blagosklonny et al 1996). In normal cells most Hsp90 is not bound to other proteins. Therefore, the effect on normal cells of the Hsp90-inhibitory drugs is not so drastic as in tumor cells. In fact, these drugs are toxic, but the toxicity of 17AAG is manageable. This strategy is interesting because of its ability to affect multiple oncogenic substrates simultaneously; in the phase I clinical trials on cancer patients, 17AAG produced in some patients stable disease, higher apoptosis, and less proliferation of the tumors but with lower potency than radiotherapy or chemotherapy. Moreover, 17AAG can be used in combination with radiotherapy or chemotherapy (enhancement of sensitization). 17AAG has some limitations such as limited solubility with low oral bioavailability, complex formulation, and modest potency on targets, and it is a substrate for P-glycoprotein. This has increased the interest in the search for other Hps90 inhibitors. Finally, HSF-1, Hsp27, Hsp70, and grp78 are also targets of antisense oligonucleotide therapies or other manipulations with possibilities for anticancer therapies. These interesting approaches are still at the preclinical level.
Use of Hsps as carriers/adjuvant to present tumor molecules to the immune system—the objective is to elicit in a cancer patient a specific and active immune response against its own tumor using the Hsps as natural adjuvants that present to the immune system the molecules (usually protein fragments, polypeptides but also relatively large molecules) that have shielded the potential epitopes from immune recognition. The immunization is carried out with tumor-derived Hps (gp96, Hsp70, and others), which bring attached the specific tumor peptides. When injected as a therapeutic vaccine, the Hsps interact with receptors on the professional antigen presenting cells (dendritic cells, macrophages). These cells introduce the antigen(s) into the MHC class I and II pathways, inducing a specific cytotoxic T lymphocyte response and the production of proinflammatory cytokines (Table 4, Srivastava et al 1998). Another approach is to use recombinant Hsps with oncoproteins such as HER-2/neu or proteins from oncogenic viruses such as E7 of HPV. The tumor-derived auto-vaccines based on Hsp or the recombinant Hsp fusion proteins induce cytokine and costimulatory molecules with activation of CD4+ and CD8+ T cells and increases in CD11c+ cells and NK cells that kill tumor cells (Table 4, Rivoltini et al 2003). So far the most promising effects are being obtained in renal cancer and melanoma patients, but several other cancer patients are being treated with the vaccines based on Hsps including patients with colorectal, gastric and pancreatic cancers, leukemia, and lymphoma. These Hsp-based vaccines exhibit minimal toxicity, and if they continue to show good results, they may ultimately be incorporated into the armamentarium against patients with limited or minimal cancer disease (the immune system has a relatively limited capacity to kill large tumor burdens).
CONCLUSIONS
Our studies indicate a profound role for Hsp in many aspects of tumor progression and response to therapy. Although at the diagnostic level Hsps are not informative, they are effective biomarkers for carcinogenesis in some tissues and signal the degree of differentiation and aggressiveness of certain cancers. In addition, the levels of Hsp and anti-Hsp antibodies in the serum of cancer patients are useful in tumor diagnosis. Furthermore, several Hsps are implicated with the prognosis of specific cancers, including Hsp27 expression, which is associated with poor prognosis in gastric, liver, and prostate carcinoma and osteosarcomas, and Hsp70, which is correlated with poor prognosis in breast, endometrial, uterine cervical, and bladder carcinomas. Hsp may also predict the response to some anticancer treatments. Implication of Hsp in tumor progression and response to therapy has led to its successful targeting in therapy by two main strategies, including: (1) pharmacological modification of Hsp expression or molecular chaperone activity and (2) use of Hsps as adjuvants to present tumor antigens to the immune system. Study of Hsp in cancer at the cell and molecular level, although promising, is still in its infancy, and we currently have little information on how Hsp regulation is subverted in cancer and how Hsp dysregulation affects the molecular events involved with tumor growth, invasiveness, and metastasis. Such studies will be essential in interpreting and directing the studies aimed at targeting Hsps in cancer therapy.
Note: we apologize to the colleagues whose works have not been cited in the present review because of space limitations and our limitations in finding their work in the literature search.
Table 1.
Continued
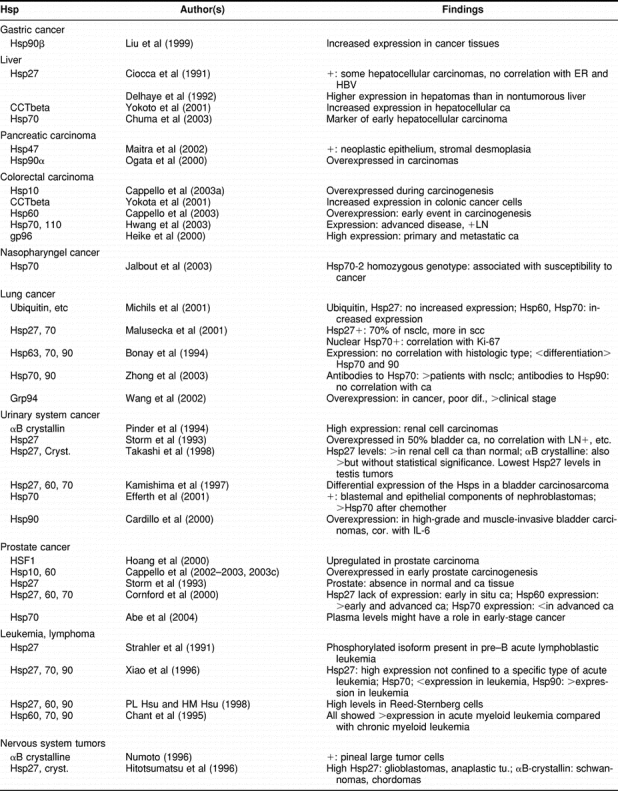
Table 1.
Continued
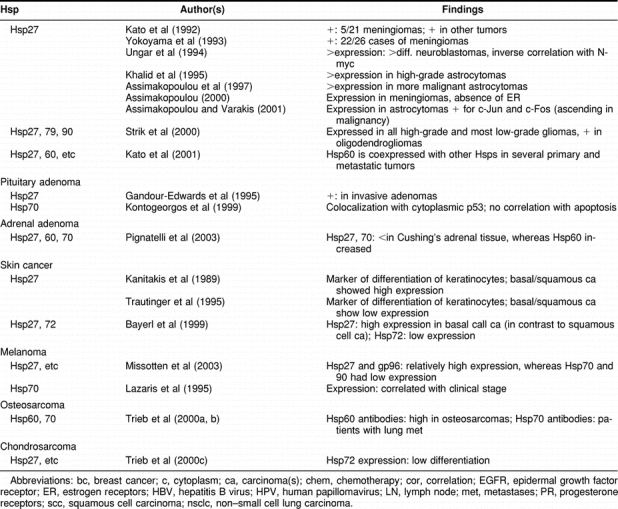
Table 2.
Continued
Acknowledgments
We thank the National Research Council (CONICET), the Argentine Foundation for Cancer Research (D.R.C.), and the National Institutes of Health CA47407, CA31303, CA50642, and CA77465 (S.K.C.) for grant support.
REFERENCES
- Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, Kantoff PW. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3:49–53. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- Abd el All H, Rey A, Duvillard P. Expression of heat shock protein 70 and c-myc in cervical carcinoma. Anticancer Res. 1998;18:1533–1536.0250-7005(1998)018[1533:EOHSPA]2.0.CO;2 [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760.0022-1767(1999)162[3757:CEREOH]2.0.CO;2 [PubMed] [Google Scholar]
- Arrigo A-P, Paul C, and Duchase C. et al. 2002 Small stress proteins: novel negative modulators of apoptosis induced independently of reactive oxygen species. Prog Mol Subcell Biol. 28:185–204. [DOI] [PubMed] [Google Scholar]
- Arts HJ, Hollema H, Lemstra W, Willemse PH, De Vries EG, Kampinga HH, Van der Zee AG. Heat-shock-protein-27 (Hsp27) expression in ovarian carcinoma: relation in response to chemotherapy and prognosis. Int J Cancer. 1999;84:234–238. doi: 10.1002/(sici)1097-0215(19990621)84:3<234::aid-ijc6>3.0.co;2-9.0020-7136(1999)084[0234:HHEIOC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Assikis VJ, Daliani D, and Pagliaro L. et al. 2003 Phase II study of an autologous tumor derived heat shock protein-peptide complex vaccine (HSPPC-96) for patients with metastatic renal cell carcinoma (mRCC). Proc ASCO 22: 386 (A1552). [Google Scholar]
- Assimakopoulou M. Human meningiomas: immunohistochemical localization of progesterone receptor and heat shock protein 27 in absence of estrogen receptor and PS2. Cancer Detect Prev. 2000;24:163–168.0361-090X(2000)024[0163:HMILOP]2.0.CO;2 [PubMed] [Google Scholar]
- Assimakopoulou M, Sotiropoulou-Bonikou G, Maraziotis T, Varakis I. Prognostic significance of Hsp27 in astrocytic brain tumors: an immunohistochemical study. Anticancer Res. 1997;17:2677–2682.0250-7005(1997)017[2677:PSOHIA]2.0.CO;2 [PubMed] [Google Scholar]
- Assimakopoulou M, Varakis J. AP-1 and heat shock protein 27 expression in human astrocytomas. J Cancer Res Clin Oncol. 2001;127:727–732. doi: 10.1007/s004320100280.0171-5216(2001)127[0727:AAHSPE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanassiadou P, Petrakakou E, Sakelariou V, Zerva C, Liossi A, Michalas S, Athanassiades P. Expression of p53, bcl-2 and heat shock protein (Hsp72) in malignant and benign ovarian tumors. Eur J Cancer Prev. 1998;7:225–231. doi: 10.1097/00008469-199806000-00007.0959-8278(1998)007[0225:EOPBAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Banerji U, O'Donnell A, and Scurr M. et al. 2003 A pharmacokinetically (PK)—pharmacodynamically (PD) guided phase I trial of the heat shock protein 90 (HSP90) inhibitor 17-allylamino, 17-demethoxy-geldanamycin (17AAG). Proc ASCO 22: 199 (A797). [Google Scholar]
- Barril X, Davis B, and Collier A. et al. 2003 Structure-based drug discovery based an a novel inhibitor of the Hsp90 molecular chaperone. Proc AACR 44: 796 (A3488). [Google Scholar]
- Bayerl C, Dorfner B, Rzany B, Fuhrmann E, Coelho CC, Jung EG. Heat shock protein HSP27 is expressed in all types of basal cell carcinoma in low and high risk UV exposure groups. Eur J Dermatol. 1999;9:281–284.1167-1122(1999)009[0281:HSPHIE]2.0.CO;2 [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LA, Welch WJ. Interaction of HSP70 with newly synthesized proteins; implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360.0193-4511(1990)248[0850:IOHWNS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Belli F, Testori A, and Rivoltini L. et al. 2002 Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunological findings. J Clin Oncol. 20:4169–4180. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Re: Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2001;93:239–240. doi: 10.1093/jnci/93.3.239-a.0027-8874(2001)093[0239:RROTHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379.0027-8424(1996)093[8379:MCOPTI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonay M, Soler P, Riquet M, Battesti JP, Hance AJ, Tazi A. Expression of heat shock proteins in human lung and lung cancers. Am J Respir Cell Mol Biol. 1994;10:453–461. doi: 10.1165/ajrcmb.10.4.8136161.1044-1549(1994)010[0453:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bostwick DG. Immunohistochemical changes in prostate cancer after androgen deprivation therapy. Mol Urol. 2000;4:101–106.1091-5362(2000)004[0101:ICIPCA]2.0.CO;2 [PubMed] [Google Scholar]
- Bubendorf L, Kolmer M, and Kononen J. et al. 1999 Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 91:1758–1764. [DOI] [PubMed] [Google Scholar]
- Buchner J. HSP 90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–142. doi: 10.1016/s0968-0004(99)01373-0.0376-5067(1999)024[0136:HCHFF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, David S, Anzalone R, Zummo G. Ten kilodalton heat shock protein (HSP10) is overexpressed during carcinogenesis of large bowel and uterine cervix. Cancer Lett. 2003a;196:35–41. doi: 10.1016/s0304-3835(03)00212-x.0304-3835(2003)196[0035:TKHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, and Palma A. et al. 2003b 60 KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 47:105–110. [DOI] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, and Palma A. et al. 2002–2003 Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology. 70:83–88. [DOI] [PubMed] [Google Scholar]
- Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003c;23:1325–1331.0250-7005(2003)023[1325:IEOPPH]2.0.CO;2 [PubMed] [Google Scholar]
- Cardillo MR, Sale P, Di Silverio F. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer Res. 2000;20:4579–4583.0250-7005(2000)020[4579:HSPIAI]2.0.CO;2 [PubMed] [Google Scholar]
- Castelli C, Rivoltini L, and Rini F. et al. 2004 Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 53:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry. Br J Haematol. 1995;90:163–168. doi: 10.1111/j.1365-2141.1995.tb03395.x.0007-1048(1995)090[0163:AOHPEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Auclair D, and Hideshima T. et al. 2003 Identification of genes regulated by 2-methoxyestradiol (2ME2) in multiple myeloma cells using oligonucleotide arrays. Blood. 101:3606–3614. [DOI] [PubMed] [Google Scholar]
- Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. A small molecule designed to bind to the adenine nucleotide pocked of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8:289–299. doi: 10.1016/s1074-5521(01)00015-1.1074-5521(2001)008[0289:ASMDTB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumor by administration of fusion protein comprising mycobacterium bovis bacille Calmette-Guerin (BCG) Hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–225. doi: 10.1046/j.1365-2249.2000.01293.x.0009-9104(2000)121[0216:IOAHPH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S. Expression profiling in multistage hepatocarcionogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022.0270-9139(2003)037[0198:EPIMHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Clark GM, Tandon AK, Fuqua SAW, Welch WJ, McGuire WL. Heath shock protein Hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570.0027-8874(1993)085[0570:HSPHIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Green S, and Elledge RM. et al. 1998 Heat shock proteins Hsp27 and Hsp70: lack of correlation with response to tamoxifen and clinical course of disease in estrogen receptor-positive metastatic breast cancer (a Southwest Oncology Group study). Clin Cancer Res. 5:1263–1266. [PubMed] [Google Scholar]
- Ciocca DR, Jorge AD, and Jorge O. et al. 1991 Estrogen receptors, progesterone receptors and heat-shock 27-kD protein in liver biopsy specimens from patients with hepatitis B virus infection. Hepatology. 13:838–844. [PubMed] [Google Scholar]
- Ciocca DR, Lo Castro G, Alonio V, Cobo MF, Lotfi H, Teyssié A. Effect of human papillomavirus infection on estrogen receptor and heat shock protein Hsp27 phenotype in human cervix and vagina. Int J Gynecol Pathol. 1992;11:113–121. doi: 10.1097/00004347-199204000-00005.0277-1691(1992)011[0113:EOHPIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Puy LA, Edwards DP, Adams DJ, McGuire WL. The presence of an estrogen-regulated protein detected by monoclonal antibody in abnormal human endometrium. J Clin Endocrinol Metab. 1985;60:137–143. doi: 10.1210/jcem-60-1-137.0021-972X(1985)060[0137:TPOAEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Puy LA, Fasoli LC. Study of estrogen receptor, progesterone receptor, and the estrogen-regulated Mr 24,000 protein in patients with carcinomas of the endometrium and cerviz. Cancer Res. 1989;49:4298–4304.0008-5472(1989)049[4298:SOERPR]2.0.CO;2 [PubMed] [Google Scholar]
- Ciocca DR, Puy LA, Lo Castro G. Localization of an estrogen-responsive protein in the human cervix during menstrual cycle, pregnancy, and menopause and in abnormal cervical epithelia without atypia. Am J Obstet Gynecol. 1986;155:1090–1096. doi: 10.1016/0002-9378(86)90357-1.0002-9378(1986)155[1090:LOAEPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Rozados VR, Cuello-Carrión FD, Gervasoni SI, Matar P, Scharovsky OG. Heat shock proteins 25 and 70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2.1466-1268(2003)008[0026:HSPAIR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Stati AO, Amprino de Castro MM. Colocalization of estrogen and progesterone receptors with an estrogen-regulated heat shock protein in paraffin sections of human breast and endometrial cancer tissue. Breast Cancer Res Treat. 1990;16:243–251. doi: 10.1007/BF01806332.0167-6806(1990)016[0243:COEAPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciupitu AMT, Petersson M, Kono K, Charo J, Kiesslin R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell response. Cancer Immunol Immunother. 2002;51:163–170. doi: 10.1007/s00262-002-0263-9.0340-7004(2002)051[0163:IWHSPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SE, Sasieni PD, Amin V, Wang DY, Smith P, Fentiman IS, Latchman DS. Antibodies to heat-shock protein 27 are associated with improved survival in patients with breast cancer. Br J Cancer. 1998a;77:1875–1879. doi: 10.1038/bjc.1998.312.0007-0920(1998)077[1875:ATHPAA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SE, Sasieni PD, Fentiman I, Latchman DS. Autoantibodies to the 90kDa heat shock protein and poor survival in breast cancer patients. Eur J Cancer. 1998b;34:942–943.0959-8049(1998)034[0942:ATTKHS]2.0.CO;2 [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, and Parsons KF. et al. 2000 Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 60:7099–7105. [PubMed] [Google Scholar]
- Damstrup L, Andersen J, Kufe DW, Hayes DF, Skovgaard Poulsen H. Immunocytochemical determination of the estrogen-regulated proteins Mr 24,000, Mr 52,000 and DF3 breast cancer associated antigen: clinical value in advanced breast cancer and correlation with estrogen receptor. Ann Oncol. 1992;3:71–77. doi: 10.1093/oxfordjournals.annonc.a058078.0923-7534(1992)003[0071:IDOTEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Daniels GA, Sanchez-Perez L, and Diaz RM. et al. 2004 A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 22:1125–1132. [DOI] [PubMed] [Google Scholar]
- Delhaye M, Gulbis B, Galand P, Mairesse N. Expression of 27-kD heat shock protein isoforms in human neoplastic and nonneoplastic liver tissues. Hepatology. 1992;16:382–389. doi: 10.1002/hep.1840160216.0270-9139(1992)016[0382:EOKHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dressler LG, Ramzy I, Sledge GW, McGuire WL. A new marker of maturation in the cervix: the estrogen-regulated 24K protein. Obstet Gynecol. 1986;68:825–831.0029-7844(1986)068[0825:ANMOMI]2.0.CO;2 [PubMed] [Google Scholar]
- Dundas SR, Lawrie LC, Rooney PH, Murray GI. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205:74–81. doi: 10.1002/path.1672.0022-3417(2005)205[0074:MIOBCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Efferth T, Schulten HG, and Thelen P. et al. 2001 Differential expression of the heat shock protein 70 in the histological compartments of nephroblastomas. Anticancer Res. 21:2915–2920. [PubMed] [Google Scholar]
- Elledge RM, Clark GM, Fuqua SAW, Yo YY, Allred DC. p53 protein accumulation detected by five different antibodies: relationship to prognosis and heat shock protein 70 in breast cancer. Cancer Res. 1994;54:3752–3757.0008-5472(1994)054[3752:PPADBF]2.0.CO;2 [PubMed] [Google Scholar]
- Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins Hsp27, Hsp70 and Hsp90 in malignant epithelial tumor of the ovaries. APMIS. 2003;111:523–530. doi: 10.1034/j.1600-0463.2003.1110411.x.0903-4641(2003)111[0523:EOHPHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Erkizan O, Kirkali G, Yorukoglu K, Kirkali Z. Significance of heat shock protein-27 expression in patients with renal cell carcinoma. Urology. 2004;64:474–478. doi: 10.1016/j.urology.2004.04.017.0090-4295(2004)064[0474:SOHSPE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Cuello Carrión FD, Dekker J, Schoemaker J, Ciocca DR. Serological detection of heat shock protein Hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:791–795.1055-9965(1998)007[0791:SDOHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–252. doi: 10.1182/blood-2002-05-1580.0006-4971(2003)101[0245:ESPETI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA, Patierno SR. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207.0167-6806(2000)059[0015:OOTGSG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fortin A, Raybaud-Diagene H, Tetu B, Deschenes R, Huot J, Landry J. Overexpression of the 27 KDa heat shock protein is associated with thermoresistance and chemoresistance but not with radioresistance. Int J Radiat Oncol Biol Phys. 2000;46:1259–1266. doi: 10.1016/s0360-3016(99)00410-1.0360-3016(2000)046[1259:OOTKHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051.0193-4511(2002)296[2232:DOTRCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gago FE, Tello OM, Diblasi AM, Ciocca DR. Integration of estrogen and progesterone receptors with pathological and molecular prognostic factors in breast cancer patients. J Steroid Biochem Mol Biol. 1998;67:431–437. doi: 10.1016/s0960-0760(98)00140-x.0960-0760(1998)067[0431:IOEAPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gandour-Edwards R, Kapadia SB, Janecka IP, Martinez AJ, Barnes L. Biologic markers of invasive pituitary adenomas involving the sphenoid sinus. Modern Pathol. 1995;8:160–164.0893-3952(1995)008[0160:BMOIPA]2.0.CO;2 [PubMed] [Google Scholar]
- Gandour-Edwards R, Trock BJ, Gumerlock P, Donald PJ. Heat shock protein and p53 expression in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 1998;118:610–615. doi: 10.1177/019459989811800508.0194-5998(1998)118[0610:HSPAPE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Geisler JP, Geisler HE, Tammela J, Miller GA, Weimann MC, Zhou Z. A study of heat shock protein 27 in endometrial carcinoma. Gynecol Oncol. 1999;72:347–350. doi: 10.1006/gyno.1998.5283.0090-8258(1999)072[0347:ASOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Geisler JP, Geisler HE, Tammela J, Wiemann MC, Zhou Z, Miller GA, Crabtree W. Heat shock protein 27: an independent prognostic indicator of survival in patients with epithelial ovarian carcinoma. Gynecol Oncol. 1998;69:14–16. doi: 10.1006/gyno.1998.4961.0090-8258(1998)069[0014:HSPAIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Georgopolis C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125.0743-4634(1993)009[0601:ROTMHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Germain I, Tetu B, Brisson J, Mondor M, Cherian MG. Markers of chemoresistance in ovarian carcinomas: an immunohistochemical study of 86 cases. Int J Gynecol Pathol. 1996;15:54–62. doi: 10.1097/00004347-199601000-00009.0277-1691(1996)015[0054:MOCIOC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0.0028-0836(1992)355[0033:PFITC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Nylandsted J, Jaattela M. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle. 2004;3:1484–1485. doi: 10.4161/cc.3.12.1287. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902.0028-4793(2002)347[1593:RFMHTC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harrison JD, Jones JA, Ellis IO, Morris DL. Oestrogen receptor D5 antibody is an independent negative prognostic factor in gastric cancer. Br J Surg. 1991;78:334–336. doi: 10.1002/bjs.1800780321.0007-1323(1991)078[0334:ORDAIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heike M, Frenzel C, Meier D, Galle PR. Expression of stress protein gp96, a tumor rejection antigen, in human colorectal cancer. Int J Cancer. 2000;86:489–493. doi: 10.1002/(sici)1097-0215(20000515)86:4<489::aid-ijc7>3.0.co;2-d.0020-7136(2000)086[0489:EOSPGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Henics T, Nagy E, Oh HJ, Csermely P, von Gabain A, Subjeck JR. Mammalian HSP70 and HSP110 proteins bind to RNA motifs involved in mRNA stability. J Biol Chem. 1999;274:17318–17324. doi: 10.1074/jbc.274.24.17318.0021-9258(1999)274[17318:MHAHPB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hermisson M, Strik H, Rieger J, Dichgans J, Meyermann R, Weller M. Expression and functional activity of heat shock proteins in human glioblastoma multiforme. Neurology. 2000;54:1357–1365. doi: 10.1212/wnl.54.6.1357.0028-3878(2000)054[1357:EAFAOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hickey E, Weber LA 1982 Preferential translation of heat shock mRNAs in HeLa cells. In: Heat Shock; from Bacteria to Man, ed Schlesinger M, Ashburner M, Tissieres A. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 199–206. [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2.0092-8674(1991)066[0191:HSSPCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu T, Iwaki T, Fukui M, Tateishi J. Distinctive immunohistochemical profiles of small heat shock proteins (heat shock protein 27 and alpha B-Crystallin) in human brain tumors. Cancer. 1996;77:352–361. doi: 10.1002/(SICI)1097-0142(19960115)77:2<352::AID-CNCR19>3.0.CO;2-0.0765-7846(1996)077[0352:DIPOSH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–864. doi: 10.1016/S0002-9440(10)64954-1.0002-9440(2000)156[0857:ANABTH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PL, Hsu SM. Abundance of heat shock proteins (Hsp89, Hsp60, and Hsp27) in malignant cells of Hodgkin's disease. Cancer Res. 1998;58:5507–5513.0008-5472(1998)058[5507:AOHSPH]2.0.CO;2 [PubMed] [Google Scholar]
- Hwang TK, Han HS, Choi HK, Lee YJ, Kim YJ, Han MY, Park YM. Differential, stage-dependent expression of Hsp70, Hsp 110, and Bcl-2 in colorectal cancer. J Gastroenterol Hepatol. 2003;18:690–700. doi: 10.1046/j.1440-1746.2003.03011.x.0815-9319(2003)018[0690:DSEOHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ito T, Kawabe R, Kurasono Y, Hara M, Kitamura H, Fujita K, Kanisawa M. Expression of heat shock proteins in squamous cell carcinoma of the tongue: an immunohistochemical study. J Oral Pathol Med. 1998;27:18–22. doi: 10.1111/j.1600-0714.1998.tb02085.x.0904-2512(1998)027[0018:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Iwaya K, Tsuda H, Fujita S, Suzuki M, Hirohashi S. Natural state of mutant p53 protein and heat shock protein 70 in breast cancer tissues. Lab Invest. 1995;72:707–714.0023-6837(1995)072[0707:NSOMPP]2.0.CO;2 [PubMed] [Google Scholar]
- Jalbout M, Bouaouina N, Gargouri J, Corbex M, Ben Ahmed S, Chouchane L. Polymorphism of the stress protein HSP70-2 gene is associated with the susceptibility to the nasopharyngeal carcinoma. Cancer Lett. 2003;193:75–81. doi: 10.1016/s0304-3835(02)00697-3.0304-3835(2003)193[0075:POTSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jameel A, Skilton RA, Cambell TA, Chander SK, Coombes RC, Luqmani YA. Clinical and biological significance of HSP89 alpha in human breast cancer. Int J Cancer. 1992;50:409–415. doi: 10.1002/ijc.2910500315.0020-7136(1992)050[0409:CABSOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8.0020-7136(2000)088[0232:IOCPWA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jones EL, Zhao MJ, Stevenson MA, Calderwood SK. The 70 kilodalton heat shock protein is an inhibitor of apoptosis in cancer. Int J Hyperthermia. 2004;20:835–849. doi: 10.1080/02656730410001721807.0265-6736(2004)020[0835:TKHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamishima T, Fukuda T, Usuda H, Takato H, Iwamoto H, Kaneko H. Carcinosarcoma of the urinary bladder: expression of epithelial markers and different expression of heat shock proteins between epithelial and sarcomatous elements. Pathol Int. 1997;47:166–173. doi: 10.1111/j.1440-1827.1997.tb03735.x.1320-5463(1997)047[0166:COTUBE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kanitakis J, Zambruno G, Viac J, Tommaselli L, Thivolet J. Expression of an estrogen receptor-associated protein (p29) in epithelial tumors of the skin. J Cutan Pathol. 1989;16:272–276. doi: 10.1111/j.1600-0560.1989.tb00051.x.0303-6987(1989)016[0272:EOAERP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kapranos N, Kominea A, Konstantinopoulos PA, Savva S, Artelaris S, Vandoros G, Sotiropoulou-Bonikou G, Papavassiliou AG. Expression of the 27-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic, and dysplastic gastric mucosa, and its prognostic significance. J Cancer Res Clin Oncol. 2002;128:426–432. doi: 10.1007/s00432-002-0357-y.0171-5216(2002)128[0426:EOTKHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimir-Bauer S, Beelen DW, Flasshove M, Noppeney R, Seeber S, Scheulen ME. Impact of the expression of P-glycoprotein, the multidrug resistance-related protein, bcl-2, mutant p53, and heat shock protein 27 on response to induction chemotherapy and long-term survival in patients with de novo acute myeloid leukemia. Exp Hematol. 2002;30:1302–1308. doi: 10.1016/s0301-472x(02)00926-8.0301-472X(2002)030[1302:IOTEOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kasimir-Bauer S, Ottinger H, Meusers P, Beelen DW, Brittinger G, Seeber S, Scheulen ME. In acute myeloid leukaemia, coexpression of at least two proteins, including P-glycoprotein, the multidrug resistance-related protein, bcl-2, mutant p53, and heat shock protein 27, is predictive of the response to induction chemotherapy. Exp Hematol. 1998;26:1111–1117.0301-472X(1998)026[1111:IAMLCO]2.0.CO;2 [PubMed] [Google Scholar]
- Kassem HSh, Sangar V, Cowan R, Clarke N, Margison GP. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder carcinoma. Int J Cancer. 2002;101:454–460. doi: 10.1002/ijc.10631.0020-7136(2002)101[0454:APROHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kato M, Herz F, Hirano A. Expression of stress-response (heat-shock) protein 27 in human brain tumors: an immunohistochemical study. Acta Neuropathol. 1992;83:420–422. doi: 10.1007/BF00713535.0001-6322(1992)083[0420:EOSHPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kato S, Kato M, Hirano A, Takikawa M, Ohama E. The immunohistochemical expression of stress-response protein (srp) 60 in human brain tumors: relationship of srp 60 to the other five srps, proliferating cell nuclear antigen and p53 protein. Histol Histopathol. 2001;16:809–820. doi: 10.14670/HH-16.809.0213-3911(2001)016[0809:TIEOSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kaur J, Das SN, Srivastava A, Ralhan R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma: correlation with clinicopathological features. Oral Oncol. 1998;34:93–98. doi: 10.1016/s1368-8375(97)00055-9.0964-1955(1998)034[0093:CSEOKH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Shiozaki H, and Doki Y. et al. 1999 Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer. 85:1649–1657. [DOI] [PubMed] [Google Scholar]
- Keeling J, McKee GT. Heat shock protein (HSP)27: a further refinement in the diagnosis of suspicious fine needle aspirates of breast. Cytopathology. 1999;10:40–49. doi: 10.1046/j.1365-2303.1999.00140.x.0956-5507(1999)010[0040:HSPHAF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Khalid H, Tsutsumi K, Yamashita H, Kishikawa M, Yasunaga A, Shibata S. Expression of the small heat shock protein (Hsp) 27 in human astrocytomas correlates with histologic grades and tumor growth fractions. Cell Mol Neurobiol. 1995;15:257–268. doi: 10.1007/BF02073332.0272-4340(1995)015[0257:EOTSHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Jang TJ, Kim JR. HSP70 and ER expression in cervical intraepithelial neoplasia and cervical cancer. J Korean Med Sci. 1998;13:383–388. doi: 10.3346/jkms.1998.13.4.383.1011-8934(1998)013[0383:HAEEIC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Enns RE, Alcaraz JE, Arboleda J, Slamon DJ, Howell SB. Correlation of the survival of ovarian cancer patients with mRNA expresión of the 60-kD heat-shock protein HSP-60. J Clin Oncol. 1993;11:891–898. doi: 10.1200/JCO.1993.11.5.891.0732-183X(1993)011[0891:COTSOO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, Lui WY. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000;88:2464–2470. doi: 10.1002/1097-0142(20000601)88:11<2464::aid-cncr6>3.0.co;2-w.0765-7846(2000)088[2464:PSOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Konstadoulakis MM, Vezeridis M, Hatziyianni E, Karakousis CP, Cole B, Bland KI, Wanebo HJ. Molecular oncogene markers and their significance in cutaneous malignant melanoma. Ann Surg Oncol. 1998;5:253–260. doi: 10.1007/BF02303782.1068-9265(1998)005[0253:MOMATS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kontogeorgos G, Kapranos N, Thodou E, Sambaziotis D, Tsagarakis S. Immunocytochemical accumulation of p53 in corticotroph adenomas: relationship with heat shock proteins and apoptosis. Pituitary. 1999;1:207–212. doi: 10.1023/a:1009929704018.1386-341X(1999)001[0207:IAOPIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Korneeva I, Bongiovanni AM, Girotra M, Caputo TA, Witkin SS. Serum antibodies to the 27-kd heat shock protein in women with gynaecologic cancers. Am J Obstet Gynecol. 2000;183:18–21. doi: 10.1067/mob.2000.105431.0002-9378(2000)183[0018:SATTKH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Suzuki T, Ooya K. Immunohistochemical analysis of inducible nitric oxide synthetase (iNOS) and heat shock proteins (HSPs) in ameloblastomas. J Oral Pathol Med. 2002;31:605–611. doi: 10.1034/j.1600-0714.2002.00014.x.0904-2512(2002)031[0605:IAOINO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lambot MA, Peny MO, Fayt I, Haot J, Noel JC. Overexpression of 27-kDa heat shock protein relates to poor histological differentiation in human oesophageal squamous cell carcinoma. Histopathology. 2000;36:326–330. doi: 10.1046/j.1365-2559.2000.00858.x.0309-0167(2000)036[0326:OOKHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during the development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461.0008-5472(1982)042[2457:SADOHS]2.0.CO;2 [PubMed] [Google Scholar]
- Langdon SP, Rabiasz GJ, Hirst GL, King RJ, Hawkins RA, Smyth JF, Miller WR. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin Cancer Res. 1995;1:1603–1609.1078-0432(1995)001[1603:EOTHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Lazaris AC, Theodoropoulos GE, Aroni K, Saetta A, Davaris PS. Immunohistochemical expression of C-myc oncogene, heat shock protein 70 and HLA-DR molecules in malignant cutaneous melanoma. Virchows Arch. 1995;426:461–467. doi: 10.1007/BF00193169.0945-6317(1995)426[0461:IEOCOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lazaris ACh, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis BCh. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275.0167-6806(1997)043[0043:PCNAAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee AS, Dong D, Reddy R, Mao C, Gomer C, Gambhir S, Bading J. Stress induction of the glucose regulated protein (GRP78): applications in cancer therapy. Proc Int Cong Stress Responses Biol Med. 2003;1:27. [Google Scholar]
- Lee MO, Kim EO, Kwon HJ, Kim YM, Kang HJ, Kang H, Lee JE. Radicicol represses the transcriptional function of the estrogen receptor by suppressing the stabilization of the receptor by heat shock protein 90. Mol Cell Endocrinol. 2002;188:47–54. doi: 10.1016/s0303-7207(01)00753-5.0303-7207(2002)188[0047:RRTTFO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lehmann TA, Bennet WP, Metcalf RA. P53 mutations, ras mutations and p53-heat shock protein complexes in human lung carcinoma cell lines. Cancer Res. 1991;51:4090–4096.0008-5472(1991)051[4090:PMRMAP]2.0.CO;2 [PubMed] [Google Scholar]
- Lemoisson E, Cren H, Goussard J. Chromatographic separation of eight progesterone receptor isoforms in human breast tumors, and detection by radioligand and monoclonal antibodies. Association with Hsp90 and Hsp70 heat shock proteins. Ann Biol Clin. 1994;52:433–442.0003-3898(1994)052[0433:CSOEPR]2.0.CO;2 [PubMed] [Google Scholar]
- Leonardi R, Pannone G, Magro G, Kudo Y, Takata T, Lo Muzio L. Differential expression of heat shock protein 27 in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma. Oncol Rep. 2002;9:261–266.1021-335X(2002)009[0261:DEOHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between the synthesis of heat shock proteins and the development of thermotolerance in CHO fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218.0027-8424(1982)079[3218:CBTSOH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu FF, Miller N, and Levin W. et al. 1996 The potential role of HSP70 as an indicator of response to radiation and hyperthermia treatments for recurrent breast cancer. Int J Hypertherm. 12:197–208. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye L, Wang J, Fan D. Expression of heat shock protein 90 beta in human gastric cancer tissue and SGD7901/VCR of MDR-type gastric cancer cell line. Chin Med J (Engl) 1999;112:1133–1137.0366-6999(1999)112[1133:EOHSPB]2.0.CO;2 [PubMed] [Google Scholar]
- Love S, King RJB. A 27 kDa heat shock protein that has anomalous prognostic powers in early and advanced breast cancer. Br J Cancer. 1994;69:743–748. doi: 10.1038/bjc.1994.140.0007-0920(1994)069[0743:AKHSPT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo LY, Herrera I, Soosaipillai A, Diamandis EP. Identification of heat shock protein 90 and other proteins as antigens by serological screening of an ovarian carcinoma expression library. Br J Cancer. 2002;87:339–343. doi: 10.1038/sj.bjc.6600439.0007-0920(2002)087[0339:IOHSPA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara Y, Oki E, Abe T, Tokunaga E, Shibahara K, Kakeji Y, Sugimachi K. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144–151. doi: 10.1159/000012091.0030-2414(2000)058[0144:OOTHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Maitra A, Iacobuzio-Donahue C, and Rahman A. et al. 2002 Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin fascin homolog and heat shock protein 47. Am J Clin Pathol. 118:52–59. [DOI] [PubMed] [Google Scholar]
- Malusecka E, Zborek A, Krzyzowska-Gruca S, Krawczyk Z. Expression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical study. Anticancer Res. 2001;21:1015–1021.0250-7005(2001)021[1015:EOHSPH]2.0.CO;2 [PubMed] [Google Scholar]
- Manjili MH, Henderson R, Chen X, Repasky E, and Subjeck JR 2003 HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. Proc AACR 44: 458 (A2009). [DOI] [PubMed] [Google Scholar]
- Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, Kazim L, Subjeck JR. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742.0008-5472(2002)062[1737:DOARNV]2.0.CO;2 [PubMed] [Google Scholar]
- Mazzaferro V, Coppa J, and Carrabba MG. et al. 2003 Vaccination with autologous tumor-derived heat shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 9:3235–3245. [PubMed] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523.0021-9258(1998)273[7523:TDOHST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mendez F, Sandigursky M, Kureekattil RP, Kenny MK, Franklin WA, Bases R. Specific stimulation of human apurinic/apyrimidinic endonuclease by heat shock protein 70. DNA Repair. 2003;2:259–271. doi: 10.1016/s1568-7864(02)00215-x.1568-7864(2003)002[0259:SSOHAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mese H, Sasaki A, Nakayama S, Yoshioka N, Yoshihama Y, Kishimoto K, Matsumura T. Prognostic significance of heat shock protein 27 (HSP27) in patients with oral squamous cell carcinoma. Oncol Rep. 2002;9:341–344.1021-335X(2002)009[0341:PSOHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Mestiri S, Bouaouina N, Ahmed SB, Khedhaier A, Jrad BB, Remani S, Chouchane L. Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein Hsp70-2: susceptibility and prognostic implications in breast carcinoma. Cancer. 2001;91:672–678. doi: 10.1002/1097-0142(20010215)91:4<672::aid-cncr1050>3.0.co;2-j.0765-7846(2001)091[0672:GVITTN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Michils A, Redivo M, Zegers de Beyl V, de Maertelaer V, Jacobovitz D, Rocmans P, Duchateau J. Increased expression of high but not low molecular weight heat shock proteins in resectable lung carcinoma. Lung Cancer. 2001;33:59–67. doi: 10.1016/s0169-5002(01)00184-2.0169-5002(2001)033[0059:IEOHBN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mileo AM, Fanuele M, Battaglia F, Scambia G, Benedetti-Panici P, Mancuso S, Ferrini U. Selective over-expression of mRNA coding for 90 KDa stress-protein in human ovarian cancer. Anticancer Res. 1990;10:903–906.0250-7005(1990)010[0903:SOOMCF]2.0.CO;2 [PubMed] [Google Scholar]
- Missotten GS, Journee-de Korver JG, de Wolff-Rouendaal D, Keunen JE, Schlingemann RO, Jager MJ. Heat shock protein expression in the eye and in uveal melanoma. Invest Ophtalmol Vis Sci. 2003;44:3059–3065. doi: 10.1167/iovs.02-1038. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, and Poulaki V. et al. 2002 Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 99:14374–14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz de Toro MM, Luque EH. Lack of relationship between the expresión of Hsp27 heat shock estrogen receptor-associated protein and estrogen receptor or progestrone receptor status in male breast carcinoma. J Steroid Biochem Mol Biol. 1997;60:277–284. doi: 10.1016/s0960-0760(96)00221-x.0960-0760(1997)060[0277:LORBTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nadin S, Vargas-Roig LM, Cuello-Carrión FD, Ciocca DR. Deoxyribonucleic acid damage induced by doxorubicin in peripheral blood mononuclear cells: possible roles for the stress response and the deoxyribonucleic acid repair process. Cell Stress Chaperones. 2003;8:361–371. doi: 10.1379/1466-1268(2003)008<0361:dadibd>2.0.co;2.1466-1268(2003)008[0361:DADIBD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbu K, Konishi I, Komatsu T, Mandai M, Yamamoto S, Kuroda H, Koshiyama M, Mori T. Expression of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Correlation with clinicopathology, sex steroid receptor status, and p53 protein expression. Cancer. 1996;77:330–338. doi: 10.1002/(SICI)1097-0142(19960115)77:2<330::AID-CNCR16>3.0.CO;2-2.0765-7846(1996)077[0330:EOHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nanbu K, Konishi I, Mandai M, Kuroda H, Hamid AA, Komatsu T, Mori T. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998;22:549–555. doi: 10.1046/j.1525-1500.1998.00069.x.0361-090X(1998)022[0549:PSOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469.0270-7306(1997)017[0469:HANMOT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kuwano H, Miyazaki T, Masuda N, Kato H. Significant correlation between expression of heat shock proteins 27, 70 and lymphocyte infiltration in esophageal squamous cell carcinoma. Cancer Lett. 2002;178:99–106. doi: 10.1016/s0304-3835(01)00825-4.0304-3835(2002)178[0099:SCBEOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003.1040-8746(2003)015[0419:HSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Netzer WF, Hartl FU. Protein folding in the cytosol: chaperonin-dependent and-independent mechanisms. Trends Biochem Sci. 1998;23:68–74. doi: 10.1016/s0968-0004(97)01171-7.0376-5067(1998)023[0068:PFITCC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Noessner E, Gastpar R, and Milani V. et al. 2002 Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 169:5424–5432. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Muller W. Expression of heat shock protein 70 in grossly resected esophageal squamous cell carcinoma. Ann Thorac Surg. 2002;74:222–226. doi: 10.1016/s0003-4975(02)03641-x.0003-4975(2002)074[0222:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Numoto RT. Pineal parenchymal tumors: cell differentiation and prognosis. J Cancer Res Clin Oncol. 1994;120:683–690. doi: 10.1007/BF01245382.0171-5216(1994)120[0683:PPTCDA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000a;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x.0077-8923(2000)926[0122:HSPIRF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 2000b;97:7871–7876. doi: 10.1073/pnas.97.14.7871.0027-8424(2000)097[7871:SDOHSP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jaattela M. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 2002;62:7139–7142.0008-5472(2002)062[7139:EOGABA]2.0.CO;2 [PubMed] [Google Scholar]
- Oesterreich S, Hilsenbeck SG, Ciocca DR, Allred DC, Clark GM, Chamness GC, Osborne CK, Fuqua SA. The small heat shock protein HSP27 is not an independent prognostic marker in axillary lymph node-negative breast cancer patients. Clin Cancer Res. 1996;2:1199–1206.1078-0432(1996)002[1199:TSHSPH]2.0.CO;2 [PubMed] [Google Scholar]
- Ogata M, Naito Z, Tanaka S, Moriyama Y, Asano G. Overexpression and localization of heat shock proteins mRNA in pancreatic carcinoma. J Nippon Med Sch. 2000;67:177–185. doi: 10.1272/jnms.67.177.0048-0444(2000)067[0177:OALOHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999;74:53–60. doi: 10.1006/gyno.1999.5429.0090-8258(1999)074[0053:AIAOHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Ferreira J, Soares P, Costa MJ, Magalhaes MC. Immunohistochemical study of heat shock proteins 27, 60 and 70 in the normal human adrenal and in adrenal tumors with suppressed ACTH production. Microsc Res Tech. 2003;61:315–323. doi: 10.1002/jemt.10341.1059-910X(2003)061[0315:ISOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pinder SE, Balsitis M, Ellis IO, Landon M, Mayer RJ, Lowe J. The expression of alpha B-crystallin in epithelial tumors: a useful tumor marker? J Pathol. 1994;174:209–215. doi: 10.1002/path.1711740310.0022-3417(1994)174[0209:TEOABI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Piura B, Rabinovich A, Yavelsky V, Wolfson M. Heat shock proteins and malignancies of the female genital tract. Harefuah. 2002;141:962–972, 1010, 1009.0017-7768(2002)141[0962:HSPAMO]2.0.CO;2 [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signalling protein function and trafficking by the Hsp90/Hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Puy LA, Lo Castro G, Olcese JE, Lotfi HO, Brandi HR, Ciocca DR. Analysis of a 24-kilodalton (KD) protein in the human uterine cervix during abnormal growth. Cancer. 1989;64:1067–1973. doi: 10.1002/1097-0142(19890901)64:5<1067::aid-cncr2820640518>3.0.co;2-h.0765-7846(1989)064[1067:AOAKKP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Gioorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci U S A. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906.0027-8424(1991)088[6906:MCAEOA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralhan R, Kaur J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin Cancer Res. 1995;1:1217–1222.1078-0432(1995)001[1217:DEOMHS]2.0.CO;2 [PubMed] [Google Scholar]
- Rau B, Gaestel M, Wust P, Stahl J, Mansmann U, Schlag PM, Benndorf R. Preoperative treatment of rectal cancer with radiation, chemotherapy and hyperthermia: analysis of treatment efficacy and heat shock response. Radiat Res. 1999;151:479–488.0033-7587(1999)151[0479:PTORCW]2.0.CO;2 [PubMed] [Google Scholar]
- Ricaniadis N, Kataki A, Agnantis N, Androulakis G, Karakousis CP. Long-term prognostic significance of HSP-70, c-myc and HLA-DR expression in patients with malignant melanoma. Eur J Surg Oncol. 2001;27:88–93. doi: 10.1053/ejso.1999.1018.0748-7983(2001)027[0088:LPSOHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rivoltini L, Castelli C, and Carrabba M. et al. 2003 Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J Immunol. 171:3467–3474. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Signaevsky M, Beraldi E, Nelson C, and Gleave M 2003 Heat shock protein 27 expression increases in prostate cancer cells after androgen ablation and plays cytoprotective role during androgen-independent progression. Proc AACR 44: 1279 (A5581). [DOI] [PubMed] [Google Scholar]
- Rocchi P, So A, and Kojima S. et al. 2004 Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 64:6595–6602. [DOI] [PubMed] [Google Scholar]
- Sagol O, Tuna B, and Coker A. et al. 2002 Immunohistochemical detection of pS2 protein and heat shock protein-70 in pancreatic adenocarcinomas. Relationship with disease extend and patient survival. Pathol Res Pract. 198:77–84. [DOI] [PubMed] [Google Scholar]
- Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997;33:873–877. doi: 10.1016/s0959-8049(97)00002-6.0959-8049(1997)033[0873:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schneider J, Jimenez E, Marenbach K, Marx D, Meden H. Co-expression of the MDR1 gene and HSP27 in human ovarian cancer. Anticancer Res. 1998;18:2967–2971.0250-7005(1998)018[2967:COTMGA]2.0.CO;2 [PubMed] [Google Scholar]
- Schneider J, Jimenez E, Marenbach K, Romero H, Marx D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res. 1999;19:2141–2146.0250-7005(1999)019[2141:IDOHIH]2.0.CO;2 [PubMed] [Google Scholar]
- Schuetz TJ, Gallo GJ, Sheldon L, Tempst P, Kingston RE. Isolation of a cDNA for HSF2; evidence for two heat shock factors in humans. Proc Natl Acad Sci U S A. 1991;88:6910–6915. doi: 10.1073/pnas.88.16.6911.0027-8424(1991)088[6910:IOACFE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L, Bezwoda WR, Meyer K. Tumor factors predicting for prognosis in metastatic breast cancer. The presence of P24 predicts for response to treatment and duration of survival. Cancer. 1990;66:2390–2394. doi: 10.1002/1097-0142(19901201)66:11<2390::aid-cncr2820661124>3.0.co;2-a.0765-7846(1990)066[2390:TFPFPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith V, Sausville EA, Camalier RF, Fiebig HH, and Burger AM 2003 17-DMA-geldamycin is a novel water-soluble, orally bioavailable Hsp90 inhibitor with potent in vitro and in vivo anticancer activity. Proc AACR 44: 180 (A786). [Google Scholar]
- Soldes OS, Kuick RD, Thompson IA, Hughes SJ, Orringer MB, Iannettoni MD, Hanash SM, Beer DG. Differential expression of Hsp27 in normal oesophagus, Barret's metaplasia and oesophageal adenocarcinomas. Br J Cancer. 1999;79:595–603. doi: 10.1038/sj.bjc.6690094.0007-0920(1999)079[0595:DEOHIN]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit DB, Anana M, and Valentin G. et al. 2003 Phase I trial of 17-AAG (17-allylamino, 17-demethoxygeldanamycin) in patients (pts) with advanced cancer. Proc ASCO 22: 198 (A795). [Google Scholar]
- Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitises tumors to taxol. Cancer Res. 2003;63:2139–2144.0008-5472(2003)063[2139:IOHSPF]2.0.CO;2 [PubMed] [Google Scholar]
- Sorger PK, Pelham HRB. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6.0092-8674(1988)054[0855:YHSFIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Spears PA, Barnes JA 2003 HSP70 enhances MCF-7 cell growth and estrogen receptor activity. Proc 1st Int Cong Stress Responses Biol Med Quebec, September 10–14. pp. 73. [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801.0732-0582(2002)020[0395:IOHSPW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1.1074-7613(1998)008[0657:HSPCOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stammler G, Volm M. Expression of heat shock proteins, glutathione peroxidase and catalase in childhood acute lymphoblastic leukemia and nephroblastoma. Cancer Lett. 1996;99:35–42. doi: 10.1016/0304-3835(95)04035-8.0304-3835(1996)099[0035:EOHSPG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Storm FK, Mahvi DM, Gilchrist KW. Hsp27 has no diagnostic or prognostic significance in prostate or bladder cancers. Urology. 1993;42:379–382. doi: 10.1016/0090-4295(93)90361-d.0090-4295(1993)042[0379:HHNDOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Strahler JR, Kuik R, Hanash SM. Diminished phosphorylation of a heat shock protein (HSP27) in infant acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1991;175:134–142. doi: 10.1016/s0006-291x(05)81211-2.0006-291X(1991)175[0134:DPOAHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Strik HM, Weller M, Frank B, Hermisson M, Deininger MH, Dichgans J, Meyermann R. Heat shock protein expression in human gliomas. Anticancer Res. 2000;20:4457–4462.0250-7005(2000)020[4457:HSPEIH]2.0.CO;2 [PubMed] [Google Scholar]
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance: a comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579.0007-1285(1982)055[0579:HSPATA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Syrigos KN, Harrington KJ, Karayiannakis AJ, Sekara E, Chatziyianni E, Syrigou EI, Waxman J. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology. 2003;61:677–680. doi: 10.1016/s0090-4295(02)02289-6.0090-4295(2003)061[0677:CSOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mikami T, and Watanabe Y. et al. 1994 Correlation of heat shock protein 70 expression with estrogen receptor levels in invasive human breast cancer. Am J Clin Pathol. 101:519–525. [DOI] [PubMed] [Google Scholar]
- Takashi M, Katsuno S, Sakata T, Ohshima S, Kato K. Different concentrations of two small stress proteins, alphaB crystalline and HSP27 in human urological tumor tissues. Urol Res. 1998;26:395–399. doi: 10.1007/s002400050075.0300-5623(1998)026[0395:DCOTSS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takeno S, Noguchi T, Kikuchi R, Sato T, Uchida Y, Yokoyama S. Analysis of the survival period in respectable stage IV gastric cancer. Ann Surg Oncol. 2001a;8:215–221. doi: 10.1007/s10434-001-0215-1.1068-9265(2001)008[0215:AOTSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takeno S, Noguchi T, Takahashi Y, Kikuchi R, Uchida Y, Yokoyama S. Immunohistochemical and clinicopathologic analysis of response to neoadjuvant therapy for esophageal squamous cell carcinoma. Dis Esophagus. 2001b;14:149–154. doi: 10.1046/j.1442-2050.2001.00174.x.1120-8694(2001)014[0149:IACAOR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tang D, Khaleque AA, Jones ER, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK. Expression of heat shock proteins and HSP messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10:46–59. doi: 10.1379/CSC-44R.1.1466-1268(2005)010[0046:EOHSPA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi K, Tsutsumi Y, Hori S, Yoshimura S, Osamura RY, Watanabe K. Expression of heat shock protein 70 and c-myc protein in human breast cancer: an immunohistochemical study. Jpn J Clin Oncol. 1991;21:256–263.0368-2811(1991)021[0256:EOHSPA]2.0.CO;2 [PubMed] [Google Scholar]
- Têtu B, Brisson J, Landry J, Huot J. Prognostic significance of heat-shock protein-27 in node-positive breast carcinoma: an immunohistochemical study. Breast Cancer Res Treat. 1995;36:93–97. doi: 10.1007/BF00690189.0167-6806(1995)036[0093:PSOHPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Têtu B, Lacasse B, Bouchard HL, Lagacé R, Huot J, Landry J. Prognostic influence of HSP-27 expression in malignant fibrous histiocytoma: a clinicopathological and immunohistochemical study. Cancer Res. 1992;52:2325–2328.0008-5472(1992)052[2325:PIOHEI]2.0.CO;2 [PubMed] [Google Scholar]
- Thanner F, Sutterlin MW, Kapp M, Rieger L, Kristen P, Dietl J, Gassel AM, Muller T. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res. 2003;23:1057–1062.0250-7005(2003)023[1057:HPAAPM]2.0.CO;2 [PubMed] [Google Scholar]
- Thor A, Benz C, and Moore D. et al. 1991 Stress response protein (srp-27) determination in primary human breast carcinomas: clinical, histologic, and prognostic correlations. J Natl Cancer Inst. 83:170–178. [DOI] [PubMed] [Google Scholar]
- Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x.0019-2805(2003)110[0001:FOHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend PA, Dublin E, and Hart IR. et al. 2002 BAG-I expression in human breast cancer: interrelationships between BAG-1 RNA, protein, HSC70 expression and clinicopathological data. J Pathol. 197:51–59. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Kindas-Mugge I, Dekrout B, Knobler RM, Metze D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: an immunohistochemical study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x.0007-0963(1995)133[0194:EOTKHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Trieb K, Gerth R, Holzer G, Grohs JG, Berger P, Kotz R. Antibodies to heat shock protein 90 in osteosarcoma patients correlate with response to neoadjuvant chemotherapy. Br J Cancer. 2000a;82:85–87. doi: 10.1054/bjoc.1999.0881.0007-0920(2000)082[0085:ATHSPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieb K, Gerth R, Windhager R, Grohs JG, Holzer G, Berger P, Kotz R. Serum antibodies against the heat shock protein 60 are elevated in patients with osteosarcoma. Immunobiology. 2000b;201:368–376. doi: 10.1016/S0171-2985(00)80091-1.0171-2985(2000)201[0368:SAATHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Trieb K, Kohlbeck R, Lang S, Klinger H, Blahovec H, Kotz R. Heat shock protein 72 expression in chondrosarcoma correlates with differentiation. J Cancer Res Clin Oncol. 2000c;126:667–670. doi: 10.1007/s004320000167.0171-5216(2000)126[0667:HSPEIC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Heat shock protein 72 expression in osteosarcomas correlated with good response to neoadjuvant chemotherapy. Human Pathol. 1998;29:1050–1055. doi: 10.1016/s0046-8177(98)90412-9.0046-8177(1998)029[1050:HSPEIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ungar DR, Hailat N, Strahler JR, Kuik RD, Brodeur GM, Seeger RC, Reynolds CP, Hanash SM. Hsp27 expression in neuroblastoma: correlation with disease stage. J Natl Cancer Inst. 1994;86:780–784. doi: 10.1093/jnci/86.10.780.0027-8874(1994)086[0780:HEINCW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, Imamura T, Machinami R. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–673. doi: 10.1016/S0344-0338(00)80118-1.0344-0338(2000)196[0665:EOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, and van't Veer LJ. et al. 2002 A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 34:1999–2009. [DOI] [PubMed] [Google Scholar]
- Vanmuylder N, Evrard L, Daelemans P, Van Reck J, Dourov N. Expression of heat shock proteins in salivary gland tumors. Immunohistochemical study of HSP27, HSP70, HSP90, and HSP110: a propos of 50 cases. Ann Pathol. 2000;20:190–195.0242-6498(2000)020[0190:EOHSPI]2.0.CO;2 [PubMed] [Google Scholar]
- van 't Veer LJ, Dai H, and van de Vijver MJ. et al. 2002 Gene expression profiling predicts clinical outcome of breast cancer. Nature. 415:530–536. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, López LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21:441–451.0361-090X(1997)021[0441:HSPACP]2.0.CO;2 [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer (Pred Oncol) 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vargas Roig LM, Lotfi H, Olcese JE, Lo Castro G, Ciocca DR. Effect of short-term tamoxifen administration in patients with invasive cervical cancer. Anticancer Res. 1993;13:2457–2464.0250-7005(1993)013[2457:EOSTAI]2.0.CO;2 [PubMed] [Google Scholar]
- Vegh GL, Fulop V, and Liu Y. et al. 1999 Differential gene expression pattern between normal human trophoblast and choriocarcinoma cell lines: downregulation of heat shock protein-27 in choriocarcinoma in vitro and in vivo. Gynecol Oncol. 75:391–396. [DOI] [PubMed] [Google Scholar]
- Vidal CI, Mintz PJ, and Lu K. et al. 2004 An HSP90-mimic peptide revealed by fingerprinting the pool of antibodies from ovarian cancer patients. Oncogene. 23:8859–8867. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4:357–401.1045-4403(1994)004[0357:TOTSSA]2.0.CO;2 [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1.1466-1268(2004)009[0122:OMTCHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch VZ, Sherman MY. Oncogenic potential of Hsp72. Oncogene. 1999;18:3648–3651. doi: 10.1038/sj.onc.1202525.0950-9232(1999)018[3648:OPOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Volm M, Rittgen W. Cellular predictive factors for the drug response of lung cancer. Anticancer Res. 2000;20:3449–3458.0250-7005(2000)020[3449:CPFFTD]2.0.CO;2 [PubMed] [Google Scholar]
- Wang Q, An L, Chen Y, Yue S. Expression of endoplasmic reticulum molecular chaperone GRP94 in human lung cancer tissues and its clinical significance. Chin Med J (Engl) 2002;115:1615–1619.0366-6999(2002)115[1615:EOERMC]2.0.CO;2 [PubMed] [Google Scholar]
- Wang XY, Chen X, Manjili MH, Repasky EA, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp110. Cancer Res. 2003a;63:2553–2560.0008-5472(2003)063[2553:TIURCC]2.0.CO;2 [PubMed] [Google Scholar]
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003b;105:226–231. doi: 10.1002/ijc.11058.0020-7136(2003)105[0226:IWTECG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang Y, Theriault JR, He H, Gong J, Calderwood SK. Expression of a dominant negative heat shock factor-1 construct inhibits aneuploidy in prostate carcinoma cells. J Biol Chem. 2004;279:32651–32659. doi: 10.1074/jbc.M401475200.0021-9258(2004)279[32651:EOADNH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wataba K, Saito T, Fukunaka K, Ashihara K, Nishimura M, Kudo R. Over-expression of heat shock proteins in carcinogenic endometrium. Int J Cancer. 2001;91:448–456. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1077>3.0.co;2-f.0020-7136(2001)091[0448:OOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Watson RWG, Lebret T, Fitzpatrick JM. Heat shock proteins in the genitourinary system. Curr Urol Rep. 2003;4:70–76. doi: 10.1007/s11934-003-0060-9.1534-6285(2003)004[0070:HSPITG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Workman P. Pharmacogenomics in cancer drug discovery and development: inhibitors of the Hsp90 molecular chaperone. Cancer Detect Prev. 2002;26:405–410. doi: 10.1016/s0361-090x(02)00126-5.0361-090X(2002)026[0405:PICDDA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Ann Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301.1081-0706(1995)011[0441:HSTFSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xia W, Hardy L, Liu L, Zhao S, Goodman M, Voellmy R, Spector NL. Concurrent exposure to heat shock and H7 synergizes to trigger breast cancer cell apoptosis while sparing normal cells. Breast Cancer Res Treat. 2003;77:233–243. doi: 10.1023/a:1021895803424.0167-6806(2003)077[0233:CETHSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xiao K, Liu W, Qu S, Sun H, Tang J. Study of heat shock protein HSP90 alpha, HSP70, HSP27 mRNA expression in human acute leukaemia cells. J Tongji Med Univ. 1996;16:212–216. doi: 10.1007/BF02888109.0257-716X(1996)016[0212:SOHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yano M, Naito Z, Yokoyama M, Shiraki Y, Ishiwata T, Inokuchi M, Asano G. Expression of Hsp90 and cyclin D1 in human breast cancer. Cancer Lett. 1999;137:45–51. doi: 10.1016/s0304-3835(98)00338-3.0304-3835(1999)137[0045:EOHACD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yokota S, Yamamoto Y, and Shimizu K. et al. 2001 Increased expression of cytosolic chaperonin CCT in human hepatocellular and colonic carcinoma. Cell Stress Chaperones. 6:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Iwaki T, Goldman JE, Tateishi J, Fukui M. Small heat-shock protein is expressed in meningiomas and in granulofilamentous inclusion bodies. Acta Neuropathol (Berl) 1993;85:248–255. doi: 10.1007/BF00227718.0001-6322(1993)085[0248:SHPIEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhao M, Tang D, Lechpammer S, Hoffman A, Asea AA, Stevenson MA, Calderwood SK. Double stranded RNA-dependent protein kinase (pkr) is essential for thermotolerance, accumulation of HSP70 and stabilization of ARE containing HSP70 mRNA during stress. J Biol Chem. 2002;277:44539–44547. doi: 10.1074/jbc.M208408200.0021-9258(2002)277[44539:DSRPKP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhong L, Peng X, Hidalgo GE, Doherty DE, Stromberg AJ, Hirschowitz EA. Antibodies to HSP70 and HSP90 in serum in non-small cell lung cancer patients. Cancer Detect Prev. 2003;27:285–290. doi: 10.1016/s0361-090x(03)00097-7.0361-090X(2003)027[0285:ATHAHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zlotta AR, Drowart A, and Huygen K. et al. 1997 Humoral response against heat shock proteins and other mycobacterial antigens after intravesical treatment with bacille Calmette-Guerin (BCG) in patients with superficial bladder cancer. Clin Exp Immunol. 109:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]


