Abstract
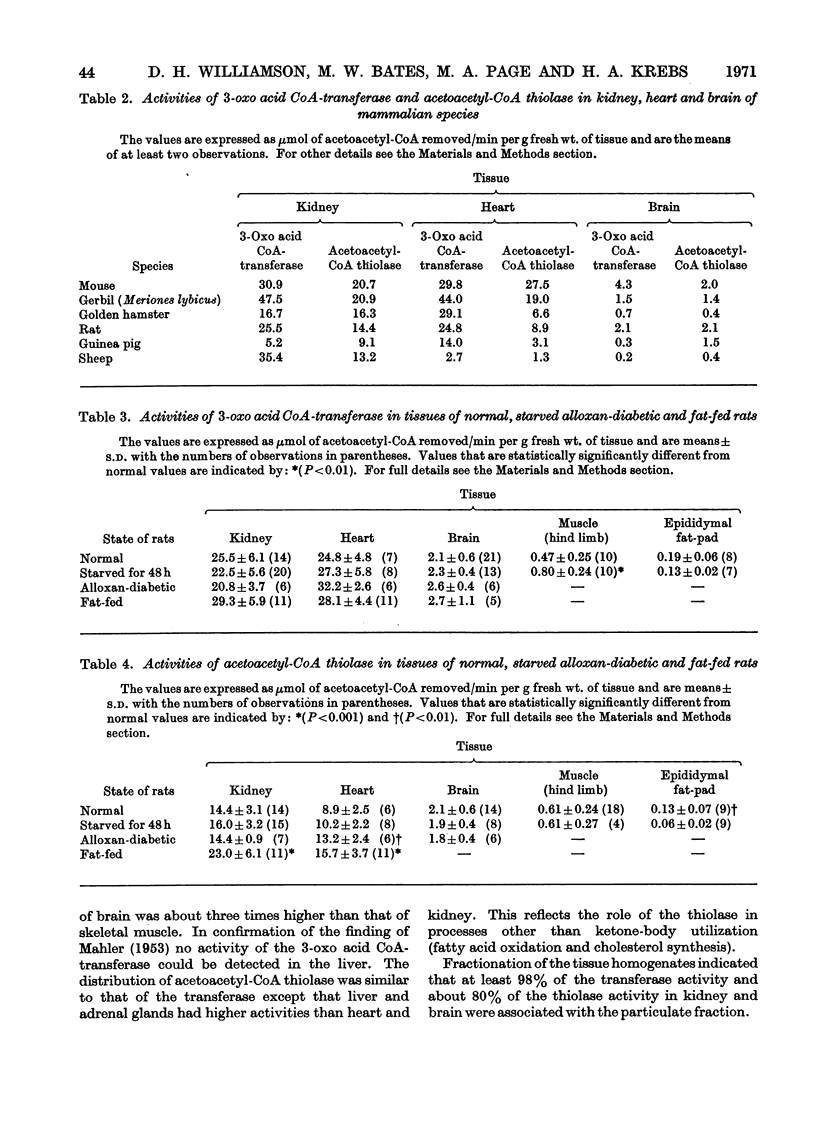
1. The activities in rat tissues of 3-oxo acid CoA-transferase (the first enzyme involved in acetoacetate utilization) were found to be highest in kidney and heart. In submaxillary and adrenal glands the activities were about one-quarter of those in kidney and heart. In brain it was about one-tenth and was less in lung, spleen, skeletal muscle and epididymal fat. No activity was detectable in liver. 2. The activities of acetoacetyl-CoA thiolase were found roughly to parallel those of the transferase except for liver and adrenal glands. The high activity in the latter two tissues may be explained by additional roles of thiolase, namely, the production of acetyl-CoA from fatty acids. 3. The activities of the two enzymes in tissues of mouse, gerbil, golden hamster, guinea pig and sheep were similar to those of rat tissues. The notable exception was the low activity of the transferase and thiolase in sheep heart and brain. 4. The activities of the transferase in rat tissues did not change appreciably in starvation, alloxan-diabetes or on fat-feeding, where the rates of ketone-body utilization are increased. Thiolase activity increased in kidney and heart on fat-feeding. 5. The activity of 3-hydroxybutyrate dehydrogenase did not change in rat brain during starvation. 6. The factors controlling the rate of ketone-body utilization are discussed. It is concluded that the activities of the relevant enzymes in the adult rat do not control the variations in the rate of ketone-body utilization that occur in starvation or alloxan-diabetes. The controlling factor in these situations is the concentration of the ketone bodies in plasma and tissues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Siliprandi D., Siliprandi N. Acetoacetate activation and oxidation in kidney and heart mitochondria. Biochim Biophys Acta. 1969 Jun 24;180(2):237–243. doi: 10.1016/0005-2728(69)90110-8. [DOI] [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Steinke J., Cahill G. F., Jr Metabolic interactions of glucose, lactate, and beta-hydroxybutyrate in rat brain slices. Am J Physiol. 1969 Sep;217(3):784–792. doi: 10.1152/ajplegacy.1969.217.3.784. [DOI] [PubMed] [Google Scholar]
- Ito T., Quastel J. H. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J. 1970 Feb;116(4):641–655. doi: 10.1042/bj1160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. The physiological role of the ketone bodies. Biochem J. 1961 Aug;80:225–233. doi: 10.1042/bj0800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- MAHLER H. R. Role of coenzyme A in fatty acid metabolism. Fed Proc. 1953 Sep;12(3):694–702. [PubMed] [Google Scholar]
- MCCANN W. P. The oxidation of ketone bodies by mitochondria from liver and peripheral tissues. J Biol Chem. 1957 May;226(1):15–22. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Ketogenesis and cholesterol synthesis in normal and neoplastic tissues of the rat. J Biol Chem. 1969 Aug 10;244(15):4251–4256. [PubMed] [Google Scholar]
- Ohe K., Morris H. P., Weinhouse S. Beta-hydroxybutyrate dehydrogenase activity in liver and liver tumors. Cancer Res. 1967 Aug;27(8):1360–1371. [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOELING H. D., GARLEPP H. J., CREUTZFELDE W. DIE WIRKUNG VON INSULIN UND GLUKOSE AUD DIE KETONKOERPERAUFNAHME TOTAL EVISCERIERTER, NORMALER, HUNGERNDER UND ALLOXANDIABETISCHER RATTEN. Biochim Biophys Acta. 1965 May 4;100:530–543. [PubMed] [Google Scholar]
- STERN J. R., COON M. J., DEL CAMPILLO A., SCHNEIDER M. C. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J Biol Chem. 1956 Jul;221(1):15–31. [PubMed] [Google Scholar]
- STERN J. R., OCHOA S. Enzymatic synthesis of citric acid. I. Synthesis with soluble enzymes. J Biol Chem. 1951 Jul;191(1):161–172. [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]


