Abstract
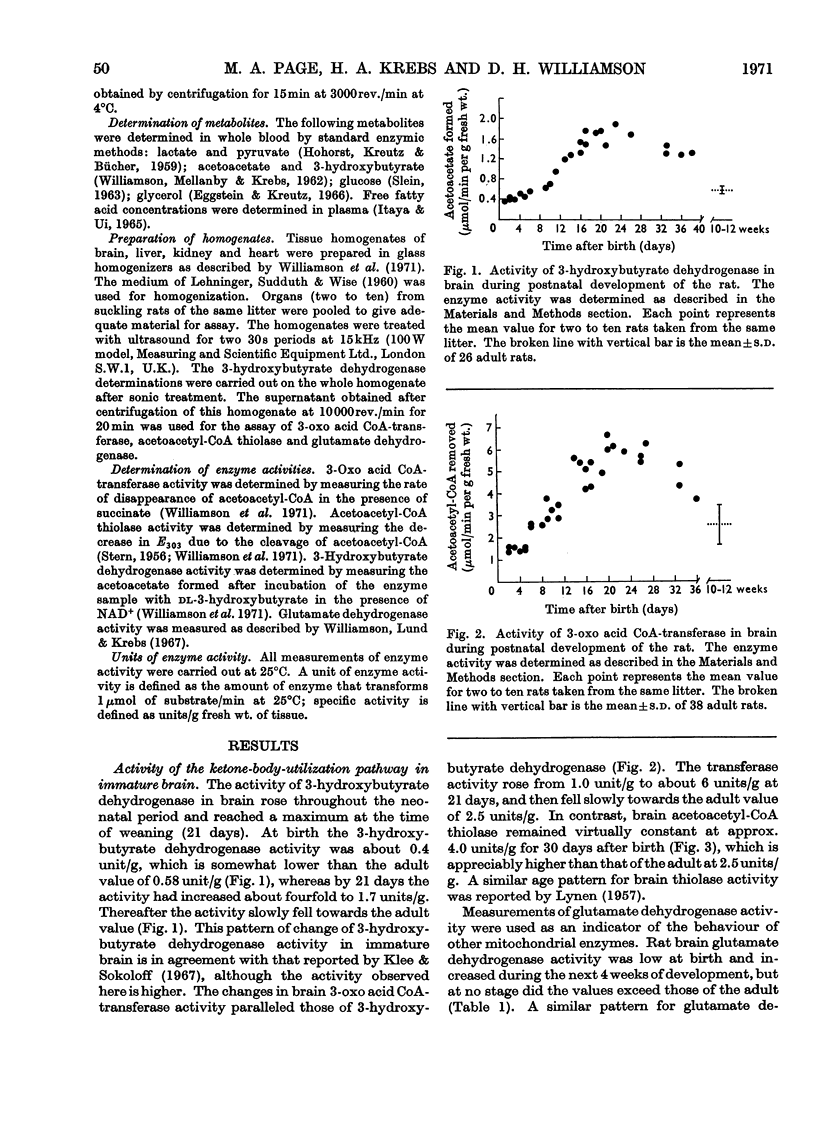
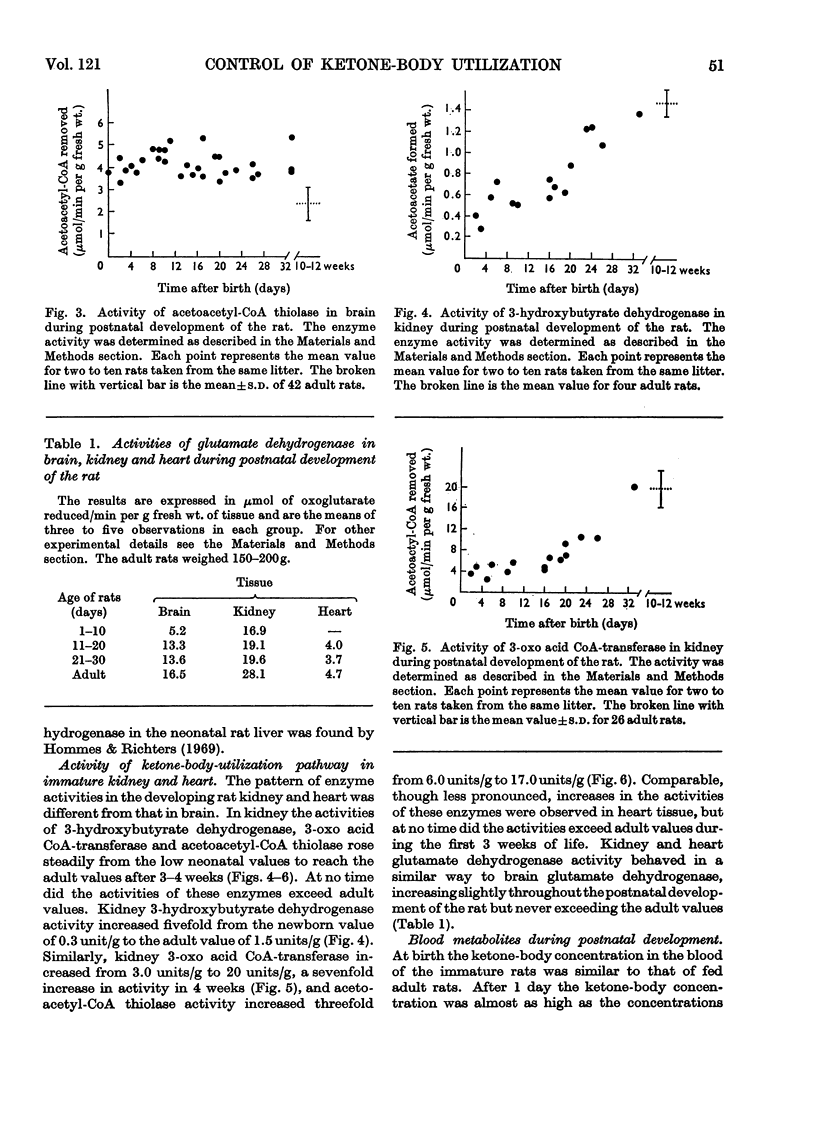
1. The activities of 3-hydroxybutyrate dehydrogenase and 3-oxo acid CoA-transferase in rat brain at birth were found to be about two-thirds of those of adult rat brain, expressed per g wet wt. The activities rose throughout the suckling period and at the time of weaning reached values about three times higher than those for adult brain. Later they gradually declined. 2. At birth the activity of acetoacetyl-CoA thiolase in rat brain was about 60% higher than in the adult. During the suckling period there was no significant change in activity. 3. In rat kidney the activities of the three enzymes at birth were less than one-third of those at maturity. They gradually rose and after 5 weeks approached the adult value. Similar results were obtained with rat heart. 4. The activity of glutamate dehydrogenase (a mitochondrial enzyme like 3-hydroxybutyrate dehydrogenase and 3-oxo acid CoA-transferase) also rose in brain and kidney during the suckling period, but at no stage did it exceed the adult value. 5. Throughout the suckling period the total ketone-body concentration in the blood was about six times higher than in adult fed rats, and the concentration of free fatty acids in the blood was three to four times higher. 6. It is concluded that the rate of ketone-body utilization in brains of suckling rats is determined by both the greater amounts of the key enzymes in the tissue and the high concentrations of ketone bodies in the blood. In addition, the low activities of the relevant enzymes in kidney and heart of suckling rats may make available more ketone bodies for the brain.
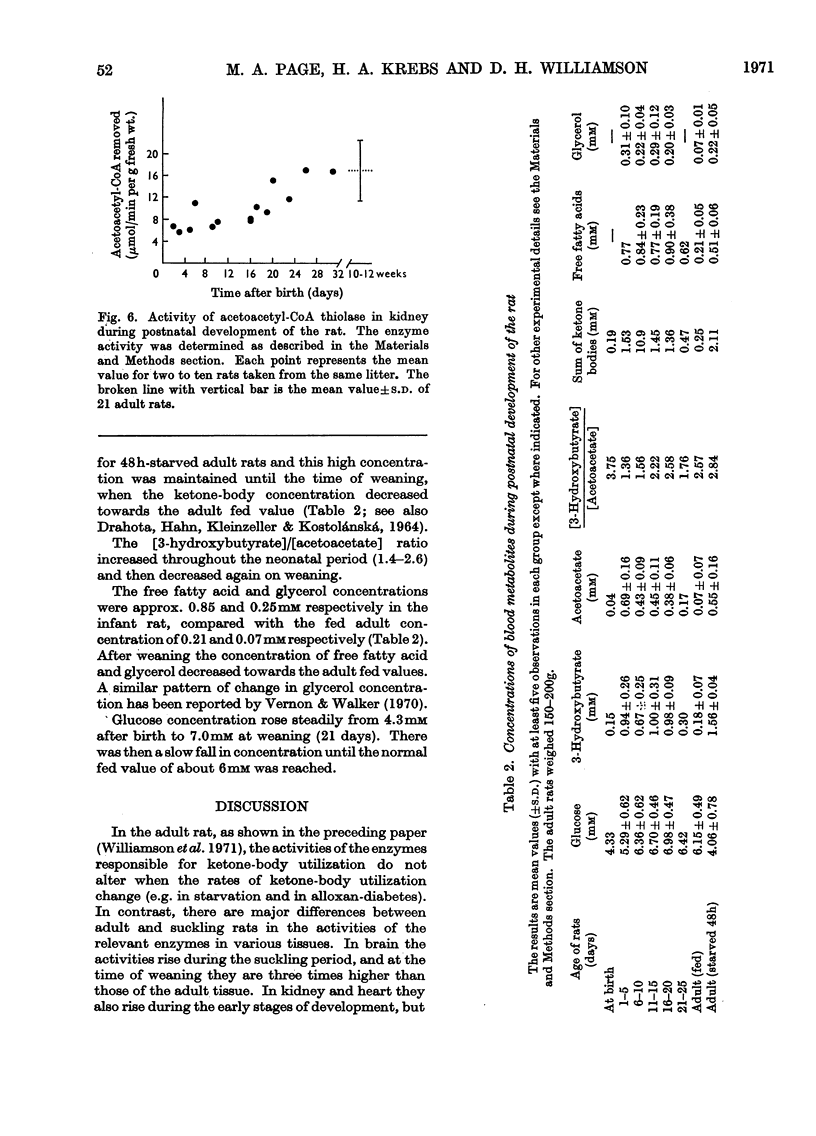
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard E., Grippo J., Menkes J. H. Fatty acid synthesis in the developing brain. Pediatr Res. 1969 Nov;3(6):590–596. doi: 10.1203/00006450-196911000-00009. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., HAHN P., MOUREK J., TROJANOVA M. THE EFFECT OF ACETOACETATE ON OXYGEN CONSUMPTION OF BRAIN SLICES FROM INFANT AND ADULT RATS. Physiol Bohemoslov. 1965;14:134–136. [PubMed] [Google Scholar]
- DYMSZA H. A., CZAJKA D. M., MILLER S. A. INFLUENCE OF ARTIFICIAL DIET ON WEIGHT GAIN AND BODY COMPOSITION OF THE NEONATAL RAT. J Nutr. 1964 Oct;84:100–106. doi: 10.1093/jn/84.2.100. [DOI] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Kleinzeller A., Kostolánská A. Acetoacetate formation by liver slices from adult and infant rats. Biochem J. 1964 Oct;93(1):61–65. doi: 10.1042/bj0930061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes F. A., Richters A. R. Mechanism of oxidation of cytoplasmic reduced nicotinamide adenine dinucleotides in the developing rat liver. Biol Neonat. 1969;14(5):359–364. doi: 10.1159/000240201. [DOI] [PubMed] [Google Scholar]
- Ito T., Quastel J. H. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J. 1970 Feb;116(4):641–655. doi: 10.1042/bj1160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Sokoloff L. Changes in D(--)-beta-hydroxybutyric dehydrogenase activity during brain maturation in the rat. J Biol Chem. 1967 Sep 10;242(17):3880–3883. [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Glycerol metabolism in the neonatal rat. Biochem J. 1970 Jul;118(3):531–536. doi: 10.1042/bj1180531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]


