Abstract
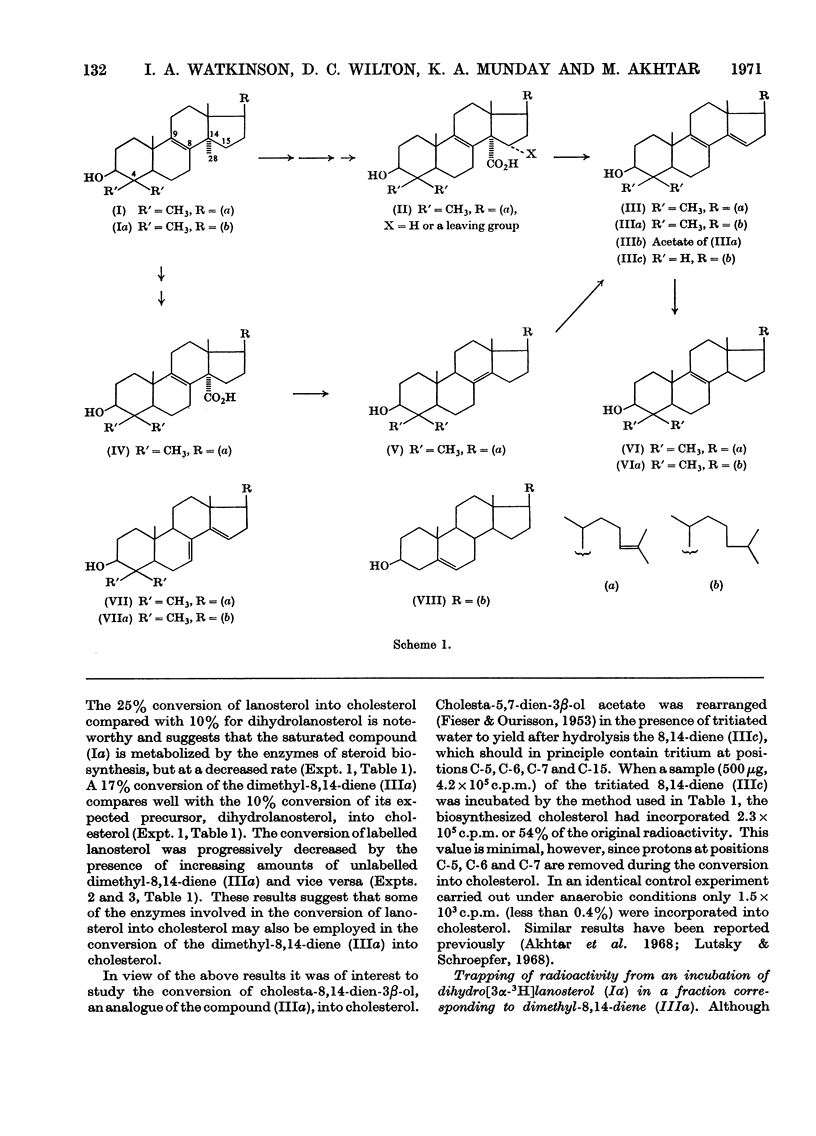
It was shown that 100μg quantities of 4,4′-dimethyl[2-3H2]cholesta-8,14-dien-3β-ol (IIIa), tritiated cholesta-8,14-dien-3β-ol, 4,4′-dimethyl[2-3H2]cholesta-7,14-dien-3β-ol, dihydro[2-3H2]lanosterol and [24-3H]lanosterol were converted by a 10000g supernatant of rat liver homogenate into cholesterol in 17%, 54%, 6%, 9.5% and 24% yields respectively. From an incubation of dihydro[3α-3H]lanosterol with a rat liver homogenate in the presence of a trap up to 38% of the radioactivity was found to be associated with a fraction that was unambiguously shown to be 4,4′-dimethylcholesta-8,14-dien-3β-ol. Another related compound, 4,4′-dimethylcholesta-7,14-dien-3β-ol was also shown to be equally effective in its ability to trap compound (IIIa) from an incubation of dihydro[3α-3H]lanosterol. The mechanism of the further conversion of the compound (IIIa) into cholesterol occurred by the reduction of the 14,15-double bond and involved the addition of a hydrogen atom from the medium to C-15 and another from the 4-position of NADPH to C-14. Two possible mechanisms for the removal of the 14α-methyl group in sterol biosynthesis are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Hunt P. F., Parvez M. A. The transfer of hydrogen from C-24 to C-25 in ergosterol biosynthesis. Biochem J. 1967 Jun;103(3):616–622. doi: 10.1042/bj1030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Rahimtula A. D., Watkinson I. A., Wilton D. C., Munday K. A. The status of C-6, C-7, C-15 and C-16 hydrogen atoms in cholesterol biosynthesis. Eur J Biochem. 1969 May 1;9(1):107–111. doi: 10.1111/j.1432-1033.1969.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Rahimtula A. D., Wilton D. C. The incorporation of a hydrogen atom at C-15 of cholesterol biosynthesized from squalene. Biochem J. 1969 Oct;114(4):801–806. doi: 10.1042/bj1140801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Watkinson I. A., Rahimtula A. D., Wilton D. C., Munday K. A. The role of a cholesta-8,14-dien-3-beta-ol system in cholesterol biosynthesis. Biochem J. 1969 Mar;111(5):757–761. doi: 10.1042/bj1110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica L., Fiecchi A., Kienle M. G., Scala A., Galli G., Paoletti E. G., Paoletti R. Evidence for the biological conversion of delta 8,14 sterol dienes into cholesterol. J Am Chem Soc. 1968 Nov 6;90(23):6532–6534. doi: 10.1021/ja01025a062. [DOI] [PubMed] [Google Scholar]
- Canonica L., Fiecchi A., Kienle M. G., Scala A., Galli G., Paoletti E. G., Paoletti R. The fate of the 15-beta hydrogen of lanosterol in cholesterol biosynthesis. J Am Chem Soc. 1968 Jun 19;90(13):3597–3598. doi: 10.1021/ja01015a074. [DOI] [PubMed] [Google Scholar]
- Caspi E., Ramm P. J., Gain R. E. Stereochemistry of tritium at carbon 15 in cholesterol derived from (3R,2R)-2T-mevalonic acid in rat livers. J Am Chem Soc. 1969 Jul 2;91(14):4012–4013. doi: 10.1021/ja01042a090. [DOI] [PubMed] [Google Scholar]
- Dempsey M. E. Pathways of enzymic synthesis and conversion to cholesterol of delta-5,7,24-cholestatrien-3 beta-ol and other naturally occurring sterols. J Biol Chem. 1965 Nov;240(11):4176–4188. [PubMed] [Google Scholar]
- Fiecchi A., Canonica L., Scala A., Cattabeni F., Paoletti E. G., Paoletti R. 4,4-dimethyl-5-alpha-cholesta-8,14-dien-3-beta-ol. A new precursor of cholesterol in mammalian tissues. Life Sci. 1969 Jun 15;8(12):629–634. doi: 10.1016/0024-3205(69)90219-7. [DOI] [PubMed] [Google Scholar]
- GAUTSCHI F., BLOCH K. Synthesis of isomeric 4,4-dimethylcholestenols and identification of a lanosterol metabolite. J Biol Chem. 1958 Dec;233(6):1343–1347. [PubMed] [Google Scholar]
- Knight J. C., Klein P. D., Szczepanik P. A. The synthesis of tritium-labeled 14-alpha-methyl-5-alpha-cholest-7-en-3-beta-ol and its enzymatic demethylation. J Biol Chem. 1966 Apr 10;241(7):1502–1508. [PubMed] [Google Scholar]
- Lutsky B. N., Schroepfer G. J., Jr Enzymatic conversion of delta8, 14-cholestadien-3beta-ol to cholesterol. Biochem Biophys Res Commun. 1968 Nov 8;33(3):492–496. doi: 10.1016/0006-291x(68)90602-5. [DOI] [PubMed] [Google Scholar]
- Wilton D. C., Munday K. A., Skinner S. J., Akhtar M. The biological conversion of 7-dehydrocholesterol into cholesterol and comments on the reduction of double bonds. Biochem J. 1968 Feb;106(4):803–810. doi: 10.1042/bj1060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C., Watkinson I. A., Akhtar M. The stereochemistry of hydrogen transfer from reduced nicotinamide-adenine dinucleotide phosphate in the reduction of ethylenic linkages during cholesterol biosynthesis. Biochem J. 1970 Oct;119(4):673–675. doi: 10.1042/bj1190673. [DOI] [PMC free article] [PubMed] [Google Scholar]


