Abstract
Hyperpolarized-13C magnetic resonance imaging (HP-13C MRI) was used to image changes in 13C-lactate signal during a visual stimulus condition in comparison to an eyes-closed control condition. Whole-brain 13C-pyruvate, 13C-lactate and 13C-bicarbonate production was imaged in healthy volunteers (N = 6, ages 24–33) for the two conditions using two separate hyperpolarized 13C-pyruvate injections. BOLD-fMRI scans were used to delineate regions of functional activation. 13C-metabolite signal was normalized by 13C-metabolite signal from the brainstem and the percentage change in 13C-metabolite signal conditions was calculated. A one-way Wilcoxon signed-rank test showed a significant increase in 13C-lactate in regions of activation when compared to the remainder of the brain ( ). No significant increase was observed in 13C-pyruvate signal ( ) or 13C-bicarbonate signal ( ). The results show an increase in 13C-lactate production in activated regions that is measurable with HP-13C MRI.
Keywords: Energy metabolism, lactate, molecular imaging, MRI, MR metabolite
Introduction
Hyperpolarized-13C magnetic resonance imaging (HP-13C MRI) is a minimally invasive technique that enables imaging of exogenously administered 13C-labelled metabolites and their downstream conversion products in vivo. The most commonly imaged metabolites are 13C-pyruvate and its downstream products 13C-lactate and 13C-bicarbonate. The production of 13C-lactate signal indicates the reduction of 13C-pyruvate to 13C-lactate in the cytosol, wheras 13C-bicarbonate production indicates the decarboxylation of 13C-pyruvate into acetyl-CoA on the mitochondrial membrane. HP-13C MRI has previously been used to image 13C-metabolite production in the human brain in healthy and disease states.1–6
Previous results showed concordant regional patterns of 13C-pyruvate, 13C-lactate and 13C-bicarbonate across individuals suggesting a highly regulated process leading to the pattern. 4 At the same time, increased lactate concentration in the brain has been measured using proton spectroscopy and a visual stimulus.7–9 However, the effect of a visual stimulus, and the concomitant increase in lactate pool size, on the 13C-metabolite pattern in the brain has not been documented previously. We hypothesized that the 13C-metabolite distribution detected with HP-13C MRI would be modulated in response to task activation.
In this study whole-brain HP-13C MRI was used to assess regional changes in 13C-lactate signal during a visual task stimulus. Blood-oxygen level dependent fMRI (BOLD-fMRI) images were acquired to delineate regions of BOLD activation in each participant. We hypothesized that the regions of activation defined by BOLD-fMRI would have higher 13C-lactate signal under stimulus conditions relative to control conditions.
Materials and methods
Written informed consent was obtained from six study participants (3 male, 3 female) and procedures were performed under a protocol approved by the Research Ethics Board of Sunnybrook Health Sciences Centre and by Health Canada under a Clinical Trial Application in compliance with the ICH-GCP and Declaration of Helsinki. The participants ranged in age from 24 to 33 (mean age of 26) and only those deemed cognitively healthy via the Montreal Cognitive Assessment 10 were scanned.
Several hours prior to scanning, two doses of 13C-pyruvic acid were prepared using a 1.47 g sample of [1-13C]pyruvic acid (Sigma Aldrich, St. Louis, MO) for each dose and polarized in a SPINLab polarizer system (GE Healthcare, Waukesha, WI) for a minimum of two hours. Immediately prior to scanning, a 20 gauge intravenous catheter was placed into the forearm of each participant before positioning them in the scanner bore.
Images were acquired using a GE MR750 3.0 T MRI scanner (GE Healthcare, Waukesha, WI) with a 13C birdcage head coil built in-house for HP-13C imaging. Volumetric images of 13C-lactate, 13C-bicarbonate, and 13C-pyruvate were acquired using a 3D echo-planar imaging (EPI) sequence. 11 This sequence, applied extensively in previous brain imaging studies,4–6,12 uses frequency-selective RF pulses to separately excite individual metabolites.13,14 This “metabolite specific” imaging approach has the advantage that each RF excitation results in signal from a single metabolite (e.g. 13C-lactate), which can be imaged using conventional fast imaging methods such as the echo-planar encoding used here. The sequence of events during the imaging process are: first, a whole-brain 3D image of 13C-lactate is acquired with 15 mm isotropic spatial resolution in 1.2 seconds. Then, the RF pulse excitation frequency is changed to the frequency of 13C-bicarbonate and a 3D image of C-bicarbonate is acquired, followed by 13C-pyruvate. This allows full brain coverage for all 3 13C-metabolites within a 5 s block, which was repeated 12 times for a net 60 s imaging time. The net flip angle was 80° for 13C-lactate and 13C-bicarbonate and 11° for 13C-pyruvate. This metabolite-specific excitation and imaging approach has the disadvantage that no spectrum is acquired, just images of the pre-chosen resonances.
Each participant underwent two HP-13C scans, consisting of a task (13C-task) and control (13C-control) scan. The visual task consisted of an 8 Hz flashing checkerboard stimulus initiated 5 s before the 13C task scan. For the 13C-control scan, the participant was asked to close their eyes (and a blank screen with a plus sign was projected onto the screen). To control for any effect of scan ordering, three of the participants had the control scan first, whereas the other three had the control scan second. All stimuli and visual prompts were delivered via a projector and projector screen setup. The projector was placed inside the MRI console room facing into the MRI room, where a projector screen was located. Prior to imaging, the projector was lined up with the projector screen and we verified with the participant in the scanner that they were able to view a test image. There was a half-hour wait period between 13C-task and 13C-control scans.
During the half-hour wait period, the 13C head coil was interchanged with an 8-channel 1H head coil (Invivo Inc, Pewaukee, WI) to perform anatomical T1-weighted and BOLD-fMRI scans. Anatomical T1-weighted images were acquired with an axial RF-spoiled gradient echo sequence (FOV , 1 mm isotropic resolution, repetition time (TR) = 7.6 ms, echo time (TE) = 2.9 ms, flip angle 11°). BOLD-fMRI data were acquired using a single-shot EPI acquisition with 3.5 mm isotropic resolution, TR = 2000 ms, TE = 29 ms, 40° flip angle, matrix size and 38 3.5 mm slices with 0 mm gap. The task block duration was the same as the HP C scan duration (one minute) and the total fMRI scan duration was 5 minutes, including 3 task blocks interleaved with 2 control blocks. The timing of the scan protocol is shown in Figure 1. To minimize head motion between C and H scans, foam was placed between the head rest and participants’ heads and participants were instructed to keep their head still and move only their eyes during the experiment (i.e. blinking, opening, and closing).
Figure 1.
Image acquisition workflow for the visual stimulus scans. Two sets of 13C-images were acquired with a thirty minute wait period between acquisitions to allow for washout of residual 13C-metabolites. During this wait period, anatomical images and BOLD-fMRI images were acquired.
The C-metabolite images were summed across time to calculate the total C-metabolite signal during the 1 minute acquisition window. All subsequent analysis was done using these summed-across-time images. The control and task C-lactate images were registered (rigid six-body) to the anatomical T1-weighted images using FMRIB’s Linear Image Registration Tool from the FMRIB software library, 15 and the resulting transformation matrix was applied to the control and task C-pyruvate and C-bicarbonate images respectively. Finally, post-registered control and task C-lactate and unprocessed BOLD-fMRI images were overlaid with the corresponding anatomical T1 images and visually inspected for misalignment. Additionally, the C-lactate control and C-task images were normalized by dividing by the brainstem signal for each participant/condition and then averaged across participants to create group-average C-control and C-task images.
The T1-weighted anatomical images were parcellated into 132 brain regions according to the BrainCOLOR labelling protocol 16 using an automated workflow. 17 These parcellation maps were then used to compute regional C-pyruvate, C-lactate, and C-bicarbonate signals for each participant using the mri segstats function. 18 Regions with volumes below the voxel volume of 3.4 mL were excluded, resulting in 90 parcellated regions for each participant.
BOLD-fMRI pre- and post-processing was done using the Analysis of Functional Images (AFNI) toolbox,19,20 and included removal of the first two TRs of each acquisition, registration across time to mitigate motion effects, and blurring with a Gaussian function (4 mm full-width half maximum). Voxelwise linear regression coefficients were fit using the stimulus block design as an independent variable, input as a repeating boxcar function with box length one minute. T-statistics were calculated for each positive regression coefficient using an alpha of 0.001. Cluster correction was used to control for multiple comparisons, with voxels in face-to-face contact comprising each cluster. 21
The BOLD-fMRI t-statistic map was used to define an an activation volume for each participant, with the remainder of the brain (including the white matter and ventricles) defined as the non-activation volume.
Each regional 13C-metabolite signal, as well as the signal from the activation volume and non-activation volume, were normalized by the 13C-metabolite signal from the brainstem region from the particular participant (referred to as ‘brainstem normalization’ below). This was done to normalize for any global signal scaling between scans, such as those caused by differing 13C-pyruvate polarization levels, while maintaining the inter-regional signal pattern.
The percentage change in 13C-metabolite signal between task and control conditions was calculated as follows:
where is the brainstem-normalized C-metabolite signal from a particular region/participant in the task condition and is the signal from the same metabolite/region/participant in the control condition (also brainstem normalized). To account for the paired data from each participant without a requirement for data normality, a one-way Wilcoxon signed-rank test was used to test for a difference in the between the activation and non-activation volumes. The one-way Wilcoxon signed-rank test was also used to test for a difference between and in each individual region.
The thresholded t-statistic maps from the BOLD-fMRI scans were also used to designate parcellated brain regions as ‘activation regions’. The remaining parcellated brain regions were categorized as ‘non-activation regions’.
Finally, the strength of BOLD-fMRI activation (t-statistic) was tested for correlation with the task-induced change in C-metabolite signal. An average t-statistic was calculated within the activation volume for each volunteer, as well as the C-metabolite signal inside the same activation volume. A test for correlation was then done using the Spearman correlation test.
Results
BOLD-fMRI activation was consistently achieved in the visual cortex over the course of the 5 minute block design in all six participants. This confirmed that the stimulus block length was sufficient for inducing activation during the HP C scans within the one minute acquisition time. Example t-statistic maps registered to the MNI152 atlas 22 are shown in Figure 2. Group average C-task and control images are shown in Figure 3. No misalignments between registered 13C-metabolite images, t-statistic maps, or anatomical images were found upon visual inspection. The for the C-metabolites in the activation and non-activation volumes are plotted in Figure 4(a).
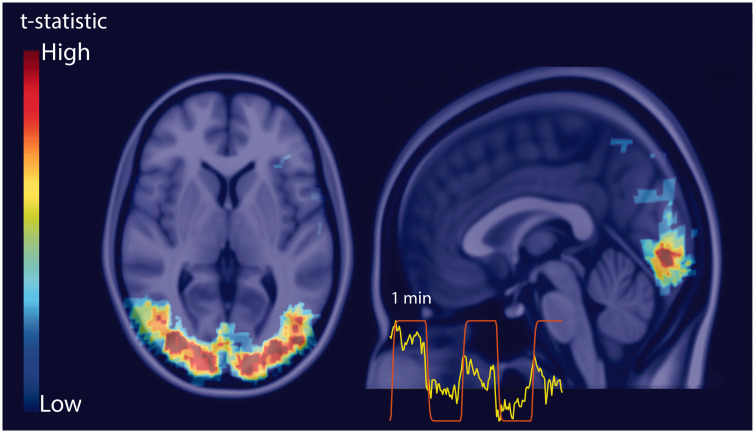
Figure 2.
Example BOLD fMRI t-statistic map for the visual stimulus registered to the MNI152 atlas. The inset is an example time series curve from a voxel with significant activation (yellow) and the stimulus regressor used in the general linear model (red).
Figure 3.
Group average 13C-lactate images overlaid on the MNI152 atlas for the control (left) and task (right) conditions. Increased 13C-lactate signal can be observed in the occipital lobe, predominantly in the left hemisphere. A sagittal slice (top) and coronal slice (bottom) are shown with the same color scale used for 13C-lactate signal. The A, P, R and L markers denote anterior, posterior, right and left.
Figure 4.
(a) The percentage change between task and control 13C-scans for the non-activated volume (left) and activated volume (right). The lines connect the data points from the same individual, the box centre line indicates the mean and whiskers at the 25th and 75th percentile and (b) the percentage change between task and control 13C-scans for each BrainCOLOR region. The BOLD-fMRI t-statistic maps were used to designate ‘activation volume regions’ and ‘non-activation volume regions’. An elevated in C-lactate is visible in the activation volume regions.
The one-way Wilcoxon signed-rank test showed a significant difference in for C-lactate signal between the activation and non-activation volumes ( , V = 21), indicating a task-induced increase in C-lactate production in the activation volume relative to the rest of the brain. The Wilcoxon signed-rank tests for C-bicarbonate and C-pyruvate showed no significant difference between the activation and non-activation volumes ( , V = 3 and , V = 17 respectively). The magnitude of the V-statistic indicates the difference between groups. None of the Wilcoxon signed-rank tests on the individual regions showed a signficant difference between and for any of the C-metabolites (after controlling for multiple testing), which was expected given the small sample size.
The parcellated regions within the activation volume for all six individuals were: the bilateral calcarine cortex, cuneus, inferior occipital gyrus, lingual gyrus, middle occipital gyrus, occipital pole, occipital fusiform gyrus, and superior occipital gyrus. These regions are highlighted in Figure 4(b), with the C-lactate signal visibly higher in these regions in comparison to the non-activation volume regions.
The correlation between the degree of BOLD activation (mean t-statistic) and the percentage change in 13C-lactate within the activation volume is plotted in Figure 5. The correlation between 13C-lactate and the mean t-statistic within the activation volume was the strongest, at 0.77, though it did not reach significance (p = 0.051). All other correlations were not significant.
Figure 5.

Correlation between the level of BOLD activation (t-statistic) within the activation volume and the percentage 13C-lactate signal change between the task and control conditions.
Discussion
In this study hyperpolarized C-MRI was used to measure the metabolic conversion of exogenously administered C-pyruvate into C-lactate and 13C-bicarbonate. Increased C-lactate signal in visual cortex regions was observed during a visual stimulus, consistent with prior studies using proton spectroscopy.8,23,24 These results demonstrate that task activation causes an increase in regional C-lactate signal measurable with HP-13C MRI. There was also a strong correlation between the percent change in 13C-lactate and t-statistic within the activation volume, suggesting a monotonic relationship between the BOLD response and the production of 13C-lactate. Elevated 13C-lactate tended to occur in areas that showed BOLD activation consistently across participants. The activation volume for each participant was defined using the BOLD signal and a significant difference in 13C-lactate signal between the activation and non-activation non-activation volumes was found ( , V = 21). C-metabolite signals were normalized by the signal from the brainstem, allowing for comparison of relative differences between conditions but not absolute quantification.
The increase in 13C-lactate signal may include 13C-lactate in the venous blood, in addition to parenchymal signal. However, there is evidence from the rodent brain that C-lactate is largely parenchymal. 25 Cerebral arteriovenous differences in lactate measured during stimulation have shown increased venous lactate consistent with local production, as opposed to arterial transport of lactate to regions of high energy demand.26,27 Moreover glutamate, the most abundant excitatory neurotransmitter in the brain, has consistently been observed to increase under stimulus conditions when measured through fMRS. 28 Glutamate is cleared from the intracellular space by astrocytes through a process that is bioenergetically supported by the non-oxidative metabolism of pyruvate into lactate. 29 However, lactate can also be used to meet transient energy needs in neurons, 30 and neuronal stimulus directly induces neuronal lactate production. 31
No significant differences between task and control conditions were detected for the C-pyruvate signal, although an increasing trend can be observed in Figure 4(a). Increased cerebral blood volume due to functional activation would be expected to increase regional C-pyruvate signal, especially as C-pyruvate signal is largely in the vascular compartment. 25 However, cerebral blood volume increases only slightly during functional activation, 32 so it is possible that the effect size was too small to discern a significant change in C-pyruvate signal with the small sample size.
Surprisingly, a task-induced increase in C-bicarbonate signal was not detected. A recent study by Zaidi et al. using HP-13C MR spectroscopy found increased 13C-bicarbonate signal in the occipital lobe during functional activation, attributed to increased pyruvate oxidation. 33 The discrepancy between the two studies may be because C-bicarbonate production is partly derived from oxidative metabolism of C-lactate, as observed in prior studies. 34 Due to the high effective flip angle used at the C-lactate frequency in our study, C-bicarbonate signal resulting from 13C-lactate would be suppressed. In the study by Zaidi et al. a reduced flip angle was used for the C-lactate frequency, so C-bicarbonate signal would not be suppressed. 33 This phenomenon will be explored in future studies.
Conclusions
HP C MRI was used to assess functional activation in a cohort of healthy volunteers. Increased C-lactate signal in visual cortex regions, defined by BOLD-fMRI activation, was observed following a visual stimulus. Thus HP C MRI may be useful for studying stimulus-induced changes in brain lactate metabolism, a distinct pathway implicated in both neuron and astrocyte bioenergetics. Future work will explore different data acquisition strategies to better understand the underlying physiological origins of the observed 13C-lactate signal.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support was provided from the Canadian Cancer Society grant 707455, Canadian Institutes of Health Research grant PJT-152928 and National Institutes of Health grant R01CA237466. K.R.K. is supported by the National Institutes of Health NIH/NCI Cancer Center Support Grant P30CA008748 and the Center for Molecular Imaging andBioengineering (CMIB) at Memorial Sloan Kettering Cancer Center.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.P.C. is employed by GE Healthcare, the manufacturer of the SPINLab polarizer. K.R.K. is co-founder of Atish Technologies and serves on the Scientific Advisory Boards of NVision Imaging Technologies and Imaginostics. He holds patents related to imaging and leveraging cellular metabolism.
Authors’ contributions: H.S. and C.H.C. are co-senior authors; B.U., N.I.C.C., F.T., and C.H.C designed the study protocol; N.M. and W.J.P. prepared pharmacy samples for injection; B.U., N.I.C.C, N.D.B, R.E., and H.S. acquired data; B.U. analyzed data; B.U., A.P., S.J.G., C.H., K.R.K., H.S., and C.H.C. interpreted results; B.U., N.I.C.C., and C.H.C. prepared figures and manuscript; B.U., N.I.C.C., S.J.G, K.R.K., A.P., H.S., and C.H.C. edited and revised manuscript; all co-authors approved final version of manuscript.
ORCID iDs: Nicole IC Cappelletto https://orcid.org/0000-0002-2181-8093
Charles H Cunningham https://orcid.org/0000-0001-9636-8130
References
- 1.Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res 2018; 78: 3755–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1–13C] pyruvate and magnetic resonance imaging. NeuroImage 2019; 189: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon JW, Chen H-Y, Autry A, et al. Translation of carbon-13 epi for hyperpolarized mr molecular imaging of prostate and brain cancer patients. Magn Reson Med 2019; 81: 2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CY, Soliman H, Geraghty BJ, et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. Neuroimage 2020; 204: 116202. [DOI] [PubMed] [Google Scholar]
- 5.Lee CY, Soliman H, Bragagnolo ND, et al. Predicting response to radiotherapy of intracranial metastases with hyperpolarized 13 13 c mri. J Neurooncol 2021; 152: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uthayakumar B, Soliman H, Bragagnolo ND, et al. Age-associated change in pyruvate metabolism investigated with hyperpolarized 13C-mri of the human brain. Hum Brain Mapp 2023; 44: 4052–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prichard J, Rothman D, Novotny E, et al. Lactate rise detected by 1H nmr in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A 1991; 88: 5829–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaller B, Mekle R, Xin L, et al. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J Neurosci Res 2013; 91: 1076–1083. [DOI] [PubMed] [Google Scholar]
- 9.Mangia S, Tkáč I, Gruetter R, et al. Sensitivity of single-voxel 1H-mrs in investigating the metabolism of the activated human visual cortex at 7 t. Magn Reson Imaging 2006; 24: 343–348. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bédirian V, et al. Montreal cognitive assessment. Am J Geriatr Psychiatry 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 11.Geraghty BJ, Lau JY, Chen AP, et al. Dual-echo epi sequence for integrated distortion correction in 3D time-resolved hyperpolarized 13C MRI. Magn Reson Med 2018; 79: 643–653. [DOI] [PubMed] [Google Scholar]
- 12.Uthayakumar B, Soliman H, Chen AP, et al. Evidence of 13C-lactate oxidation in the human brain from hyperpolarized 13C-MRI. Magn Reson Med 2024; 91: 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau AZ, Chen AP, Hurd RE, et al. Spectral–spatial excitation for rapid imaging of dnp compounds. NMR Biomed 2011; 24: 988–996. [DOI] [PubMed] [Google Scholar]
- 14.Gordon JW, Chen H-Y, Dwork N, et al. Fast imaging for hyperpolarized MR metabolic imaging. J Magn Reson Imaging 2021; 53: 686–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 16.Klein A, Tourville J. 101 Labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 2012; 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo Y, Xu Z, Xiong Y, et al. 3D whole brain segmentation using spatially localized atlas network tiles. NeuroImage 2019; 194: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B. FreeSurfer. Neuroimage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed 1997; 10: 171–178. [DOI] [PubMed] [Google Scholar]
- 21.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 2014; 91: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazziotta JC, Toga AW, Evans A, et al. A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage 1995; 2: 89–101. [DOI] [PubMed] [Google Scholar]
- 23.Fox PT, Raichle ME, Mintun MA, et al. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988; 241: 462–464. [DOI] [PubMed] [Google Scholar]
- 24.Schaller B, Xin L, O'Brien K, et al. Are glutamate and lactate increases ubiquitous to physiological activation? A 1H functional MR spectroscopy study during motor activation in human brain at 7 tesla. Neuroimage 2014; 93 Pt 1: 138–145. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, Jhajharia A, Josan S, et al. Investigating the origin of the 13C lactate signal in the anesthetized healthy rat brain in vivo after hyperpolarized [1-13C] pyruvate injection. NMR Biomed 2024; 37: e5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen PL, Hasselbalch SG, Hagemann LP, et al. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety–Schmidt technique. J Cereb Blood Flow Metab 1995; 15: 485–491. [DOI] [PubMed] [Google Scholar]
- 27.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012; 32: 1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasanta D, He JL, Ford T, et al. Functional MRS studies of gaba and glutamate/glx – a systematic review and meta-analysis. Neurosci Biobehav Rev 2023; 144: 104940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed 2001; 14: 389–396. [DOI] [PubMed] [Google Scholar]
- 30.Boumezbeur F, Petersen KF, Cline GW, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 2010; 30: 13983–13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz-García CM, Mongeon R, Lahmann C, et al. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 2017; 26: 361–374.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim T, Hendrich KS, Masamoto K, et al. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: Implication for bold fMRI. J Cereb Blood Flow Metab 2007; 27: 1235–1247. [DOI] [PubMed] [Google Scholar]
- 33.Zaidi1 M, Chen J, Ma1 J, et al. Assessment of human brain pyruvate oxidation using functional hyperpolarized 13C MRS. In: Proceedings of the joint annual ISMRM-ESMRMB 2022, and ISMRT annual meeting, London, UK.
- 34.Bøgh N, Grist JT, Rasmussen CW, et al. Lactate saturation limits bicarbonate detection in hyperpolarized 13C-pyruvate MRI of the brain. Magn Reson Med 2022; 18: 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]