Abstract
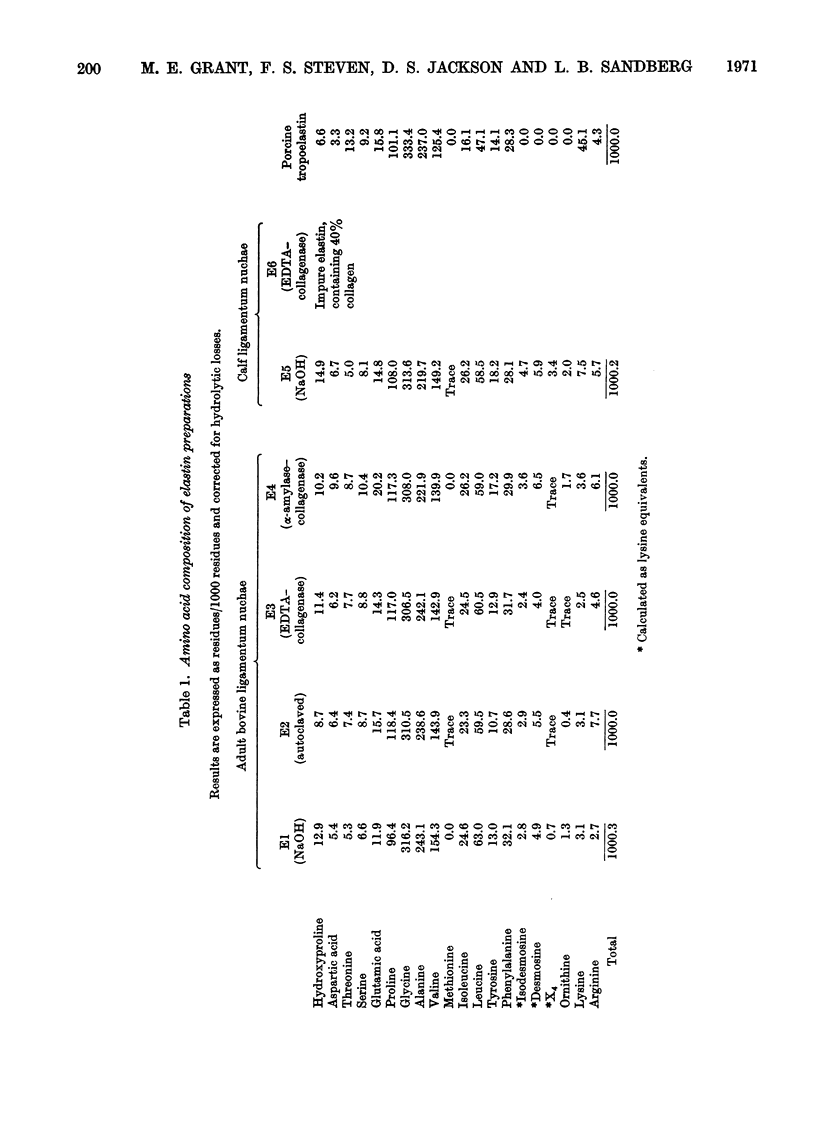
1. Insoluble elastin has been prepared by several different methods from adult bovine and calf ligamentum nuchae. Highly purified tropoelastin has been prepared from copper-deficient porcine aorta. 2. Amino acid analyses indicated that all preparations, except that obtained from calf ligamentum nuchae by using an EDTA extraction followed by collagenase digestion (preparation E6), were typical of pure elastin having high concentrations of hydrophobic and low concentrations of hydrophilic amino acids. Preparation E6 was found to contain approx. 40% collagen. 3. The determination and composition of the carbohydrates associated with these preparations is reported. With the exception of preparation E6, the insoluble elastins contained only trace amounts of neutral sugars (0.13–0.35%, w/w) and amino sugars (0.01–0.06%, w/w). The porcine tropoelastin contained virtually no carbohydrate. 4. The results suggest that carbohydrate analyses can yield valuable information about the purity of elastin preparations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANASTASSIADIS P. A., COMMON R. H. Liberation of hexosamine, hexuronic acid, and hydroxyproline from tissues by resin hydrolysis. Can J Biochem Physiol. 1958 Apr;36(4):413–424. [PubMed] [Google Scholar]
- Anwar R. A. Comparison of elastins from various sources. Can J Biochem. 1966 Jun;44(6):725–734. doi: 10.1139/o66-090. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Etherington D. J. Action of crude bacterial alpha-amylase on tropocollagen. Biochim Biophys Acta. 1970 Jul 27;214(1):238–241. doi: 10.1016/0005-2795(70)90093-0. [DOI] [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary E. G., Sandberg L. B., Jackson D. S. The changes in chemical composition during development of the bovine nuchal ligament. J Cell Biol. 1967 Jun;33(3):469–479. doi: 10.1083/jcb.33.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary E. G., Sandberg L. B., Jackson D. S. The incorporation of lysine into growing elastin. Biochem Biophys Res Commun. 1966 Apr 19;23(2):139–144. doi: 10.1016/0006-291x(66)90518-3. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- FULLER K. W., NORTHCOTE D. H. A micro method for the separation and determination of polysaccharides by zone electrophoresis. Biochem J. 1956 Dec;64(4):657–663. doi: 10.1042/bj0640657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTE L., STERN P., ELSDEN D. F., PARTRIDGE S. M. The chemistry of connective tissues. 8. The composition of elastin from three bovine tissues. Biochem J. 1963 May;87:344–351. doi: 10.1042/bj0870344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Freeman I. L., Schofield J. D., Jackson D. S. Variations in the carbohydrate content of human and bovine polymeric collagens from various tissues. Biochim Biophys Acta. 1969 May 6;177(3):682–685. doi: 10.1016/0304-4165(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Jackson D. S. Carbohydrate content of bovine collagen preparations. Biochem J. 1968 Jul;108(4):587–591. doi: 10.1042/bj1080587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSPELHORN V. D., FITZPATRICK M. J. The isolation of elastic tissue from lung. Biochem Biophys Res Commun. 1961 Nov 20;6:191–195. doi: 10.1016/0006-291x(61)90127-9. [DOI] [PubMed] [Google Scholar]
- LANSING A. I., ROSENTHAL T. B., ALEX M., DEMPSEY E. W. The structure and chemical characterization of elastic fibers as revealed by elastase and by electron microscopy. Anat Rec. 1952 Dec;114(4):555–575. doi: 10.1002/ar.1091140404. [DOI] [PubMed] [Google Scholar]
- LaBella F. S., Vivian S., Thornhill D. P. Amino acid composition of human aortic elastin as influenced by age. J Gerontol. 1966 Oct;21(4):550–555. doi: 10.1093/geronj/21.4.550. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- MORET V., SERAFINI-FRACASSINI A., GOTTE L. THE CARBOHYDRATE COMPOSITION OF THE NAC1-SOLUBLE FRACTION FROM AUTOCLAVED ELASTIN. J Atheroscler Res. 1964 Mar-Apr;4:184–188. doi: 10.1016/s0368-1319(64)80038-7. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin. Biochem J. 1955 Sep;61(1):11–21. doi: 10.1042/bj0610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE S. M., ELSDEN D. F., THOMAS J. Constitution of the cross-linkages in elastin. Nature. 1963 Mar 30;197:1297–1298. doi: 10.1038/1971297a0. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B., Weissman N., Smith D. W. The purification and partial characterization of a soluble elastin-like protein from copper-deficient porcine aorta. Biochemistry. 1969 Jul;8(7):2940–2945. doi: 10.1021/bi00835a037. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S. Isolation and amino acid composition of insoluble elastin. Bovine foetal and adult aorta and ligamentum nuchae. Biochim Biophys Acta. 1968 Oct 21;168(2):334–340. doi: 10.1016/0005-2795(68)90155-4. [DOI] [PubMed] [Google Scholar]
- Steven F. S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967 Aug 15;140(3):522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]


