Abstract
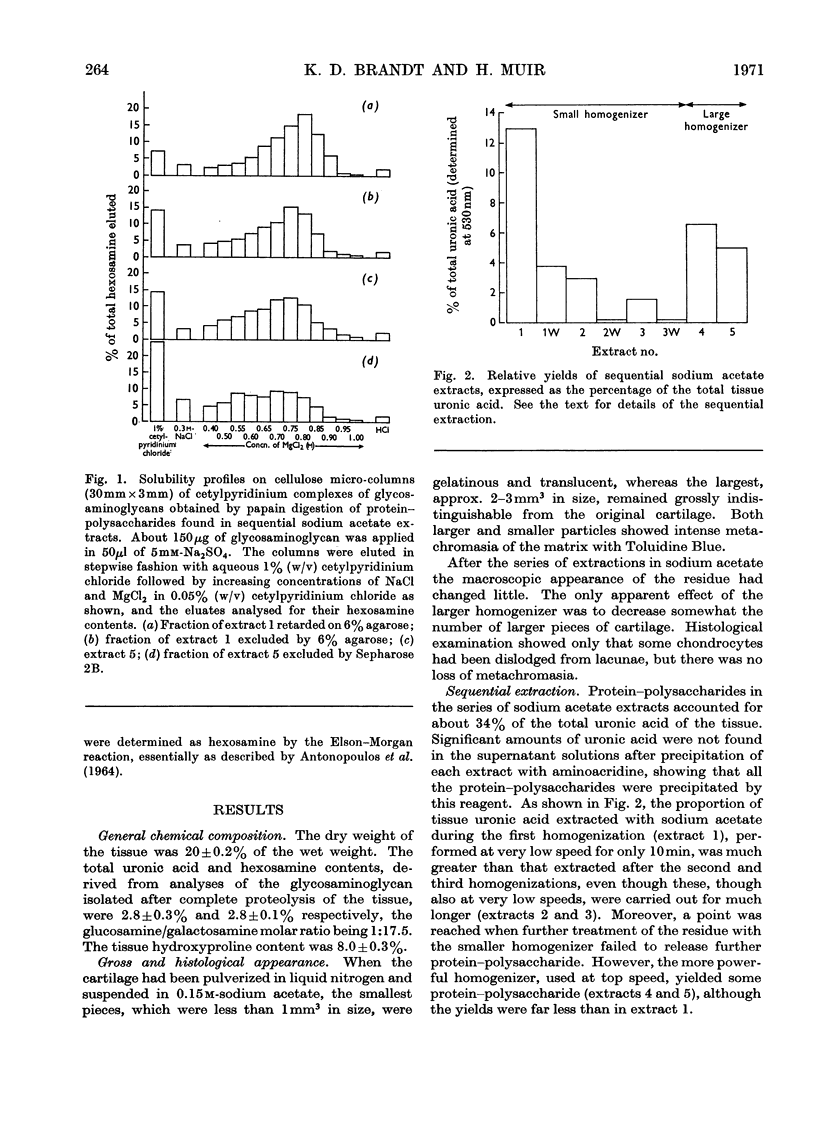
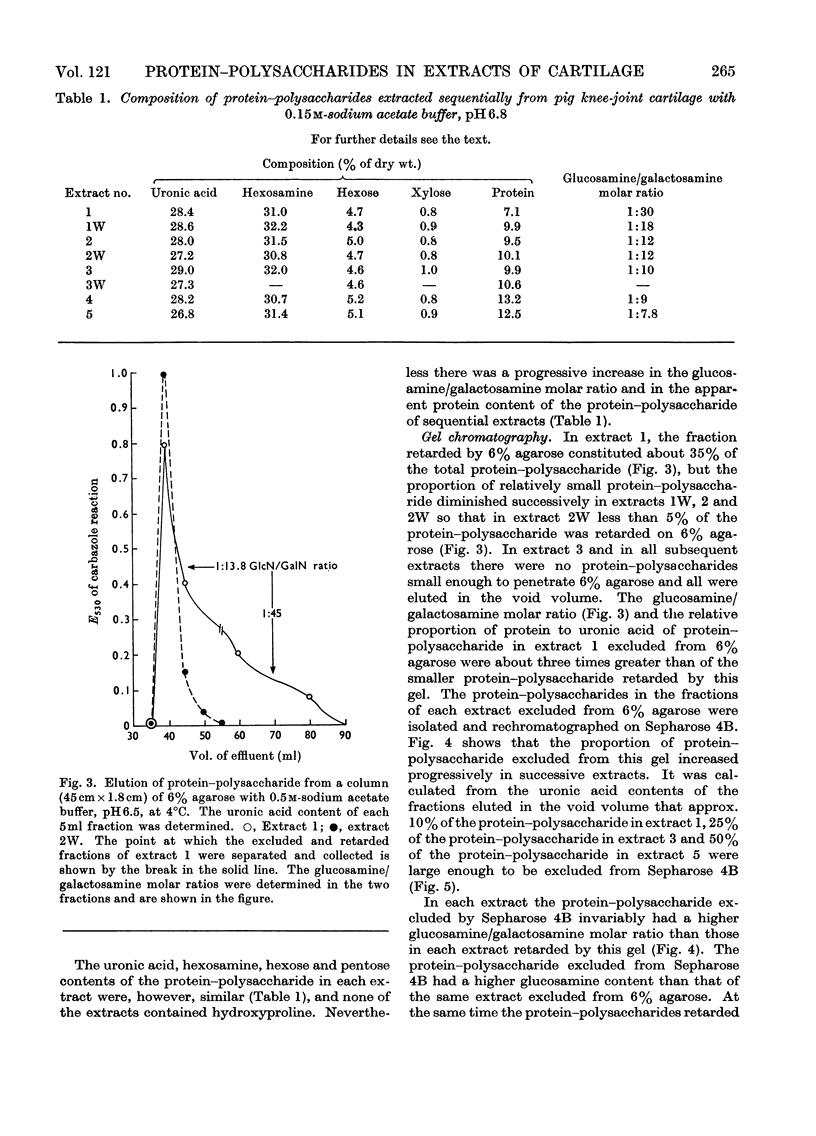
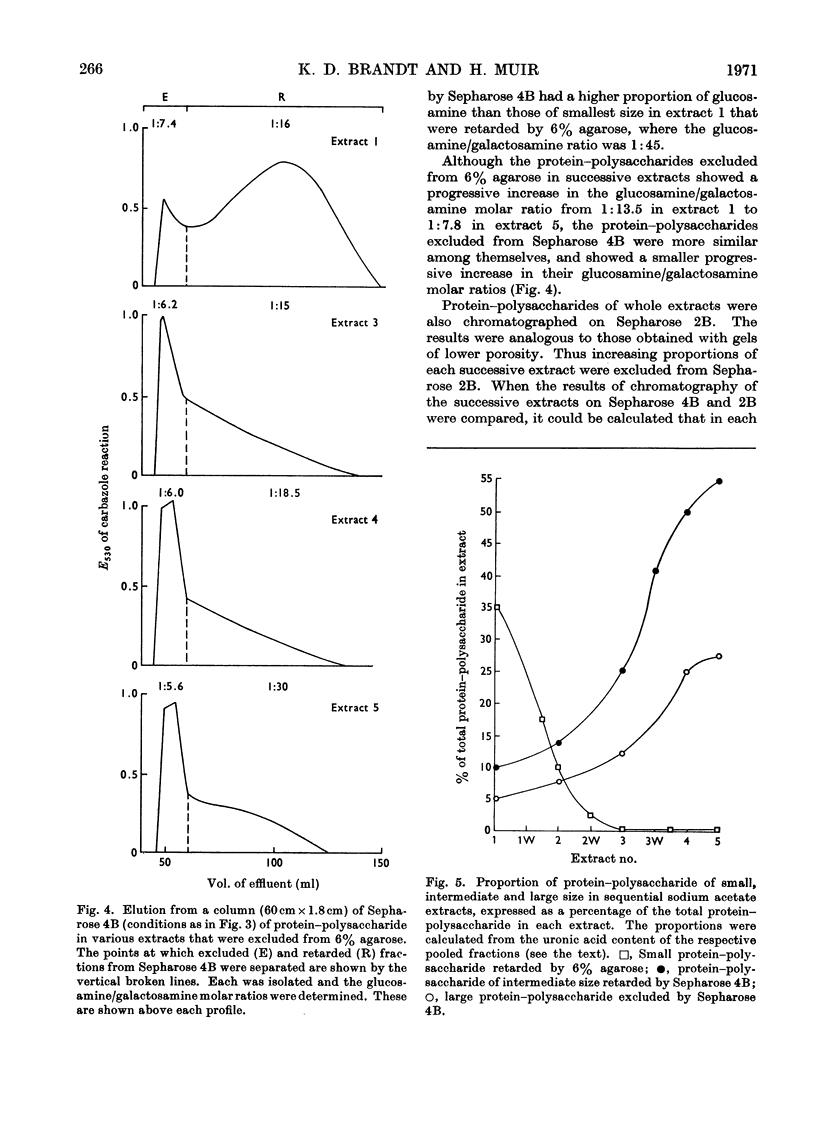
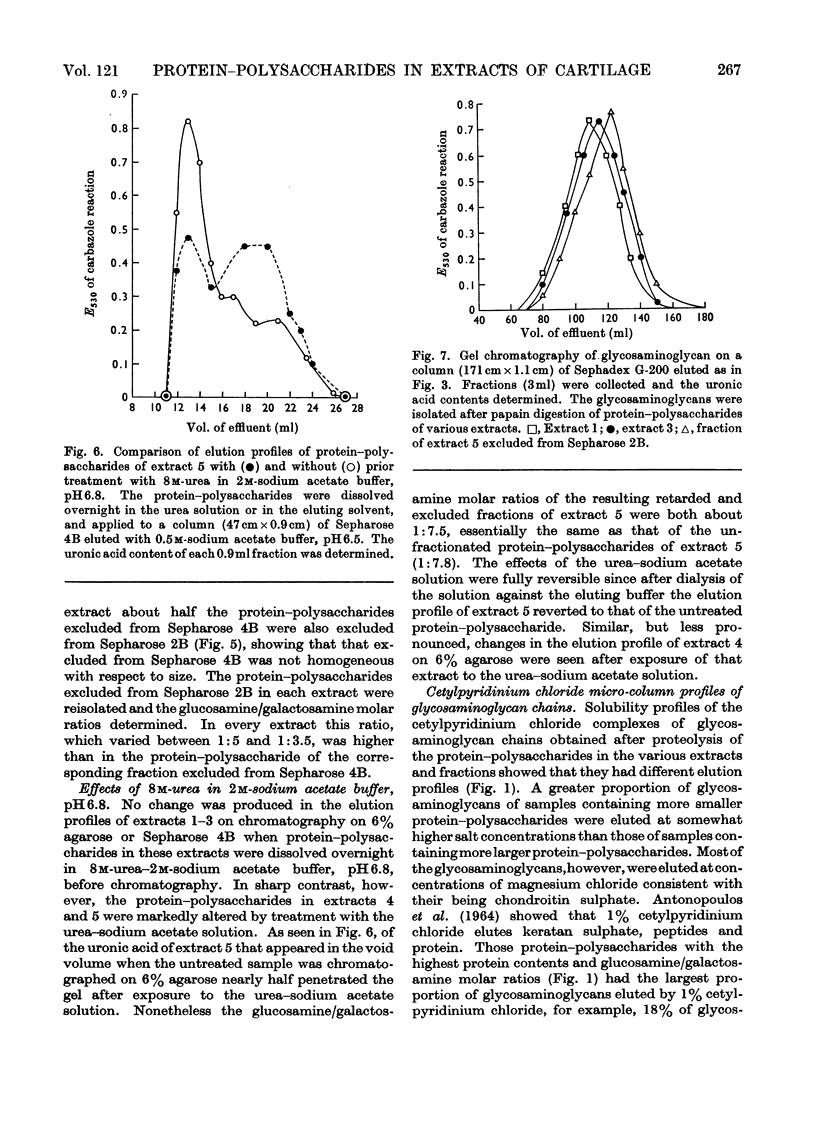
Protein–polysaccharides of knee-joint cartilage of 9-month-old pigs were extracted sequentially with neutral iso-osmotic sodium acetate after five repeated homogenizations. One-third of the uronic acid originally present in the tissue was brought into solution, about half being in the first extract. The protein–polysaccharides, which were purified by precipitation with 9-aminoacridine, were heterogeneous in size on gel chromatography. The smallest (retarded by 6% agarose) were the most easily extracted since they were most prevalent in the initial extracts and absent from later ones, whereas the proportion of larger molecules increased progressively in successive extracts. Nevertheless a small proportion of the largest molecules (excluded from Sepharose 2B) was present even in the first extract. None of the protein–polysaccharide preparations contained hydroxyproline, and the analyses of their constituent sugars were the same, although there was a progressive increase in the protein content and in the glucosamine/galactosamine molar ratio of successive extracts. In each preparation this molar ratio was invariably greater in larger than in smaller molecules separated by gel filtration. From galactosamine/pentose molar ratios it appeared that the chondroitin sulphate chains were on average about 29 disaccharide units in length in the protein–polysaccharides of each extract, although gel-chromatography and cetylpyridinium chloride elution profiles showed that a somewhat higher proportion of shorter chondroitin sulphate chains occurred in the larger protein–polysaccharides. In the last extract, where the largest molecules predominated, about half could be reversibly dissociated by urea, whereas this had no effect on the protein–polysaccharides of earlier extracts even though these contained some large molecules.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Characterization of protein-polysaccharides of articular cartilage from mature and immature pigs. Biochem J. 1969 Oct;114(4):871–876. doi: 10.1042/bj1140871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLANAN M. J., CARROLL W. R., MITCHELL E. R. Physical and chemical properties of protamine from the sperm of salmon (Oncorhynchus tschawytscha). J Biol Chem. 1957 Nov;229(1):279–287. [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hoffman P., Mashburn T. A., Jr, Meyer K., Bray B. A. Proteinpolysaccharide of bovine cartilage. I. Extraction and electrophoretic studies. J Biol Chem. 1967 Sep 10;242(17):3799–3804. [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALAWISTA I., SCHUBERT M. Chondromucoprotein: new extraction method and alkaline degradation. J Biol Chem. 1958 Jan;230(1):535–544. [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- Muir H., Jacobs S. Protein-polysaccharides of pig laryngeal cartilage. Biochem J. 1967 May;103(2):367–374. doi: 10.1042/bj1030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Rodén L., Armand G. Structure of the chondroitin 4-sulfate-protein linkage region. Isolation and characterization of the disaccharide 3-O-beta-D-glucuronosyl-D-galactose. J Biol Chem. 1966 Jan 10;241(1):65–70. [PubMed] [Google Scholar]
- Rosenberg L., Schubert M. The proteinpolysaccharides of bovine nucleus pulposus. J Biol Chem. 1967 Oct 25;242(20):4691–4701. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Serafini-Fracassini A., Peters T. J., Floreani L. The protein-polysaccharide complex of bovine nasal cartilage. Studies on the protein core. Biochem J. 1967 Nov;105(2):569–575. doi: 10.1042/bj1050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Estimation of pentoses and methylpentoses in biopolymers, in particular of fucose and xylose. Anal Biochem. 1966 Dec;17(3):495–501. doi: 10.1016/0003-2697(66)90184-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Extraction and purification. Biochem J. 1969 Aug;113(5):879–884. doi: 10.1042/bj1130879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of molecular weight dispersion in chondroitin sulphate on a microgram level. Biochim Biophys Acta. 1969 Feb 18;177(1):152–154. doi: 10.1016/0304-4165(69)90076-2. [DOI] [PubMed] [Google Scholar]


