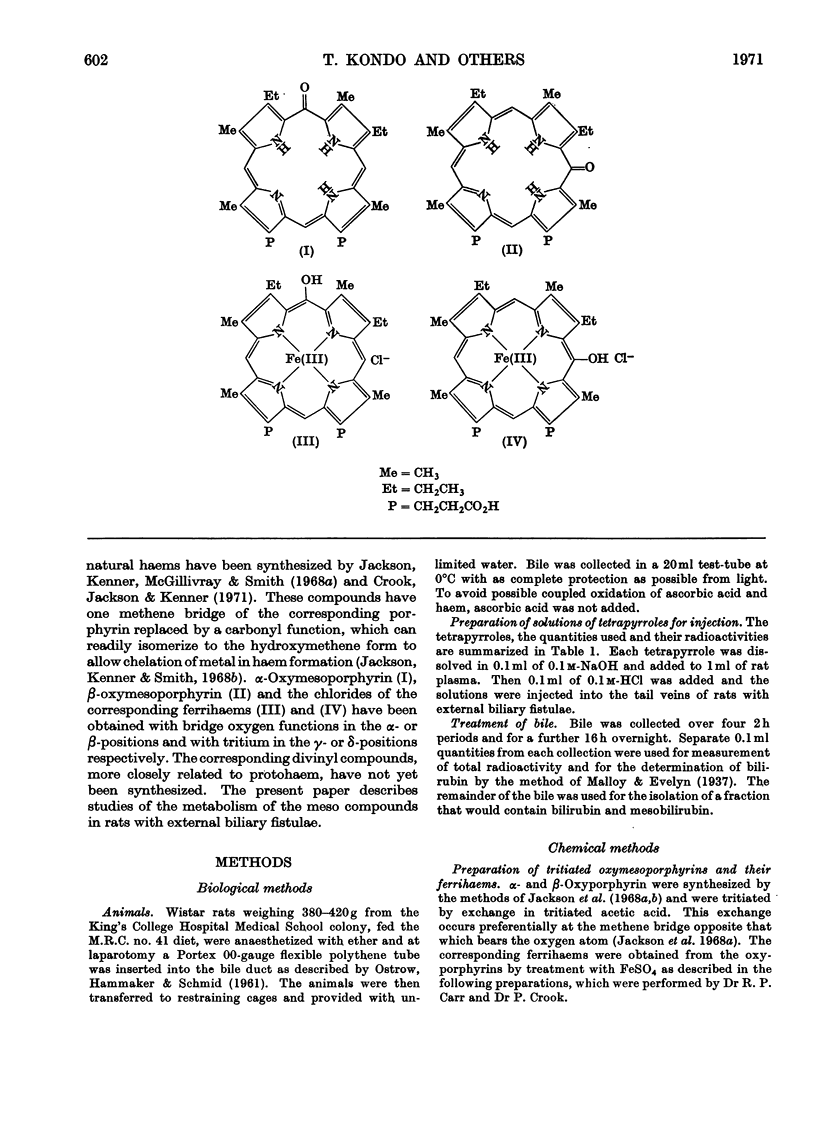
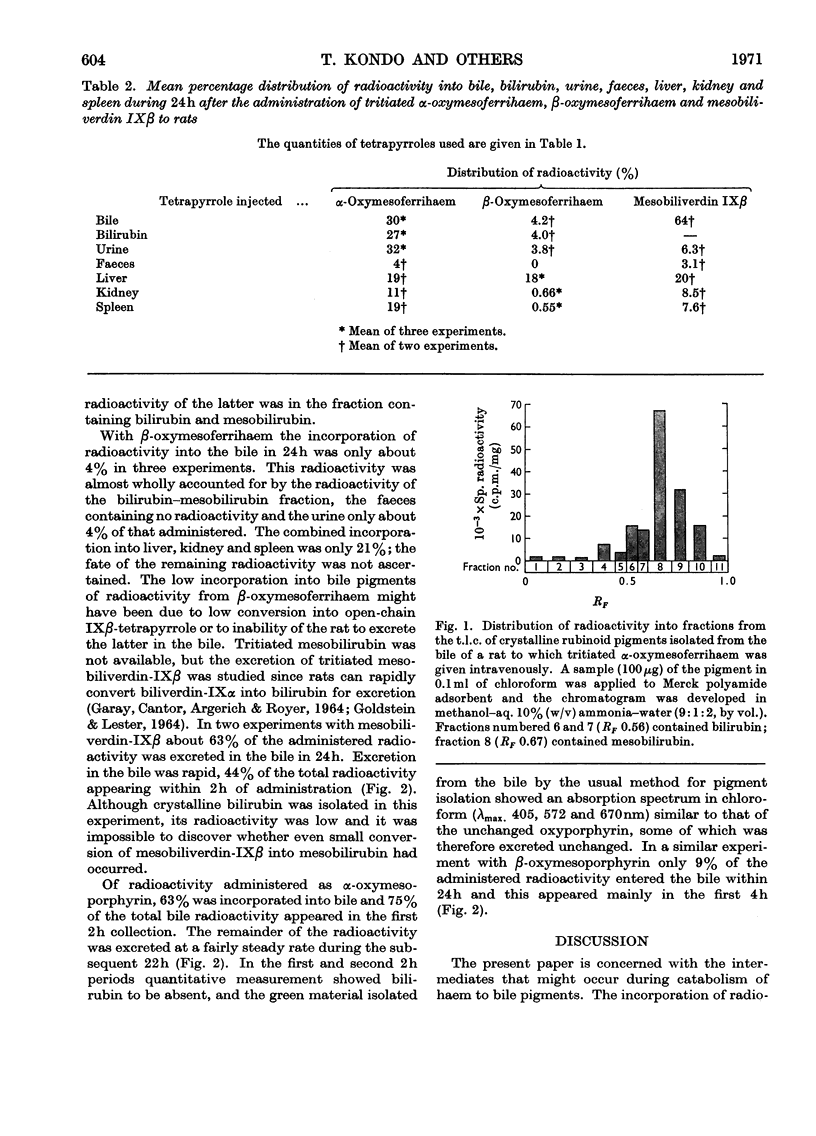
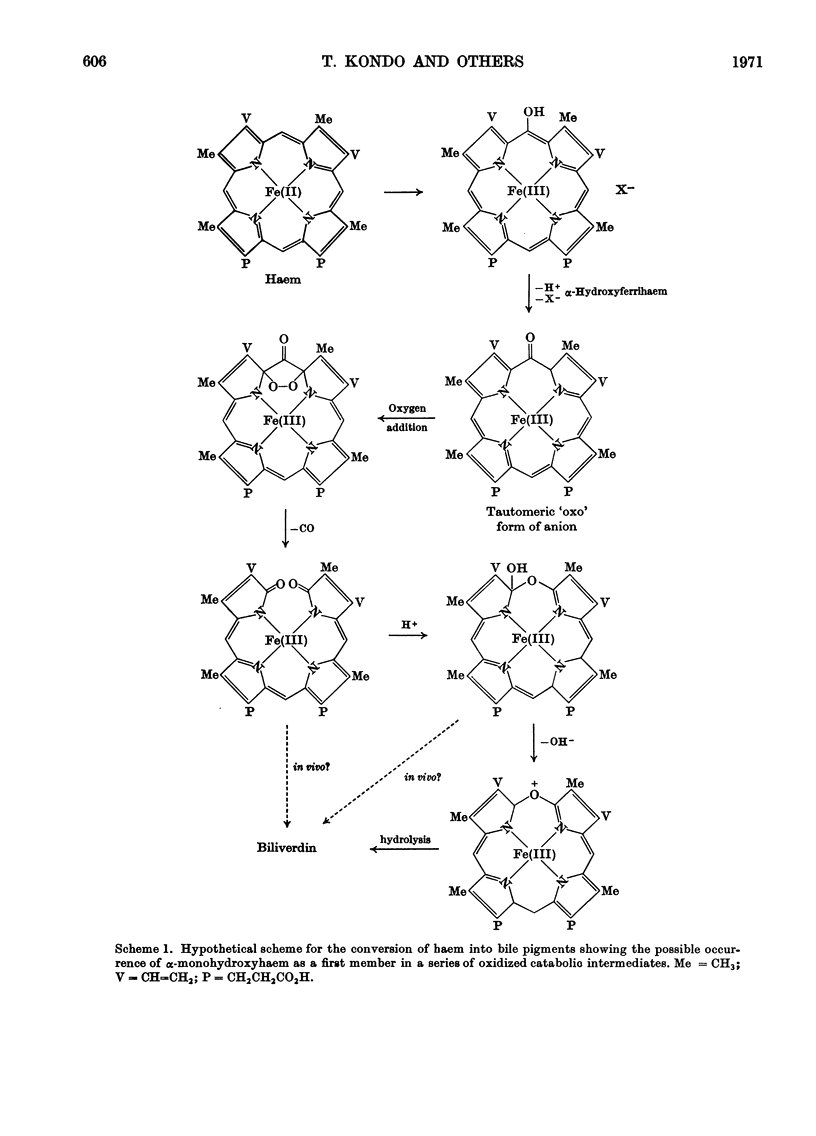
Abstract
1. Tritiated oxymesoporphyrins and their ferrihaems were tested as possible intermediates in the catabolism of haemoglobin. The tritiated compounds were injected into rats with biliary fistulae and the incorporation of the isotope into bile, bile pigment, urine, faeces, liver, kidney and spleen was measured. 2. α-Oxymesoferrihaem was extensively converted into bile pigment and specifically to the expected mesobilirubin. 3. β-Oxymesoferrihaem was poorly converted into bile pigment and was not converted into mesobiliverdin IXβ. The latter was independently shown to be excreted rapidly in bile. 4. The free oxyporphyrins were also poor precursors of bile pigment, and α-oxymesoporphyrin competed with bilirubin for excretion by the liver. 5. By analogy with the results obtained with α-oxymesoferrihaem it is concluded that α-oxyprotoferrihaem is an intermediate in the catabolism of haemoglobin, undergoing further oxidation to bile pigment under the catalysis of an enzyme of definite specificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN G. W., LESTER R. REDUCTION OF BILIVERDIN-C-14 TO BILIRUBIN-C-14 IN VIVO. Proc Soc Exp Biol Med. 1964 Dec;117:681–683. doi: 10.3181/00379727-117-29666. [DOI] [PubMed] [Google Scholar]
- GRAY C. H., NICHOLSON D. C., NICOLAUS R. A. The IX-alpha structure of the common bile pigments. Nature. 1958 Jan 18;181(4603):183–185. doi: 10.1038/181183b0. [DOI] [PubMed] [Google Scholar]
- KENCH J. E. Bile pigment formation in vitro from haematin and haem derivatives. Biochem J. 1954 Apr;56(4):669–677. doi: 10.1042/bj0560669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA H., TAKEMURA T., NAKAJIMA O., YAMAOKA K. STUDIES ON HEME ALPHA-METHENYL OXYGENASE. I. THE ENZYMATIC CONVERSION OF PYRIDINE-HEMICHROMOGEN AND HEMOGLOBIN-HAPTOGLOBIN INTO A POSSIBLE PRECURSOR OF BILIVERDIN. J Biol Chem. 1963 Nov;238:3784–3796. [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryka Z. J., Watson C. J. Separation of bile pigments by thin layer chromatography. J Chromatogr. 1968 Sep 24;37(1):76–82. doi: 10.1016/s0021-9673(01)99073-9. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- VAN PUTTEN L. M. The life span of red cells in the rat and the mouse as determined by labeling with DFP32 in vivo. Blood. 1958 Aug;13(8):789–794. [PubMed] [Google Scholar]


