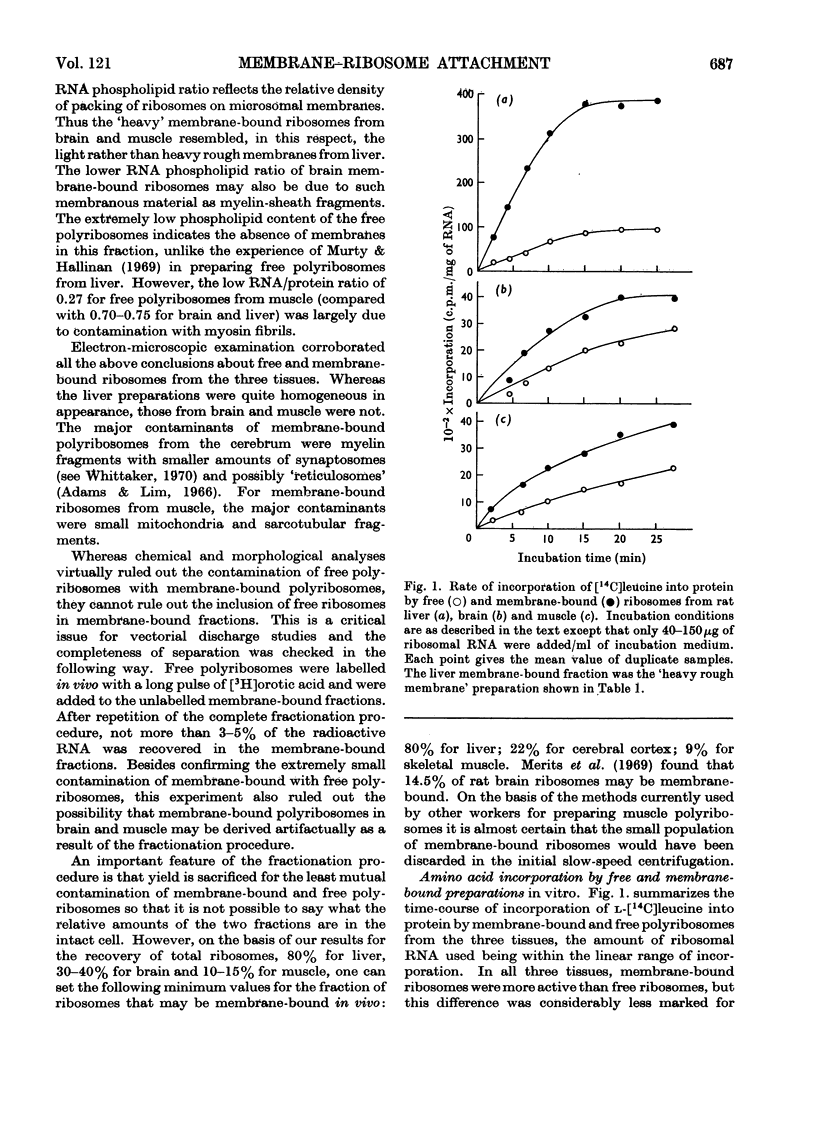
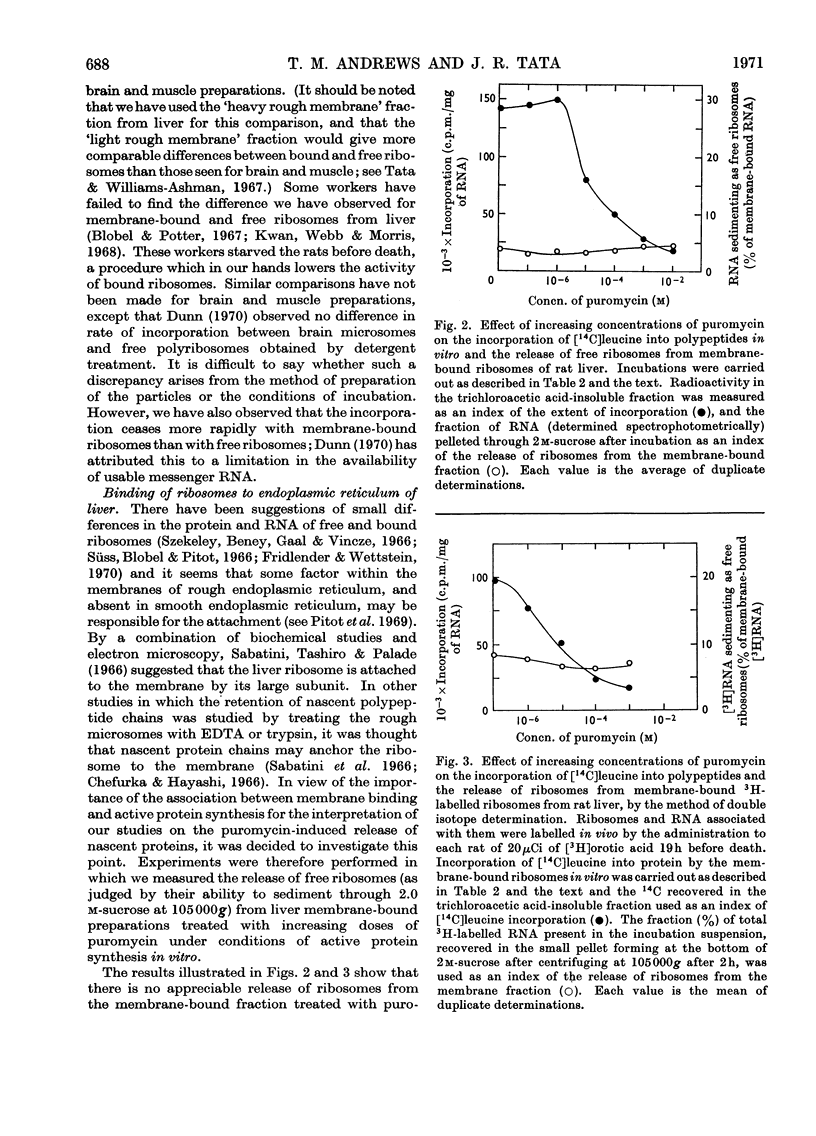
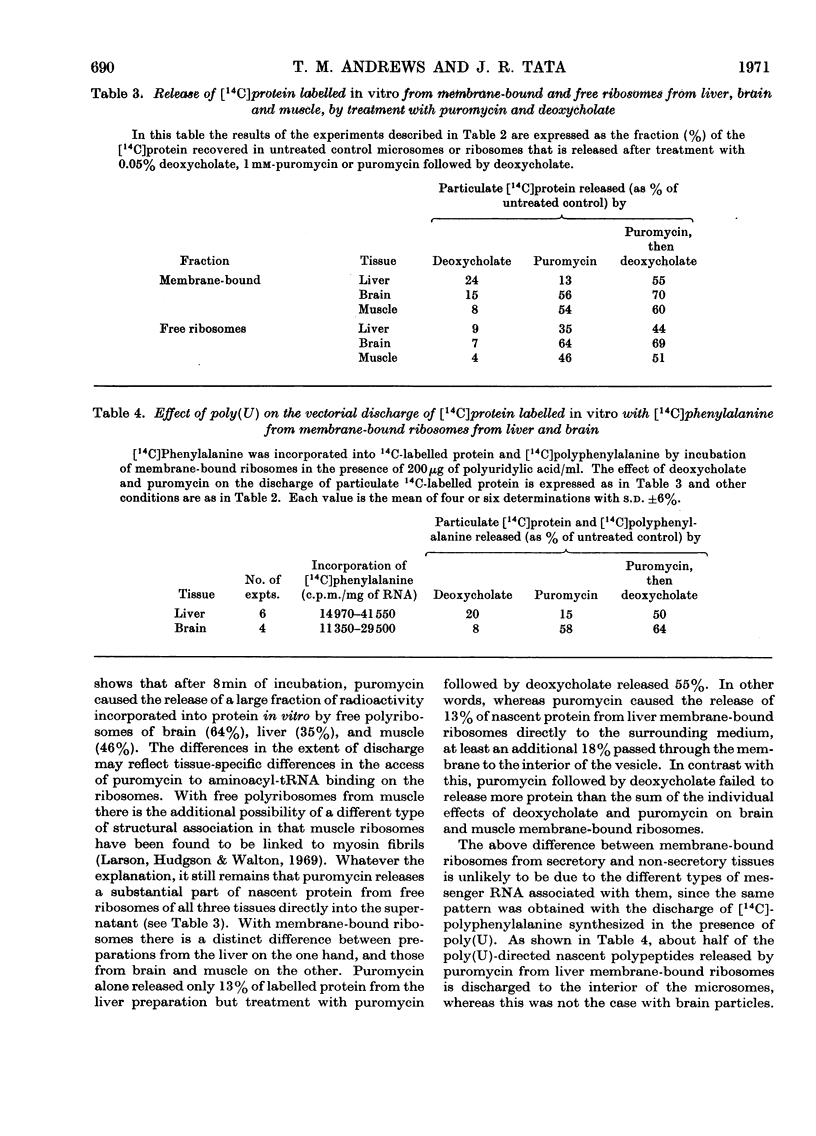
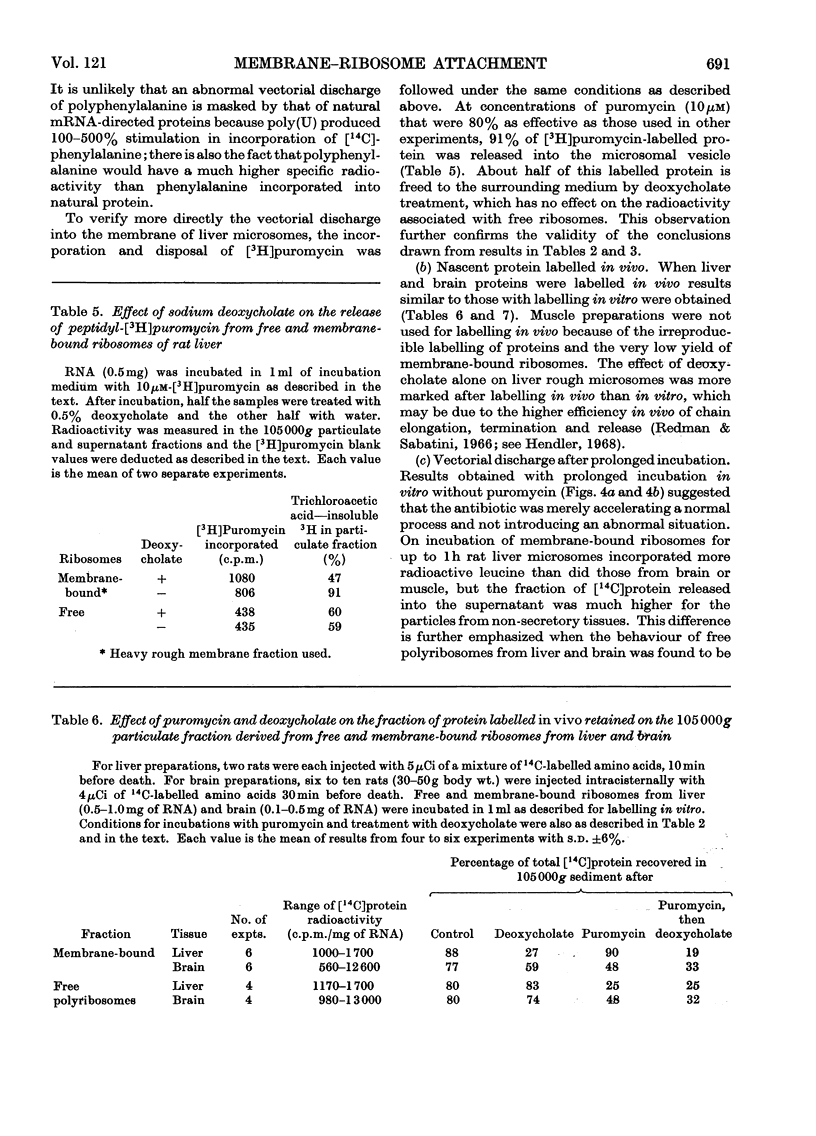
Abstract
1. Methods for the separation of membrane-bound and free ribosomes from rat brain (cortex) and skeletal muscle were described and the preparations characterized by chemical analysis and electron microscopy. The attachment of ribosomes to membranes is not an artifact of the separation procedure. 2. The rate of incorporation of l-[14C]leucine into protein in vitro by the membrane-bound and free ribosomes from these two predominantly non-protein-secreting tissues is compared with that by similar preparations from rat liver. With all three tissues the initial rate was higher for the membrane-bound preparations. 3. By using the technique of discharging nascent polypeptide chains by incubation with puromycin followed by treatment with sodium deoxycholate (Redman & Sabatini, 1966), a major difference was observed for the vectorial discharge of nascent protein synthesized both in vivo and in vitro on membrane-bound ribosomes from liver, on the one hand, and brain and muscle, on the other. Whereas a large part of nascent protein synthesized on membrane-bound liver ribosomes was discharged into the membranous vesicles (presumably destined for export from the cell), almost all nascent protein from membrane-bound ribosomes from brain and muscle was released directly into the supernatant. Incorporation of [3H]puromycin into peptidyl-[3H]puromycin confirmed these findings. There was thus no difference between membrane-bound and free ribosomes from brain on the one hand, and from free polyribosomes from liver on the other, as far as the vectorial release of newly synthesized protein was concerned. 4. Incubation with puromycin also showed that the nascent chains, pre-formed in vivo and in vitro, are not involved in the attachment of ribosomes to membranes of the endoplasmic reticulum. 5. The differences in vectorial discharge from membrane-bound ribosomes from liver as compared with brain and muscle are not due to the different types of messenger RNA in the different tissues. Polyphenylalanine synthesized on incubation with polyuridylic acid was handled in the same way as polypeptides synthesized with endogenous messenger. 6. It is concluded that there is a major difference in the attachment of ribosomes to the membranes of the endoplasmic reticulum of secretory and non-secretory tissues, which results in a tissue-specific difference in the vectorial discharge of nascent proteins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Adams D. H., Lim L. Amino acid incorporation by preparations from the developing rat brain. Biochem J. 1966 May;99(2):261–265. doi: 10.1042/bj0990261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. M., Tata J. R. Difference in vectorial release of nascent protein from membrane-bound ribosomes of secretory and non-secretory tissues. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1050–1056. doi: 10.1016/0006-291x(68)90136-8. [DOI] [PubMed] [Google Scholar]
- BREUER C. B., DAVIES M. C., FLORINI J. R. AMINO ACID INCORPORATION INTO PROTEIN BY CELL-FREE PREPARATIONS FROM RAT SKELETAL MUSCLE. II. PREPARATION AND PROPERTIES OF MUSCLE RIBOSOMES AND POLYRIBOSOMES. Biochemistry. 1964 Nov;3:1713–1719. doi: 10.1021/bi00899a020. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., de Vries M., Benedetti E. L. Isolation and properties of polyribosomes and fragments of the endoplasmic reticulum from rat liver. Biochem J. 1967 Apr;103(1):177–182. doi: 10.1042/bj1030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock G., White A. M., Worthington J. The effects of catabolic and anabolic steroids on amino acid incorporation by skeletal-muscle ribosomes. Biochem J. 1968 Jul;108(3):417–425. doi: 10.1042/bj1080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burka E. R., Schreml W., Kick C. J. Membrane-bound ribonucleic acid in mammalian erythroid cells. Biochemistry. 1967 Sep;6(9):2840–2847. doi: 10.1021/bi00861a026. [DOI] [PubMed] [Google Scholar]
- Campbell M. K., Mahler H. R., Moore W. J., Tewari S. Protein synthesis systems from rat brain. Biochemistry. 1966 Apr;5(4):1174–1184. doi: 10.1021/bi00868a009. [DOI] [PubMed] [Google Scholar]
- Campbell P. N., Lowe E., Serck-Hanssen G. Protein synthesis by microsomal particles from regenerating rat liver. Biochem J. 1967 Apr;103(1):280–288. doi: 10.1042/bj1030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. C., Young V. R. Preparation and some properties of rat skeletal-muscle polyribosomes. Biochem J. 1968 Jan;106(1):61–67. doi: 10.1042/bj1060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Ernster L. Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem. 1968 Oct;16(10):611–632. doi: 10.1177/16.10.611. [DOI] [PubMed] [Google Scholar]
- Dunn A. J. The limiting factors of a cell-free protein-synthesizing system from rat brain. Biochem J. 1970 Jan;116(1):135–145. doi: 10.1042/bj1160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl D. C., Morgan H. E. An improved preparation of ribosomes and polysomes from cardiac muscle. Arch Biochem Biophys. 1968 Nov;128(2):460–469. doi: 10.1016/0003-9861(68)90052-0. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fridlender B. R., Wettstein F. O. Differences in the ribosomal protein of free and membrane bound polysomes of chick embryo cells. Biochem Biophys Res Commun. 1970 Apr 24;39(2):247–253. doi: 10.1016/0006-291x(70)90785-0. [DOI] [PubMed] [Google Scholar]
- González-Cadavid N. F., Bravo M., Campbell P. N. The significance of cytochrome c redistribution during the subcellular fractionation of rat liver. Biochem J. 1968 Apr;107(4):523–529. doi: 10.1042/bj1070523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson R., Tata J. R., Lindberg O., Ernster L. The relationship between the structure and activity of rat skeletal muscle mitochondria after thyroidectomy and thyroid hormone treatment. J Cell Biol. 1965 Aug;26(2):555–578. doi: 10.1083/jcb.26.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. A study of muscle polyribosomes and the coprecipitation of polyribosomes with myosin. Biochemistry. 1968 Sep;7(9):3289–3296. doi: 10.1021/bi00849a036. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B. Biosynthesis of cytochrome c. The sites of synthesis of apoprotein and holoenzyme. Eur J Biochem. 1970 Feb;12(2):392–398. doi: 10.1111/j.1432-1033.1970.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Kwan S. W., Webb T. E., Morris H. P. Diversity and nature of ribosomal pools in hepatoma 7800 and host liver. Biochem J. 1968 Oct;109(4):617–623. doi: 10.1042/bj1090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson P. F., Hudgson P., Walton J. N. Morphological relationship of polyribosomes and myosin filaments in deeloping and regenerating skeletal muscle. Nature. 1969 Jun 21;222(5199):1168–1169. doi: 10.1038/2221168a0. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Brown B. J. Protein synthesis by cerebral cortex polysomes: characterization of the system. Arch Biochem Biophys. 1968 May;125(2):387–400. doi: 10.1016/0003-9861(68)90595-x. [DOI] [PubMed] [Google Scholar]
- Merits I., Cain J. C., Rdzok E. J., Minard F. N. Distribution between free and membrane-bound ribosomes in rat brain. Experientia. 1969;25(7):739–740. doi: 10.1007/BF01897596. [DOI] [PubMed] [Google Scholar]
- Murty C. N., Hallinan T. Agranular membranes in free polysome preparations and their possible interference in studies of protein biosynthesis. Biochem J. 1969 Apr;112(3):269–274. doi: 10.1042/bj1120269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. E. Structure and function at the cellular level. JAMA. 1966 Nov 21;198(8):815–825. [PubMed] [Google Scholar]
- Pollak J. K., Ward D. B. Changes in the chemical composition and the enzymic activities of hepatic microsomes of the chick embryo during development. Biochem J. 1967 Jun;103(3):730–738. doi: 10.1042/bj1030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley G. C., Pruyn M. L., Malt R. A. Glycoprotein synthesis by membraneound ribosomes and smooth membranes in kidney. Biochim Biophys Acta. 1969 Sep 17;190(1):154–160. doi: 10.1016/0005-2787(69)90164-6. [DOI] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Redman C. M., Sabatini D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A. 1966 Aug;56(2):608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- Redman C. M. Studies on the transfer of incomplete polypeptide chains across rat liver microsomal membranes in vitro. J Biol Chem. 1967 Feb 25;242(4):761–768. [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Székely M., Beney L., Gaál O., Vincze S. Electrophoretic studies on proteins and ribonucleic acids of free and membrane-bound ribosomes. Biochim Biophys Acta. 1966 Sep;123(3):574–584. doi: 10.1016/0005-2787(66)90224-3. [DOI] [PubMed] [Google Scholar]
- Süss R., Blobel G., Pitot H. C. Rat liver and hepatoma polysome-membrane interaction in vitro. Biochem Biophys Res Commun. 1966 May 3;23(3):299–304. doi: 10.1016/0006-291x(66)90545-6. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Co-ordination between membrane phospholipid synthesis and accelerated biosynthesis of cytoplasmic ribonucleic acid and protein. Biochem J. 1970 Feb;116(4):617–630. doi: 10.1042/bj1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. Hormonal regulation of growth and protein synthesis. Nature. 1968 Jul 27;219(5152):331–337. doi: 10.1038/219331a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The formation, distribution and function of ribosomes and microsomal membranes during induced amphibian metamorphosis. Biochem J. 1967 Nov;105(2):783–801. doi: 10.1042/bj1050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R., Williams-Ashman H. G. Effects of growth hormone and tri-iodothyronine on amino acid incorporation by microsomal subfractions from rat liver. Eur J Biochem. 1967 Oct;2(3):366–374. doi: 10.1111/j.1432-1033.1967.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Uchida T., Yoneda M. Evidence for the association of membrane with the site of toxin synthesis in Corynebacterium diphtheriae. Biochim Biophys Acta. 1967 Aug 22;145(1):210–213. doi: 10.1016/0005-2787(67)90681-8. [DOI] [PubMed] [Google Scholar]
- Wool I. G., Stirewalt W. S., Kurihara K., Low R. B., Bailey P., Oyer D. Mode of action of insulin in the regulation of protein biosynthesis in muscle. Recent Prog Horm Res. 1968;24:139–213. doi: 10.1016/b978-1-4831-9827-9.50010-1. [DOI] [PubMed] [Google Scholar]
- Work T. S., Coote J. L., Ashwell M. Biogenesis of mitochondria. Fed Proc. 1968 Sep-Oct;27(5):1174–1179. [PubMed] [Google Scholar]
- ZOMZELY C. E., ROBERTS S., RAPAPORT D. REGULATION OF CEREBRAL METABOLISM OF AMINO ACIDS-3. CHARACTERISTICS OF AMINO ACID INCORPORATION INTO PROTEIN OF MICROSOMAL AND RIBOSOMAL PREPARATIONS OF RAT CEREBRAL CORTEX. J Neurochem. 1964 Aug;11:567–582. doi: 10.1111/j.1471-4159.1964.tb11454.x. [DOI] [PubMed] [Google Scholar]
- van Dijk-Salkinoja M. S., Stoof T. J., Planta R. J. The distribution of polysomes, ribosomes and ribosomal subunits in exponential-phase cells of Bacillus licheniformis. Eur J Biochem. 1970 Feb;12(3):474–482. doi: 10.1111/j.1432-1033.1970.tb00875.x. [DOI] [PubMed] [Google Scholar]


