Abstract
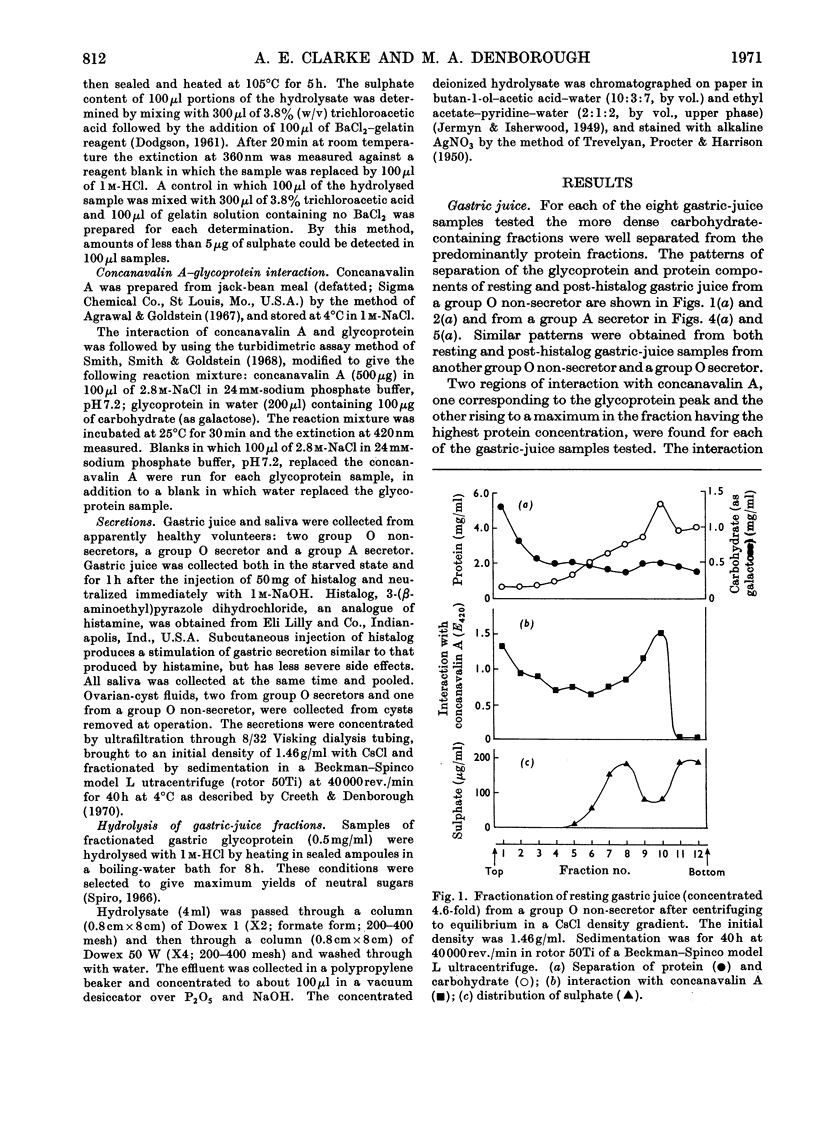
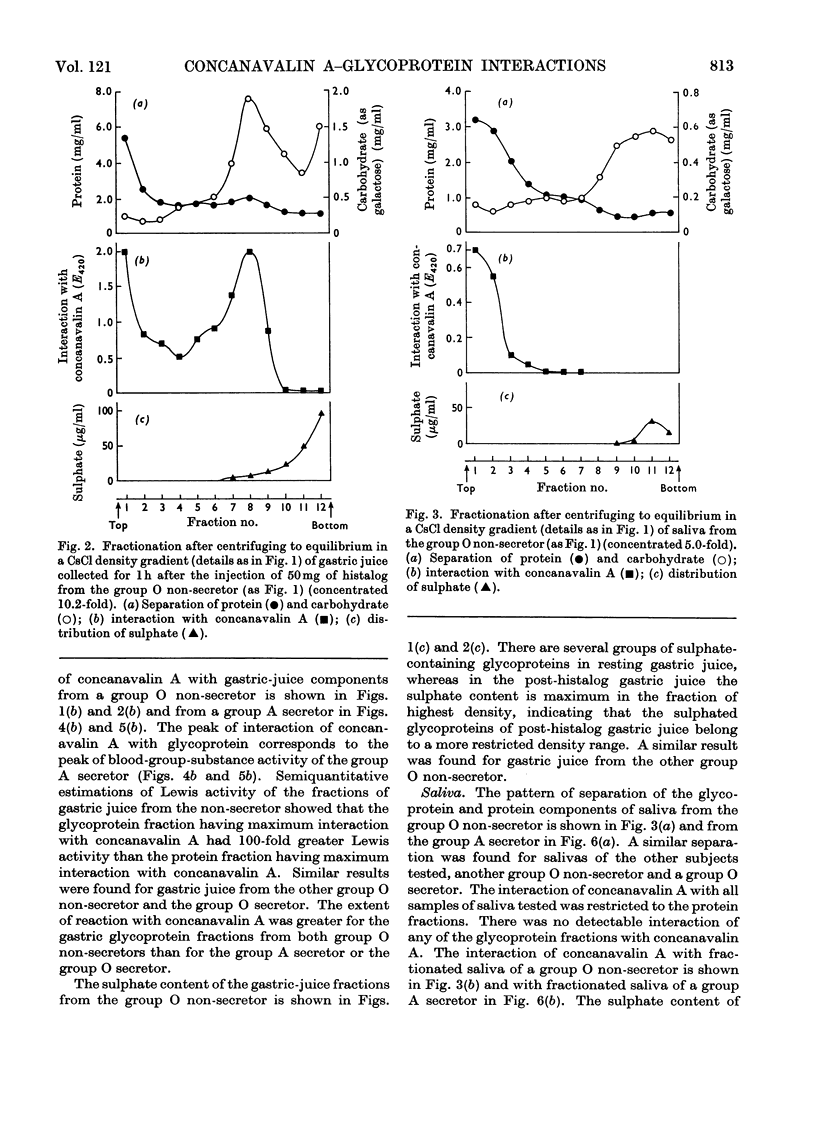
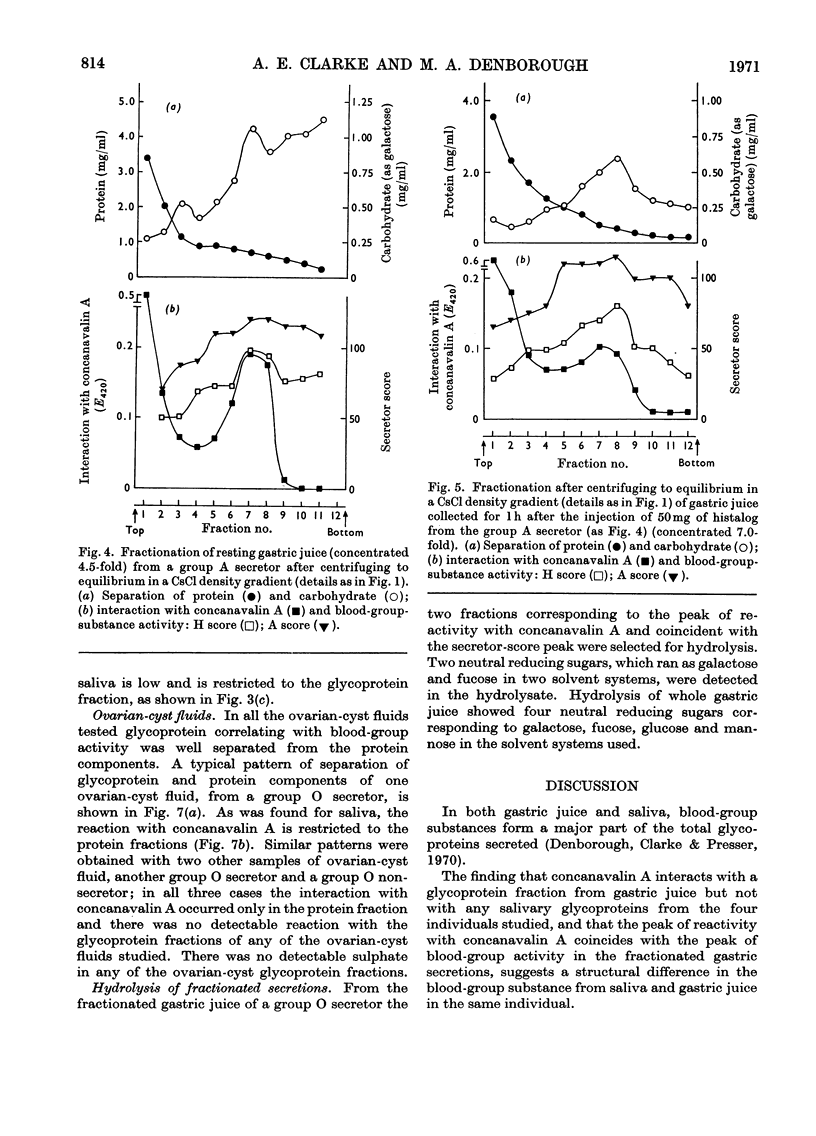
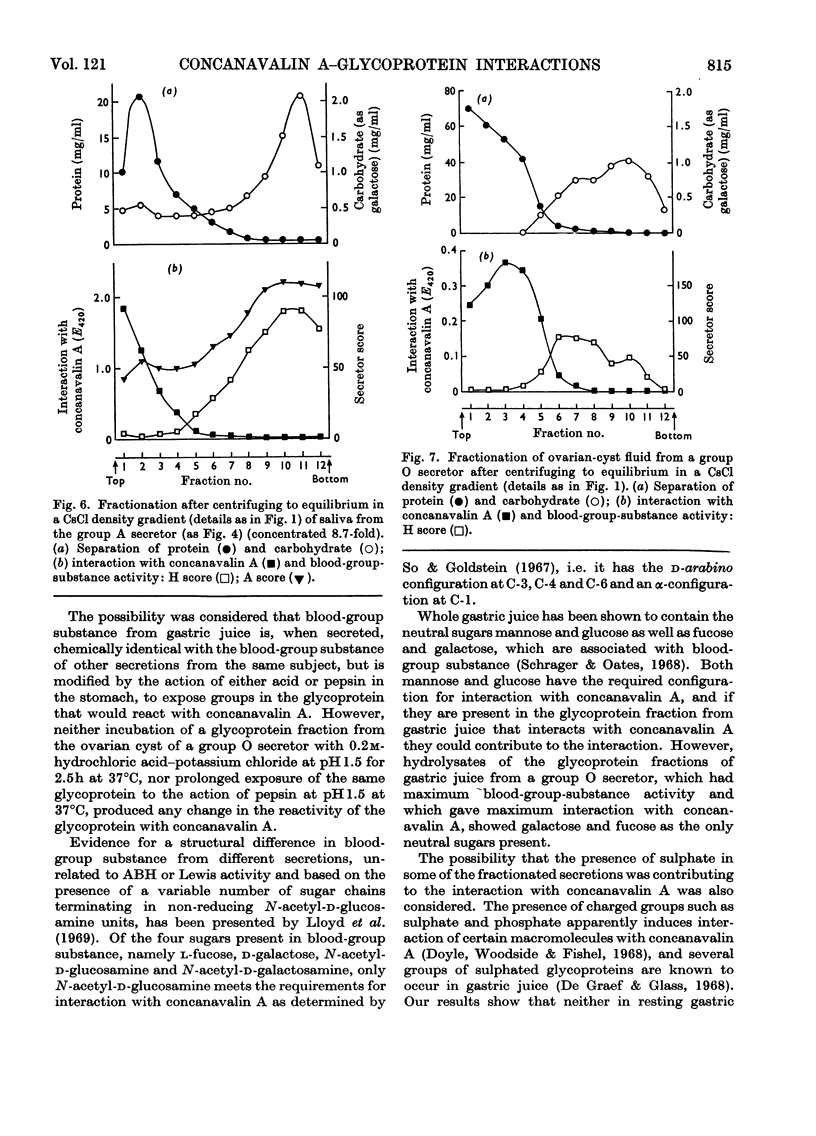
1. Gastric juice, saliva and ovarian-cyst fluid were fractionated into glycoprotein components by centrifuging to equilibrium in a caesium chloride density gradient. 2. The glycoprotein fractions from the gastric juice of two group O non-secretors, two group O secretors and three group A secretors all formed insoluble complexes with concanavalin A. 3. Fractions showing maximum interaction with concanavalin A had maximum blood-group activity measured by the haemagglutination-inhibition technique. The sulphate content of the gastric glycoproteins was unrelated to the capacity to interact with concanavalin A. 4. No interaction was found between concanavalin A and the glycoprotein fractions from any of the saliva or ovarian-cyst-fluid samples tested, implying that there is a structural difference in blood-group-substance glycoproteins in gastric juice when compared with those in saliva and ovarian-cyst fluid. 5. The protein components of each of the secretions tested, gastric juice, saliva and ovarian-cyst fluid, interacted with concanavalin A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraef J., Glass G. B. Chondroitin sulfate A and sulfated tlycoproteins in dog gastric secretion from the fundus. I. Electrophoretic and chemical characterization. Gastroenterology. 1968 Nov;55(5):584–593. [PubMed] [Google Scholar]
- Denborough M. A., Downing H. J., Doig A. G. Serum blood group substances and ABO haemolytic disease. Br J Haematol. 1969 Jan-Feb;16(1):103–109. doi: 10.1111/j.1365-2141.1969.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Woodside E. E., Fishel C. W. Protein-polyelectrolyte interactions. The concanavalin A precipitin reaction with polyelectrolytes and polysaccharide derivatives. Biochem J. 1968 Jan;106(1):35–40. doi: 10.1042/bj1060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L., Yang Y., Callies Q. C. Protein-carbohydrate interaction. XIX. The interaction of concanavalin A with IgM and the glycoprotein phytohemagglutinins of the waxbean and the soybean. J Immunol. 1969 Oct;103(4):695–698. [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Beychok S. Immunochemical studies on blood groups. 43. The interaction of blood group substances from various sources with a plant lectin, concanavalin A. J Immunol. 1969 Jun;102(6):1354–1362. [PubMed] [Google Scholar]
- Morgan W. T., Watkins W. M. Genetic and biochemical aspects of human blood-group A-, B-, H-, Le-a- and Le-b-specificity. Br Med Bull. 1969 Jan;25(1):30–34. doi: 10.1093/oxfordjournals.bmb.a070666. [DOI] [PubMed] [Google Scholar]
- Morse J. H. Immunological studies of phytohaemagglutinin. I. Reaction between phytohaemagglutinin and normal sera. Immunology. 1968 May;14(5):713–724. [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA S., TANAKA K., MURAKAWA S. Specific protein of legumes which reacts with animal proteins. Nature. 1960 Oct 8;188:144–145. doi: 10.1038/188144b0. [DOI] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The carbohydrate components of hydrolysates of gastric secretion and extracts from mucous glands of the gastric body mucosa and antrum. Biochem J. 1968 Jan;106(2):523–529. doi: 10.1042/bj1060523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. E., Smith Z. H., Goldstein I. J. Protein-carbohydrate interaction. A turbidimetric study of the interaction of concanavalin A with amylopectin and glycogen and some of their enzymic and chemical degradation products. Biochem J. 1968 May;107(5):715–724. doi: 10.1042/bj1070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]


