Abstract
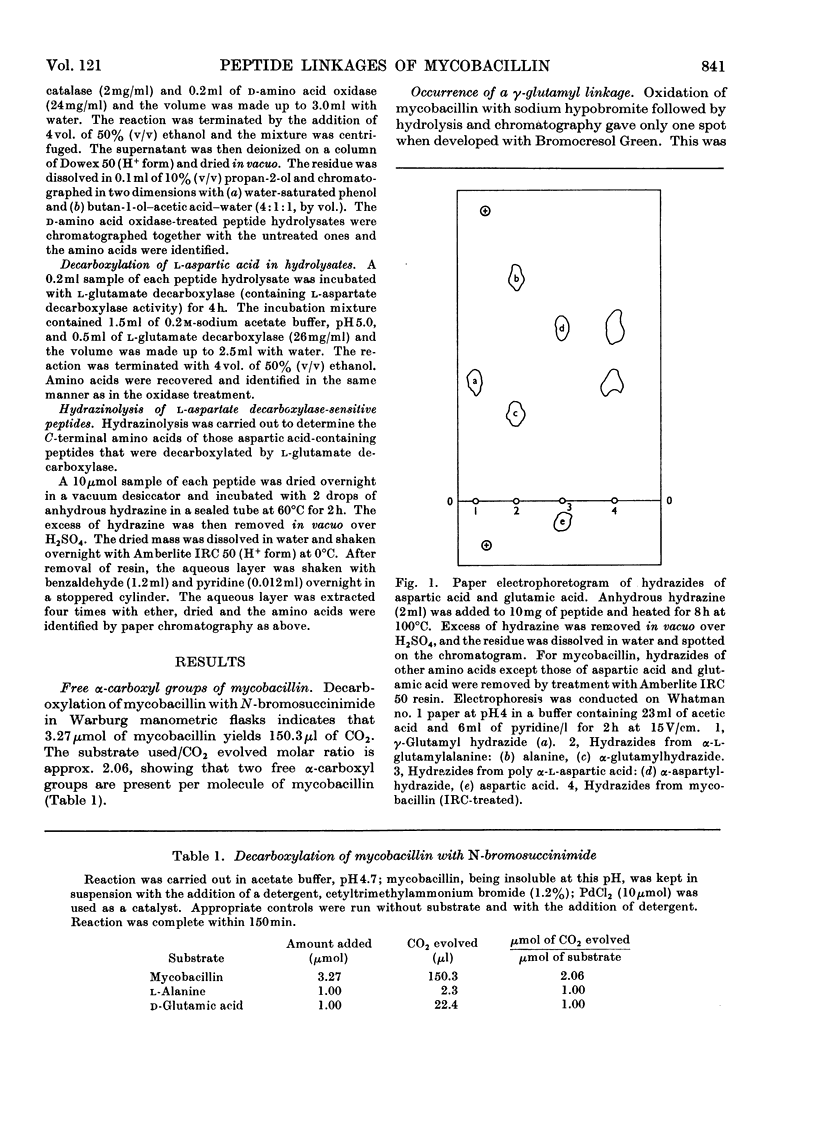
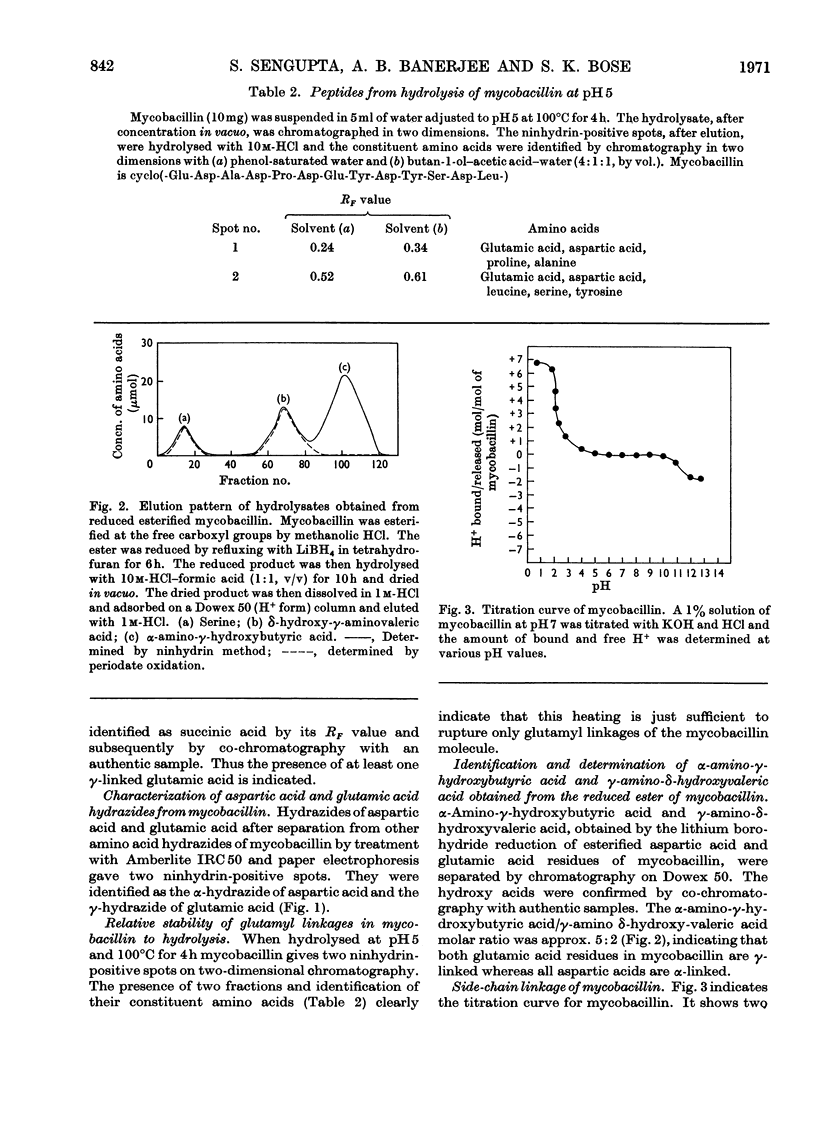
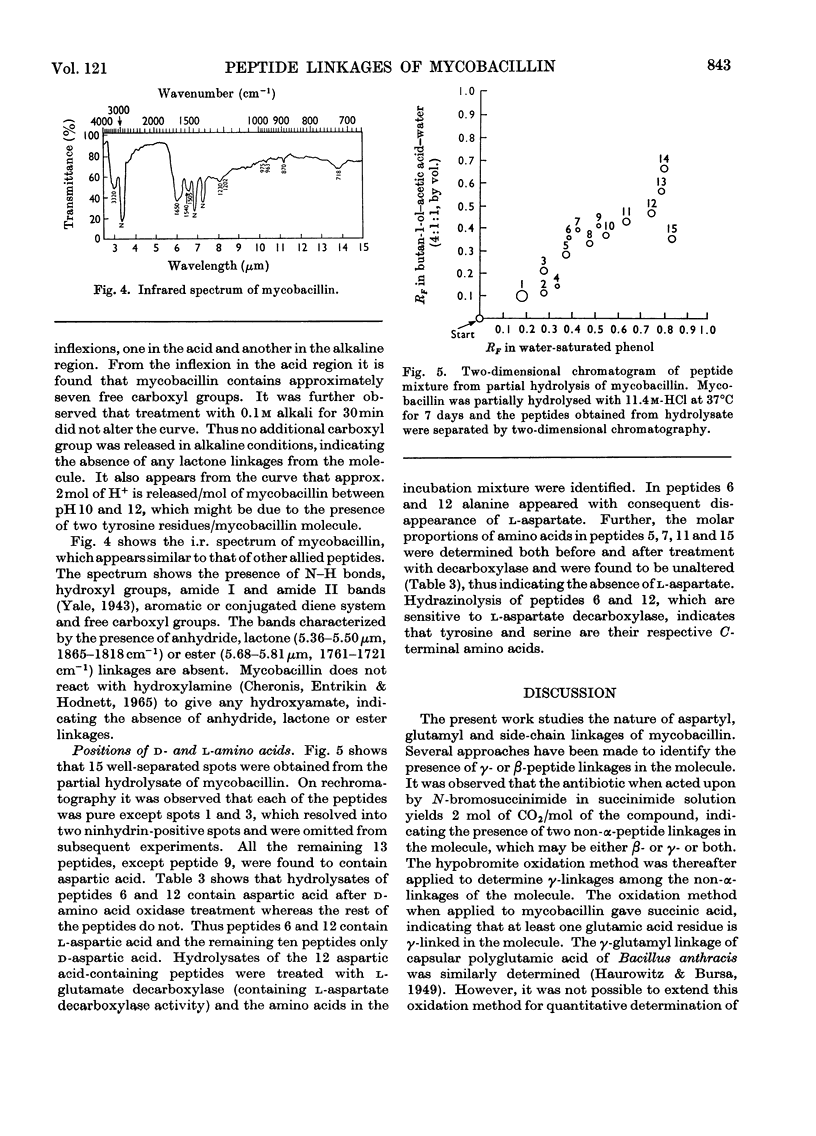
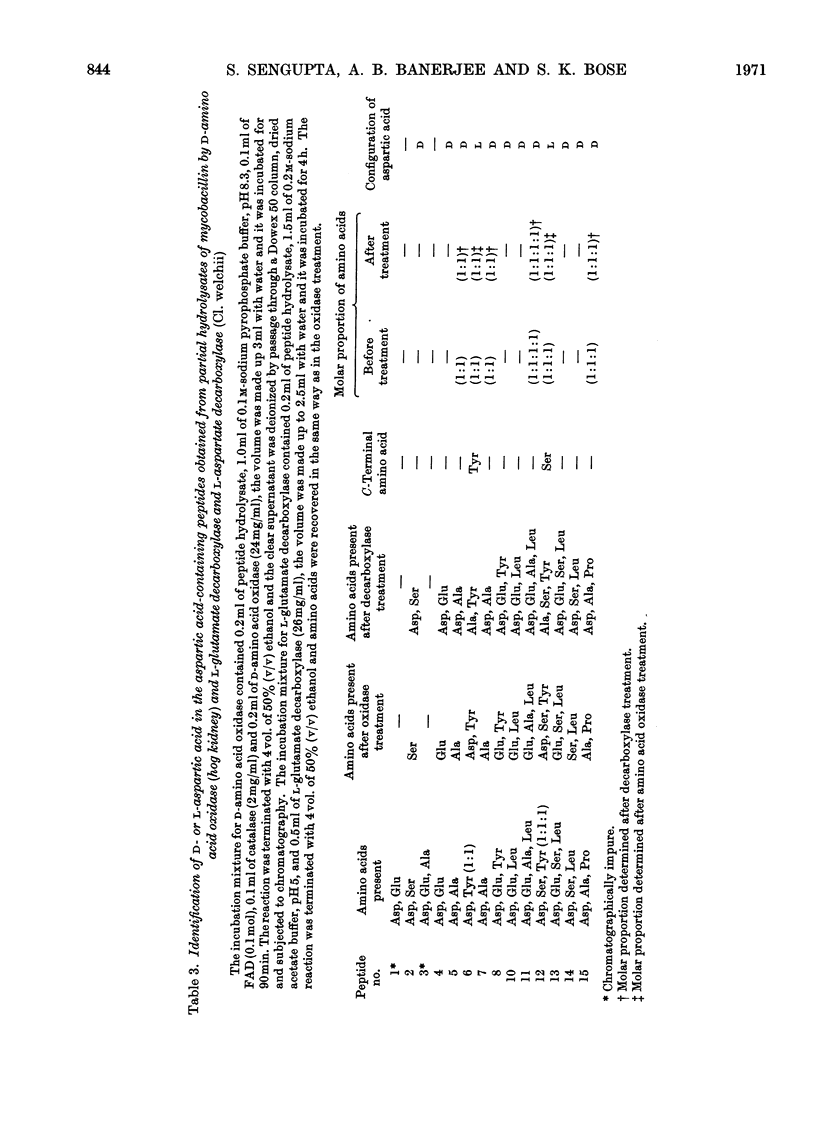
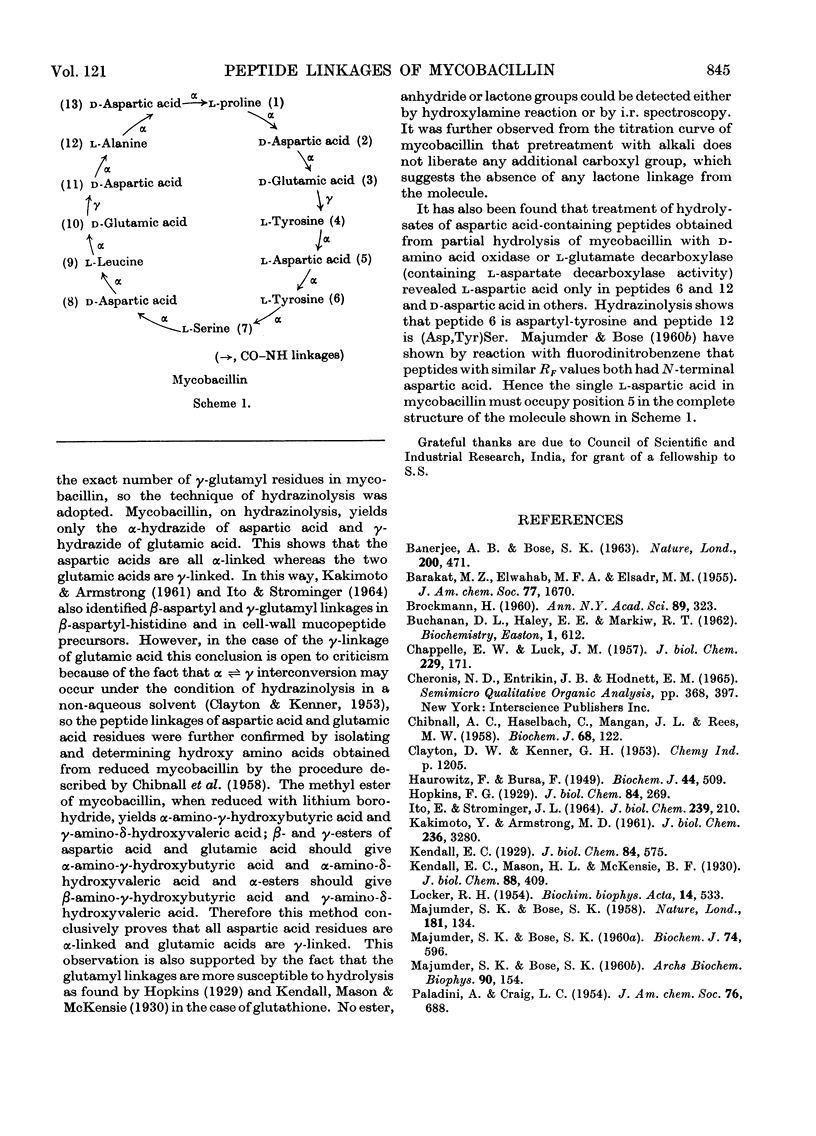
Mycobacillin lacks amino groups but contains two free α-carboxyl groups, indicating the presence of two side-chain peptide linkages. The five aspartic acid residues of mycobacillin are all in α-peptide linkage whereas the two glutamic acid residues are in γ-linkage. Mycobacillin does not react with hydroxylamine to give hydroxamate, indicating the absence of anhydride, lactone and ester linkages. This is also confirmed by i.r. spectroscopy and titration of the molecule. Of the 15 peptides obtained from partial hydrolysates of mycobacillin, 12 contain aspartic acid. Results obtained by treatment of hydrolysates of aspartic acid-containing peptides with d-amino acid oxidase and l-glutamate decarboxylase (containing l-aspartate decarboxylase activity) indicate that residue 5 is l-aspartic acid and residues 2, 8, 11 and 13 are d-aspartic acid. The d- or l-peptide sequence and nature of peptide linkages in mycobacillin are proposed on the basis of these findings and the amino acid sequence reported earlier.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEE A. B., BOSE S. K. AMINO-ACID CONFIGURATION OF MYCOBACILLIN. Nature. 1963 Nov 2;200:471–471. doi: 10.1038/200471a0. [DOI] [PubMed] [Google Scholar]
- BUCHANAN D. L., HALEY E. E., MARKIW R. T. Occurrence of beta-aspartyl and gamma-glutamyl oligopeptides in human urine. Biochemistry. 1962 Jul;1:612–620. doi: 10.1021/bi00910a011. [DOI] [PubMed] [Google Scholar]
- CHAPPELLE E. W., LUCK J. M. The decarboxylation of amino acids, proteins, and peptides by N-bromosucclnimide. J Biol Chem. 1957 Nov;229(1):171–179. [PubMed] [Google Scholar]
- CHIBNALL A. C., HASELBACH C., MANGAN J. L., REES M. W. Studies on the amide and C-terminal residues in proteins. 5. Estimation of asparagine and glutamine residues. Biochem J. 1958 Jan;68(1):122–128. doi: 10.1042/bj0680122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurowitz F., Bursa F. The linkage of glutamic acid in protein molecules. Biochem J. 1949;44(4):509–512. doi: 10.1042/bj0440509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO E., STROMINGER J. L. ENZYMATIC SYNTHESIS OF THE PEPTIDE IN BACTERIAL URIDINE NUCLEOTIDES. III. PURIFICATION AND PROPERTIES OF L-LYSIN-ADDING ENZYME. J Biol Chem. 1964 Jan;239:210–214. [PubMed] [Google Scholar]
- KAKIMOTO Y., ARMSTRONG M. D. beta-L-Aspartyl-L-histidine, a normal constituent of human urine. J Biol Chem. 1961 Dec;236:3280–3282. [PubMed] [Google Scholar]
- LOCKER R. H. C-Terminal groups in myosin, tropomyosin and actin. Biochim Biophys Acta. 1954 Aug;14(4):533–542. doi: 10.1016/0006-3002(54)90233-4. [DOI] [PubMed] [Google Scholar]
- MAJUMDAR S. K., BOSE S. K. Amino acid sequence in mycobacillin. Biochem J. 1960 Mar;74:596–599. doi: 10.1042/bj0740596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJUMDAR S. K., BOSE S. K. Mycobacillin, a new antifungal antibiotic produced by B. subtilis. Nature. 1958 Jan 11;181(4602):134–135. doi: 10.1038/181134a0. [DOI] [PubMed] [Google Scholar]
- REES M. W. Studies on the amide and C-terminal residues in proteins. 4. Separation and quantitative determination of beta-amino alcohols. Biochem J. 1958 Jan;68(1):118–122. doi: 10.1042/bj0680118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWALLOW D. L., ABRAHAM E. P. The amide and carboxyl groups of bacitracin A. Biochem J. 1959 Jun;72(2):326–332. doi: 10.1042/bj0720326. [DOI] [PMC free article] [PubMed] [Google Scholar]


