Abstract
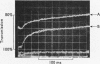
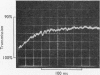
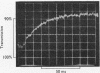
Transient kinetic studies of the reversible oxidative phosphorylation of d-glyceraldehyde 3-phosphate catalysed by d-glyceraldehyde 3-phosphate dehydrogenase show that all four sites of the tetrameric lobster enzyme are simultaneously active, apparently with equal reactivity. The rate-determining step of the oxidative phosphorylation is NADH release at high pH and phosphorolysis of the acyl-enzyme at low pH. For the reverse reaction the rate-determining step is a process associated with NADH binding, probably a conformation change, at high pH and d-glyceraldehyde 3-phosphate release at low pH. NADH has previously been shown to be a competitive inhibitor of the enzyme with respect to d-glyceraldehyde 3-phosphate and vice versa. This is consistent with the mechanism deduced from transient experiments given the additional proviso that 1-arseno-3-phosphoglycerate has a half-life of about 1min or longer at pH7. The dissociation constants of d-glyceraldehyde 3-phosphate and 1,3-diphosphoglycerate to the NAD+-bound enzyme are too large to measure but are nevertheless consistent with the low Km values of these substrates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard S. A., Dunn M. F., Luisi P. L., Schack P. Mechanistic studies on equine liver alcohol dehydrogenase. I. The stoichiometry relationship of the coenzyme binding sites to the catalytic sites active in the reduction of aromatic aldehydes in the transient state. Biochemistry. 1970 Jan 6;9(1):185–192. doi: 10.1021/bi00803a024. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- FISHER H. F., CONN E. E., VENNESLAND B., WESTHEIMER F. H. The enzymatic transfer of hydrogen. I. The reaction catalyzed by alcohol dehydrogenase. J Biol Chem. 1953 Jun;202(2):687–697. [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Heck H. D., McMurray C. H., Gutfreund H. The resolution of some steps of the reactions of lactate dehydrogenase with its substrates. Biochem J. 1968 Aug;108(5):793–796. doi: 10.1042/bj1080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra O. P., Bernhard S. A. Spectrophotometric identification of an active site-specific acyl glyceraldehyde 3-phosphate dehydrogenase. The regulation of its kinetic and equilibrium properties by coenzyme. J Biol Chem. 1968 Mar 25;243(6):1243–1252. [PubMed] [Google Scholar]
- SEGAL H. L., BOYER P. D. The role of sulfhydryl groups in the activity of D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1953 Sep;204(1):265–281. [PubMed] [Google Scholar]
- Shore J. D., Gutfreund H. Transients in the reactions of liver alcohol dehydrogenase. Biochemistry. 1970 Nov 24;9(24):4655–4659. doi: 10.1021/bi00826a006. [DOI] [PubMed] [Google Scholar]
- Teipel J., Koshland D. E., Jr The effect of NAD+ on the catalytic efficiency of glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. Biochim Biophys Acta. 1970 Feb 11;198(2):183–191. doi: 10.1016/0005-2744(70)90050-1. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Reactions of D-glyceraldehyde 3-phosphate dehydrogenase facilitated by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1971 Mar;122(1):59–69. doi: 10.1042/bj1220059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON H. C., BANASZAK L. J. STRUCTURE OF GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE. STRUCTURE SYMMETRY WITHIN THE MOLECULE. Nature. 1964 Dec 5;204:918–920. doi: 10.1038/204918a0. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., Hilvers A. G., Van Lis J. M., Slater E. C. Function and role of NAD+ in mechanism of action of rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1969 Nov 4;191(2):221–228. doi: 10.1016/0005-2744(69)90241-1. [DOI] [PubMed] [Google Scholar]