Abstract
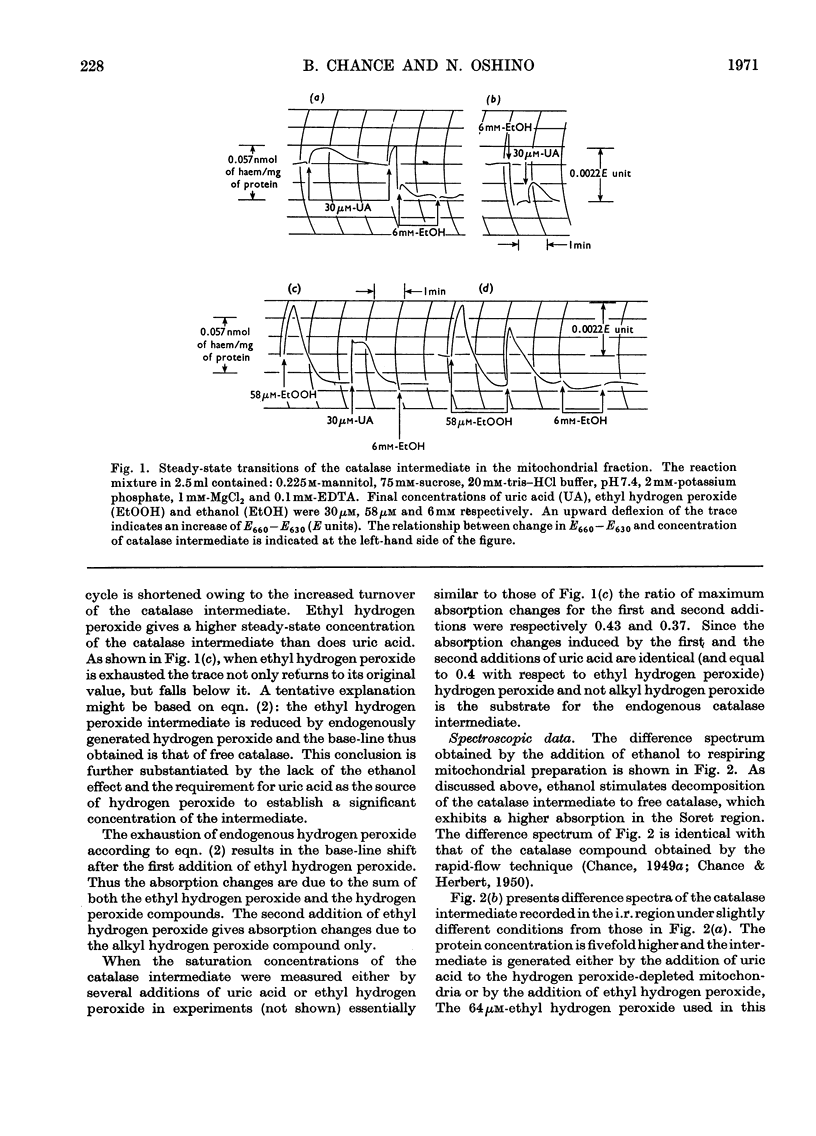
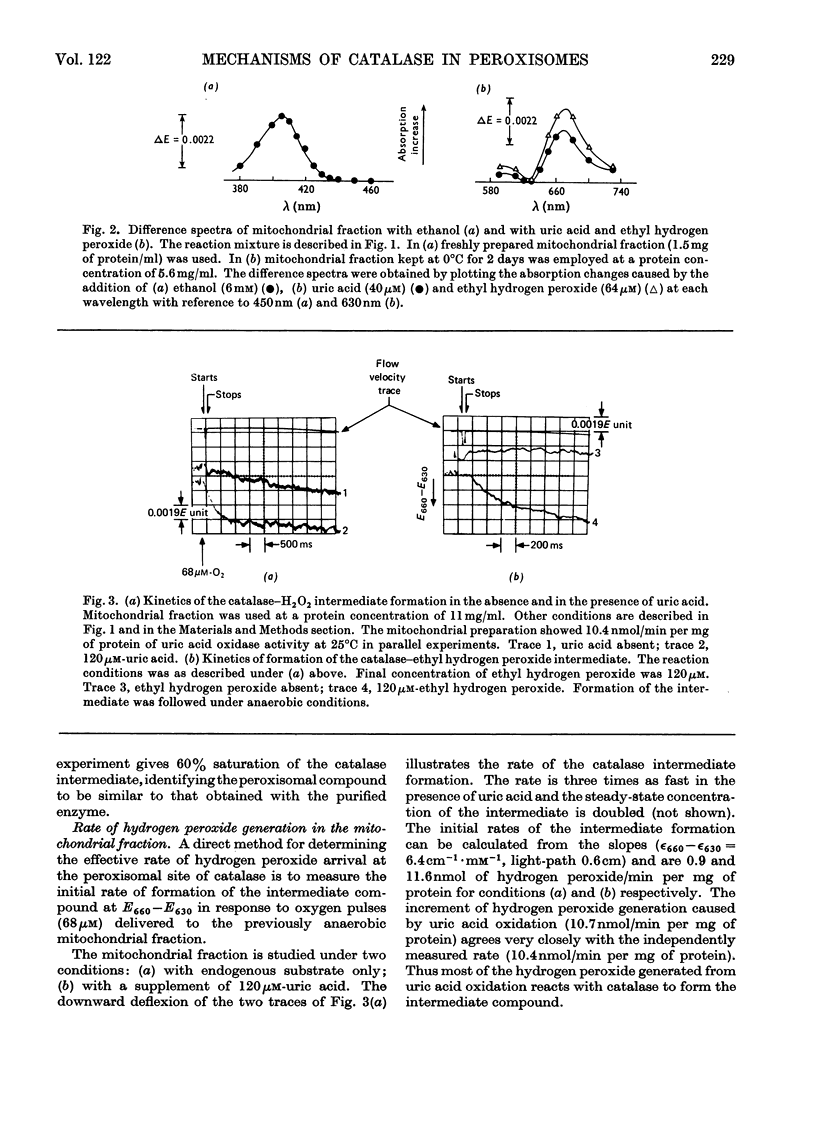
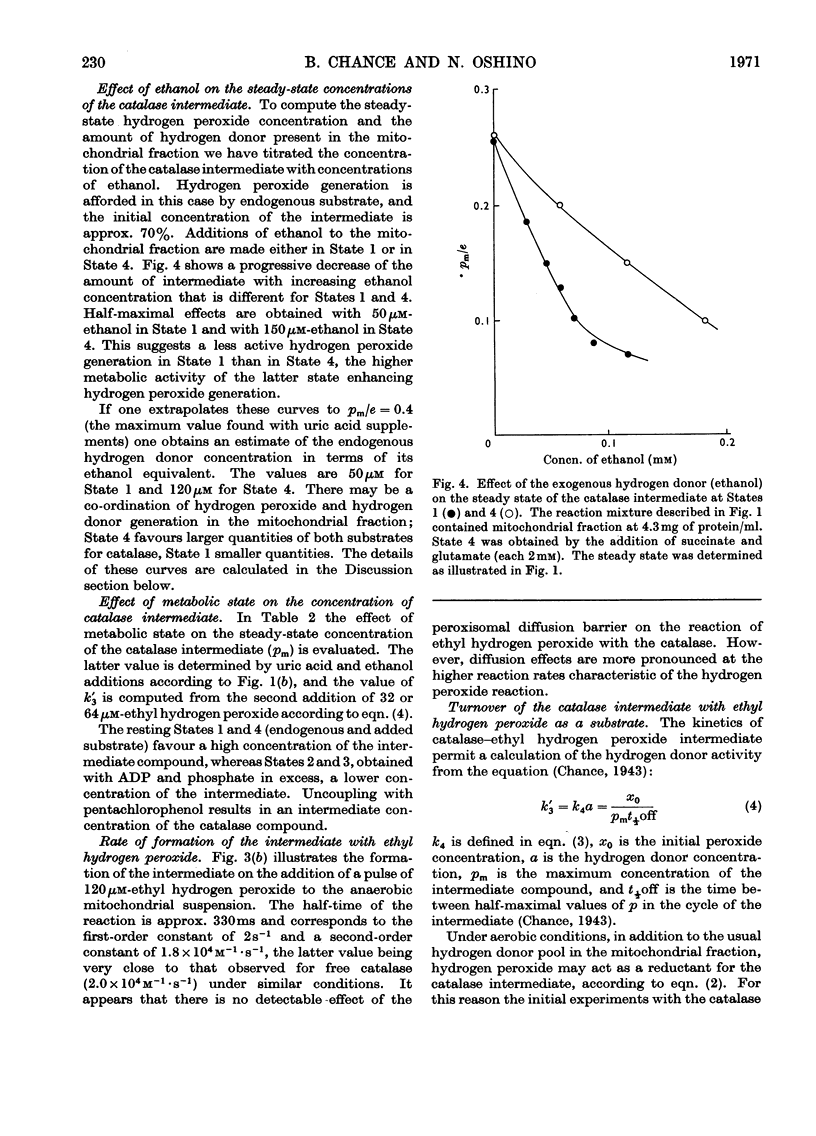
1. The primary intermediate of catalase and hydrogen peroxide was identified and investigated in peroxisome-rich mitochondrial fractions of rat liver. On the basis of kinetic constants determined in vitro, it is possible to calculate with reasonable precision the molecular statistics of catalase action in the peroxisomes. 2. The endogenous hydrogen peroxide generation is adequate to sustain a concentration of the catalase intermediate (pm/e) of 60–70% of the hydrogen peroxide saturation value. Total amount of catalase corresponds to 0.12–0.15nmol of haem iron/mg of protein. In State 1 the rate of hydrogen peroxide generation corresponds to 0.9nmol/min per mg of protein or 5% of the mitochondrial respiratory rate in State 4. 3. Partial saturation of the catalase intermediate with hydrogen peroxide (pm/e) in the mitochondrial fraction suggests its significant peroxidatic activity towards its endogenous hydrogen donor. A variation of this value (pm/e) from 0.3 in State 4 to 0 under anaerobic conditions is observed. 4. For a particular preparation the hydrogen peroxide generation rate in the substrate-supplemented State 4 corresponds to 0.17s−1 (eqn. 6), the hydrogen peroxide concentration to 2.5nm and the hydrogen-donor concentration (in terms of ethanol) to 0.12mm. The reaction is 70% peroxidatic and 30% catalatic. 5. A co-ordinated production of both oxidizing and reducing substrates for catalase in the mitochondrial fraction is suggested by a 2.2-fold increase of hydrogen peroxide generation and a threefold increase in hydrogen-donor generation in the State 1 to State 4 transition. 6. Additional hydrogen peroxide generation provided by the urate oxidase system of peroxisomes (8–12nmol of uric acid oxidized/min per mg of protein) permits saturation of the catalase with hydrogen peroxide to haem occupancy of 40% compared with values of 36% for a purified rat liver catalase ofk1=1.7×107m−1·s−1 and k′4=2.6×107m−1· s−1(Chance, Greenstein & Roughton, 1952). 7. The turnover of the catalase ethyl hydrogen peroxide intermediate (k′3) in the peroxisomes is initially very rapid since endogenous hydrogen peroxide acts as a hydrogen donor. k′3 decreases fivefold in the uncoupled state of the mitochondria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. Enzyme-substrate compounds. Adv Enzymol Relat Subj Biochem. 1951;12:153–190. doi: 10.1002/9780470122570.ch3. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HERBERT D. The enzymesubstrate compounds of bacterial catalase and peroxides. Biochem J. 1950 Apr;46(4):402–414. doi: 10.1042/bj0460402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HIGGINS J. Peroxidase kinetics in coupled oxidation; an experimental and theoretical study. Arch Biochem Biophys. 1952 Dec;41(2):432–441. doi: 10.1016/0003-9861(52)90472-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., SCHONBAUM G. R. The nature of the primary complex of catalase. J Biol Chem. 1962 Jul;237:2391–2395. [PubMed] [Google Scholar]
- CHANCE B. The reactions of catalase in the presence of the notatin system. Biochem J. 1950 Apr;46(4):387–402. doi: 10.1042/bj0460387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J Biol Chem. 1955 Nov;217(1):395–407. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B. The behavior of catalase and peroxidase in coupled reactions. Ann N Y Acad Sci. 1969 Dec 19;168(2):354–355. doi: 10.1111/j.1749-6632.1969.tb43122.x. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalase-hydrogen peroxide system. A theoretical appraisal of the mechanism of catalase action. Biochem J. 1968 Dec;110(4):621–629. doi: 10.1042/bj1100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of azide-catalase. Biochem J. 1945;39(2):148–157. doi: 10.1042/bj0390148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer M. L. Peroxidatic activity of catalase. Biochim Biophys Acta. 1970 Feb 11;198(2):199–209. doi: 10.1016/0005-2744(70)90052-5. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of enzymes. IX. Certain purine-metabolizing enzymes. J Biol Chem. 1952 Mar;195(1):161–166. [PubMed] [Google Scholar]
- Scholz R., Thurman R. G., Williamson J. R., Chance B., Bücher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969 May 10;244(9):2317–2324. [PubMed] [Google Scholar]
- Sies H., Brauser B., Bücher T. On the state of mitochondria in perfused liver: Action of sodium azide on respiratory carriers and respiration. FEBS Lett. 1969 Dec 30;5(5):319–323. doi: 10.1016/0014-5793(69)80346-7. [DOI] [PubMed] [Google Scholar]
- Sies H., Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970 Dec;11(3):172–176. doi: 10.1016/0014-5793(70)80521-x. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Yamane T. Proton magnetic resonance studies of human cyanomethemoglobin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1199–1206. doi: 10.1073/pnas.61.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T. Cytochrome c peroxidase. Adv Enzymol Relat Areas Mol Biol. 1970;33:309–335. doi: 10.1002/9780470122785.ch6. [DOI] [PubMed] [Google Scholar]


