Abstract
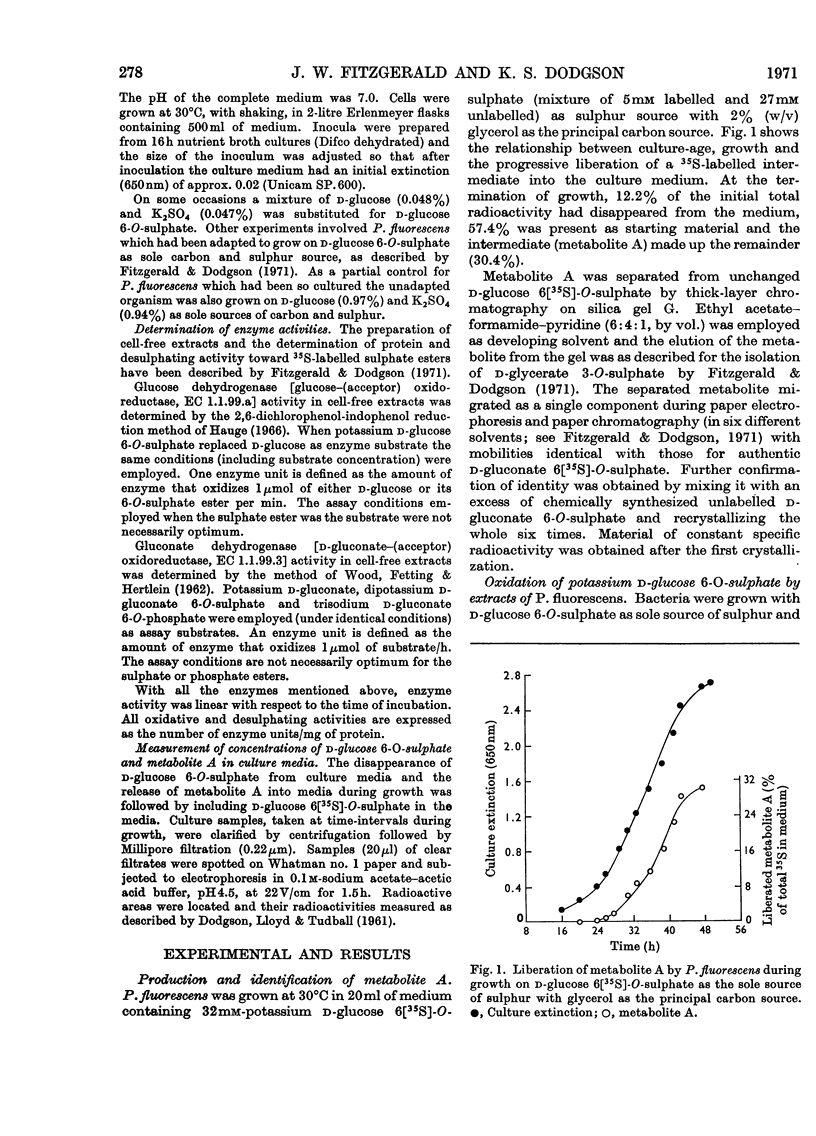
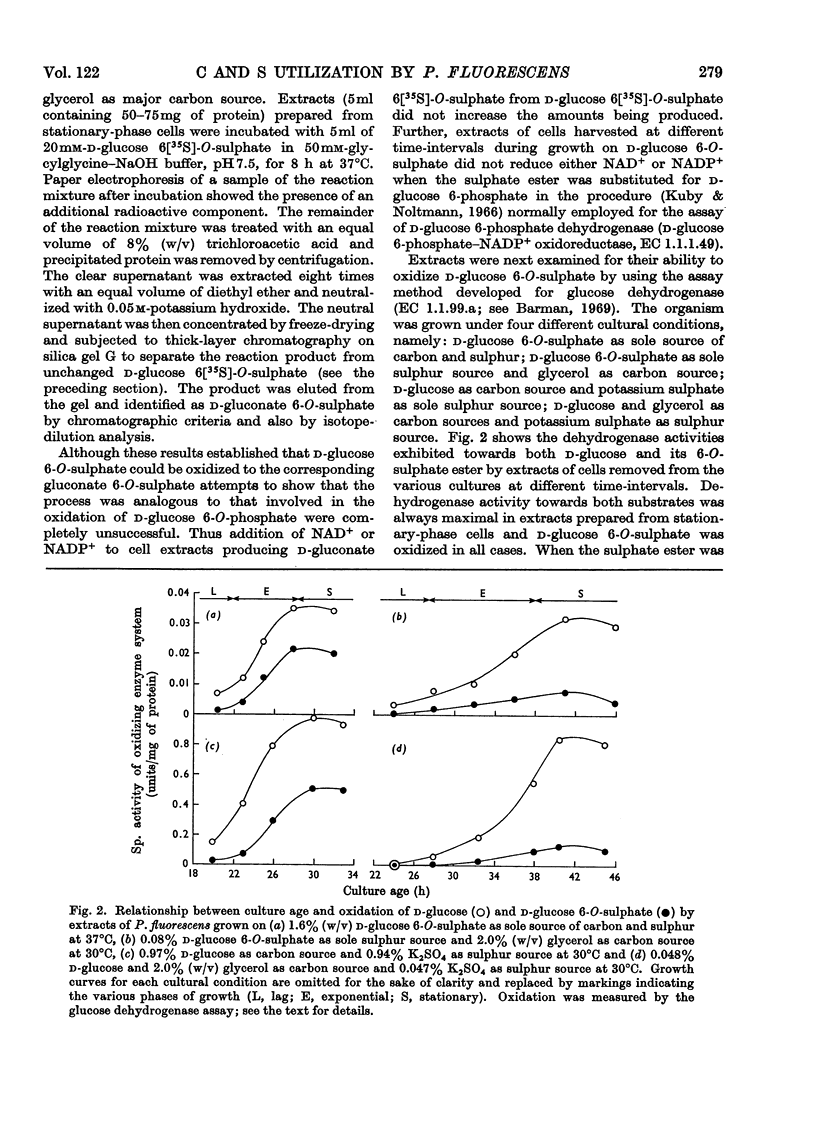
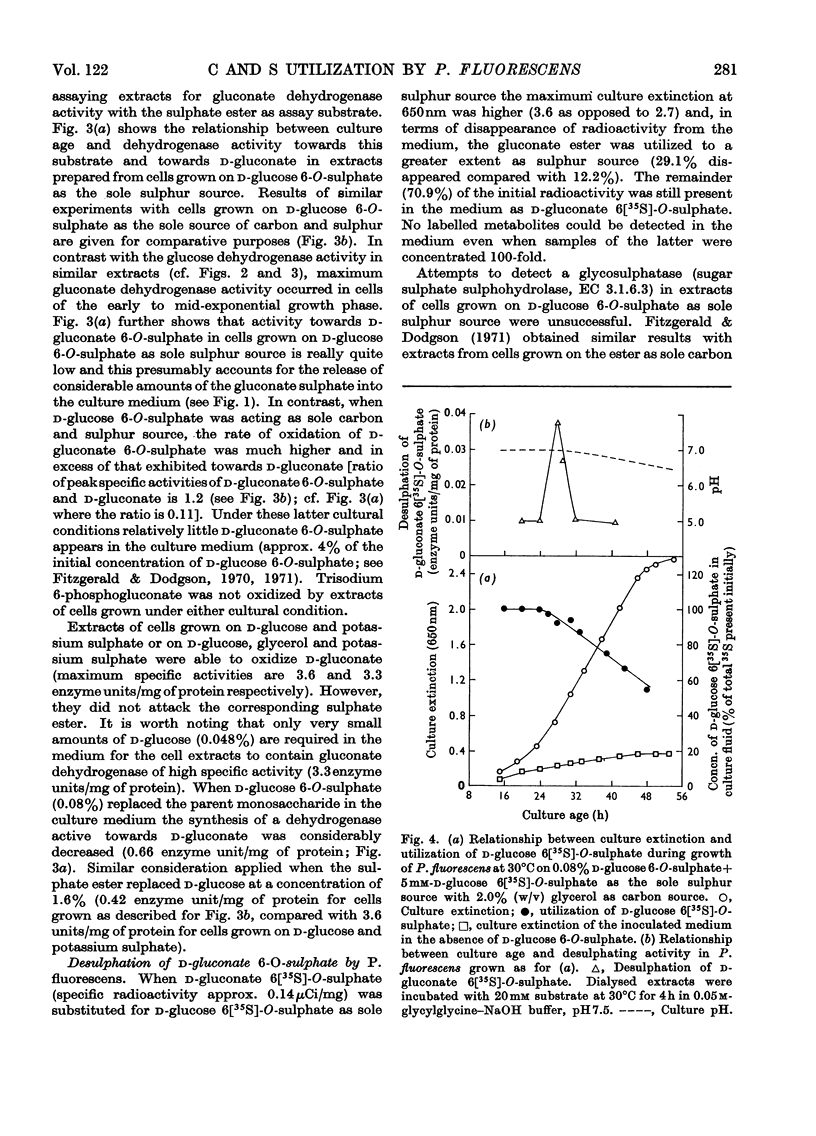
Pseudomonas fluorescens N.C.I.B. 8248, cultured on potassium d-glucose 6[35S]-O-sulphate as the sole sulphur source, liberated the 6-O-sulphate ester of d-gluconate into the culture medium. Extracts of bacteria grown under this cultural condition oxidized d-glucose 6-O-sulphate to yield the gluconate ester. Results suggest the involvement of a glucose dehydrogenase-like enzyme. The gluconate ester was apparently not oxidized further to any significant extent; however, it served as substrate for a desulphating enzyme found in extracts. Growth on d-glucose 6-O-sulphate as the sole source of sulphur was not associated with the appearance of a true glycosulphatase. Collectively, these results suggest that d-gluconate 6-O-sulphate, rather than the glucose ester, supplied the necessary sulphur for growth. Oxidative activities toward d-glucose 6-O-sulphate, d-glucose, d-gluconate 6-O-sulphate and d-gluconate found in extracts of P. fluorescens adapted to grow on d-glucose 6-O-sulphate as the sole source of carbon and sulphur are presented for comparative purposes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE LEY J. Comparative carbohydrate metabolism and localization of enzymes in Pseudomonas and related microorganisms. J Appl Bacteriol. 1960 Dec;23:400–441. doi: 10.1111/j.1365-2672.1960.tb00215.x. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., LLOYD A. G., TUDBALL N. O-sulphate esters of L-serine, L-threonine and L-hydroxyproline. Biochem J. 1961 Apr;79:111–117. doi: 10.1042/bj0790111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Production of sulphated intermediates by Pseudomonas fluorescens during growth on potassium D-glucose 6-O-sulphate. Biochem J. 1970 Sep;119(3):30P–30P. doi: 10.1042/bj1190030p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Sulphur utilization during growth of pseudomonas fluorescens on potassium D-glucose 6-O-sulphate. Biochem J. 1971 Feb;121(3):521–528. doi: 10.1042/bj1210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Thomas P., Tudball N., Winterburn P. J. Preparation of D-glycerate 3-O-sulphate, DL- glycerate 3( 35 S)-O-sulphate and D-glucose 6-O-sulphate. Biochem J. 1971 Feb;121(3):529–530. doi: 10.1042/bj1210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. G., Large P. J., Davies M., Olavesen A. H., Dodgson K. S. The glycosulphatase of Trichoderma viride. Biochem J. 1968 Jul;108(3):393–399. doi: 10.1042/bj1080393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood L. B., Tabenkin B., Ward G. E. The Production of Gluconic Acid and 2-Keto-Gluconic Acid from Glucose by Species of Pseudomonas and Phytomonas. J Bacteriol. 1941 Jul;42(1):51–61. doi: 10.1128/jb.42.1.51-61.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS A. K., EAGON R. G. Oxidation of 2-deoxy-D-glucose to 2-deoxy-D-gluconic acid by extracts of pseudomonas aeruginosa. J Bacteriol. 1959 Feb;77(2):167–172. doi: 10.1128/jb.77.2.167-172.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]


